Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
1.3. Trial Design
1.4. Randomization and Blinding
2. Materials and Methods
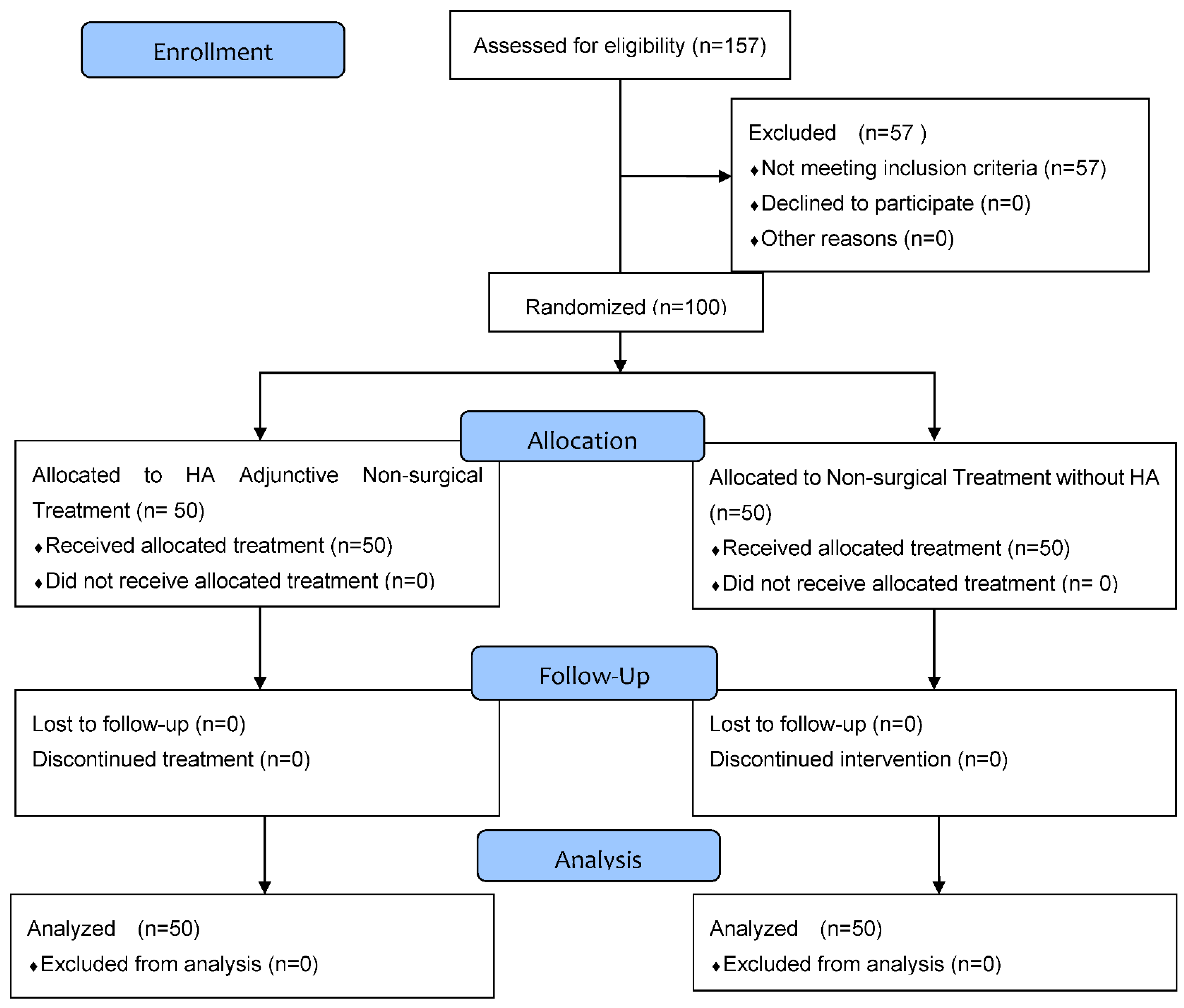
2.1. Participants
2.2. Data Collection
2.3. Clinical Parameters
2.4. Intervention
2.5. Hyaluronic Acid Gel
2.6. Safety Monitoring
2.7. Statistical Methods
3. Results
3.1. Adverse Events
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Monsarrat, P.; Vergnes, J.-N.; Cantagrel, A.; Algans, N.; Cousty, S.; Kemoun, P.; Bertrand, C.; Arrive, E.; Bou, C.; Sedarat, C.; et al. Effect of periodontal treatment on the clinical parameters of patients with rheumatoid arthritis: Study protocol of the randomized, controlled ESPERA trial. Trials 2013, 14, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, J.; Papapanou, P.N.; Philips, K.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J. Physiol. 2016, 595, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Walker, C.B. Treating chronic periodontitis: Current status, challenges, and future directions. Clin. Cosmet. Investig. Dent. 2010, 2, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- De Angelis, N.; Hanna, R.; Signore, A.; Amaroli Benedicenti, S. Effectiveness of dual-wavelength (Diodes 980 Nm and 635 Nm) laser approach as a non-surgical modality in the management of periodontally diseased root surface: A pilot study. Biotechnol. Biotechnol. Equip. 2018, 32, 1575–1582. [Google Scholar] [CrossRef]
- Amaroli, A.; Barbieri, R.; Signore, A.; Marchese, A.; Parker, S.; De Angelis, N.; Benedicenti, S. Simultaneous photoablative and photodynamic 810-nm diode laser therapy as an adjunct to non-surgical periodontal treatment: An in-vitro study. Minerva Stomatol. 2020, 69, 1–7. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef]
- Sukumar, S.; Drízhal, I. Hyaluronic Acid and Periodontitis. Acta Med. 2007, 50, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef]
- Cardoso, J.F.; Perasoli, F.B.; Almeida, T.C.; Marques, M.B.D.F.; Toledo, C.R.; Gil, P.O.; Tavares, H.D.S.; Da Paz, M.C.; Mussel, W.D.N.; Magalhães, J.T.; et al. Vancomycin-loaded N,N-dodecyl, methyl-polyethylenimine nanoparticles coated with hyaluronic acid to treat bacterial endophthalmitis: Development, characterization, and ocular biocompatibility. Int. J. Biol. Macromol. 2021, 169, 330–341. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Agiwal, S.; Srivastava, A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int. J. Biol. Macromol. 2020, 165, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0). Available online: http://www.randomizer.org/ (accessed on 9 October 2020).
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Klinische Bewertungsverahren zur Objektivierung der Mundhygiene [Clinical methods for the objective evaluation of oral hygiene]. Dtsch Zahnarztl Z. 1977, 32, 44–47. [Google Scholar]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. Pro-resolving mediators in the regulation of periodontal disease. Mol. Asp. Med. 2017, 58, 21–36. [Google Scholar] [CrossRef]
- Salvi, G.E.; Lang, N.P. The Effects of Non-Steroidal Anti-Inflammatory Drugs (Selective and Non-Selective) on the Treatment of Periodontal Diseases. Curr. Pharm. Des. 2005, 11, 1757–1769. [Google Scholar] [CrossRef]
- Pagnacco, A.; Vangelisti, R.; Erra, C.; Poma, A. Double-blind clinical trial versus placebo of a new sodium-hyaluronate-based gingival gel. Attual. Ter. Internazionale 1997, 15, 1–7. [Google Scholar]
- Eliezer, M.; Imber, J.-C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Periodontal Disease Mechanisms: Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2008, 6, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.T.; Illés, J.; Székely, Z.; Forrai, E.; Gere, A. Effect of different metal ions on the oxidative damage and antioxidant capacity of hyaluronic acid. Arch. Biochem. Biophys. 2003, 410, 76–82. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Kobayashi, E.; Hernandez, M.; Zhang, Y.; Miron, R.J. Hyaluronic Acid Gel-Based Scaffolds as Potential Carrier for Growth Factors: An In Vitro Bioassay on Its Osteogenic Potential. J. Clin. Med. 2016, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Fujioka-Kobayashi, M.; Müller, H.-D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2018, 23, 1133–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awartani, F.A.; Tatakis, D.N. Interdental papilla loss: Treatment by hyaluronic acid gel injection: A case series. Clin. Oral Investig. 2015, 20, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Mdala, I.; Olsen, I.; Haffajee, A.D.; Socransky, S.S.; Thoresen, M.; De Blasio, B.F. Comparing clinical attachment level and pocket depth for predicting periodontal disease progression in healthy sites of patients with chronic periodontitis using multi-state M arkov models. J. Clin. Periodontol. 2014, 41, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Göcke, R. Treatment of gingivitis with hyaluronan. J. Clin. Periodontol. 2003, 30, 159–164. [Google Scholar] [CrossRef]
- Pistorius, A.; Martin, M.; Willershausen, B.; Rockmann, P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005, 36, 531–538. [Google Scholar]
- Xu, Y.; Höfling, K.; Fimmers, R.; Frentzen, M.; Jervøe-Storm, P.M. Clinical and Microbiological Effects of Topical Subgingival Application of Hyaluronic Acid Gel Adjunctive to Scaling and Root Planing in the Treatment of Chronic Periodontitis. J. Periodontol. 2004, 75, 1114–1118. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Eriani, J.; Serroni, I.; Liani, G.; Borelli, V.; Castronovo, G.; Di Lenarda, R. Effectiveness of adjunctive subgingival administration of amino acids and sodium hyaluronate gel on clinical and immunological parameters in the treatment of chronic periodontitis. Ann. Stomatol. 2012, 3, 75–81. [Google Scholar]
- Eick, S.; Renatus, A.; Heinicke, M.; Pfister, W.; Stratul, S.-I.; Jentsch, H. Hyaluronic Acid as an Adjunct After Scaling and Root Planing: A Prospective Randomized Clinical Trial. J. Periodontol. 2013, 84, 941–949. [Google Scholar] [CrossRef]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local Delivery of Hyaluronan as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Wan, P. A Clinical Trial of Local Delivery of Hyaluronic Acid Gel as an Adjunct to Non-Surgical Treatment of Chronic Peri-Odontitis; The University of Hong Kong: Hong Kong, China, 2004. [Google Scholar]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal Res. 2018, 54, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Baramappa, R.; Rao, N.M.; Pavaluri, A.K. Hyaluronic Acid as an Adjunct to Scaling and Root Planing in Chronic Periodontitis. A Randomized Clinical Trail. J. Clin. Diagn. Res. 2014, 8, ZC11–ZC14. [Google Scholar] [CrossRef] [PubMed]
| Control Group | Study HA Group | Difference † (95% CI) | p * | |
|---|---|---|---|---|
| Before therapy | ||||
| BoP (%) | 31 (22.8–40.3) | 33.5 (23.8–42) | 0 (−4 to 5) | 0.79 |
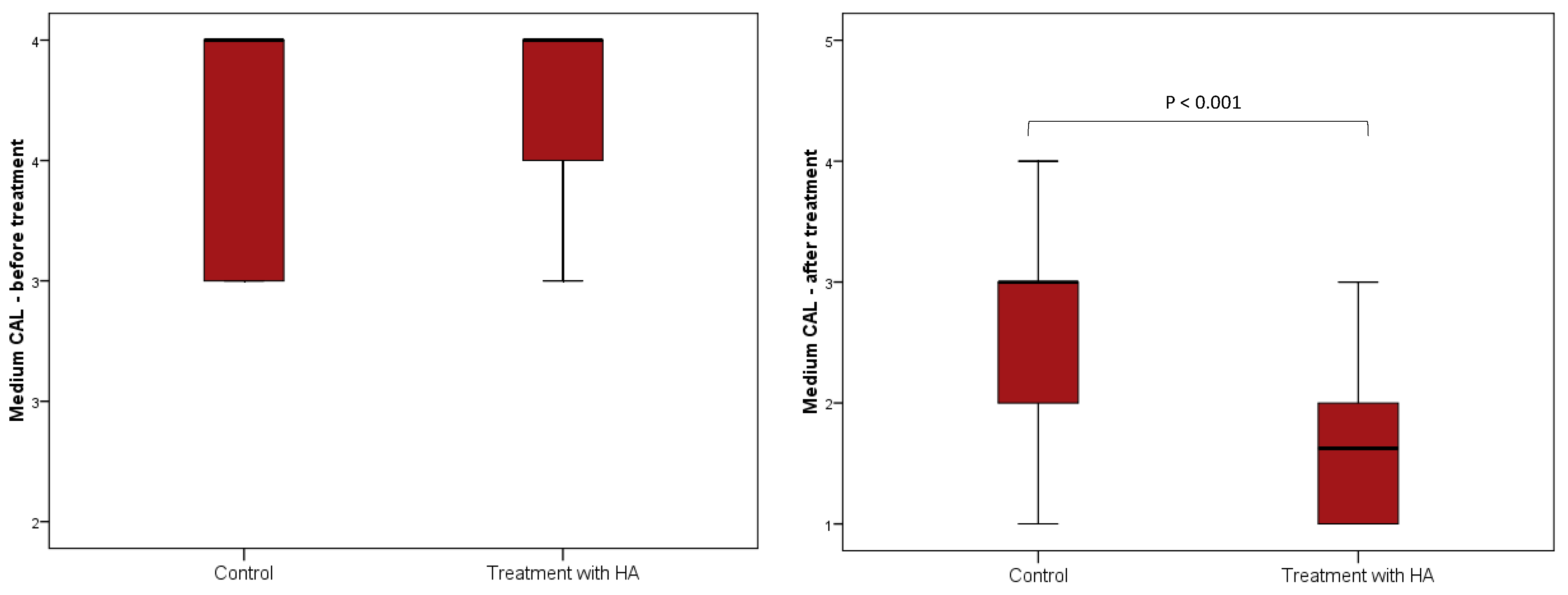
| CAL (mm) | 4 (3–4) | 4 (3.5–4) | 0 (0–0) | 0.90 |
| PPD (mm) | 4.25 (4–4.5) | 4.75 (4.4–5) | 0.25 (0–0.5) | 0.001 |
| After therapy | ||||
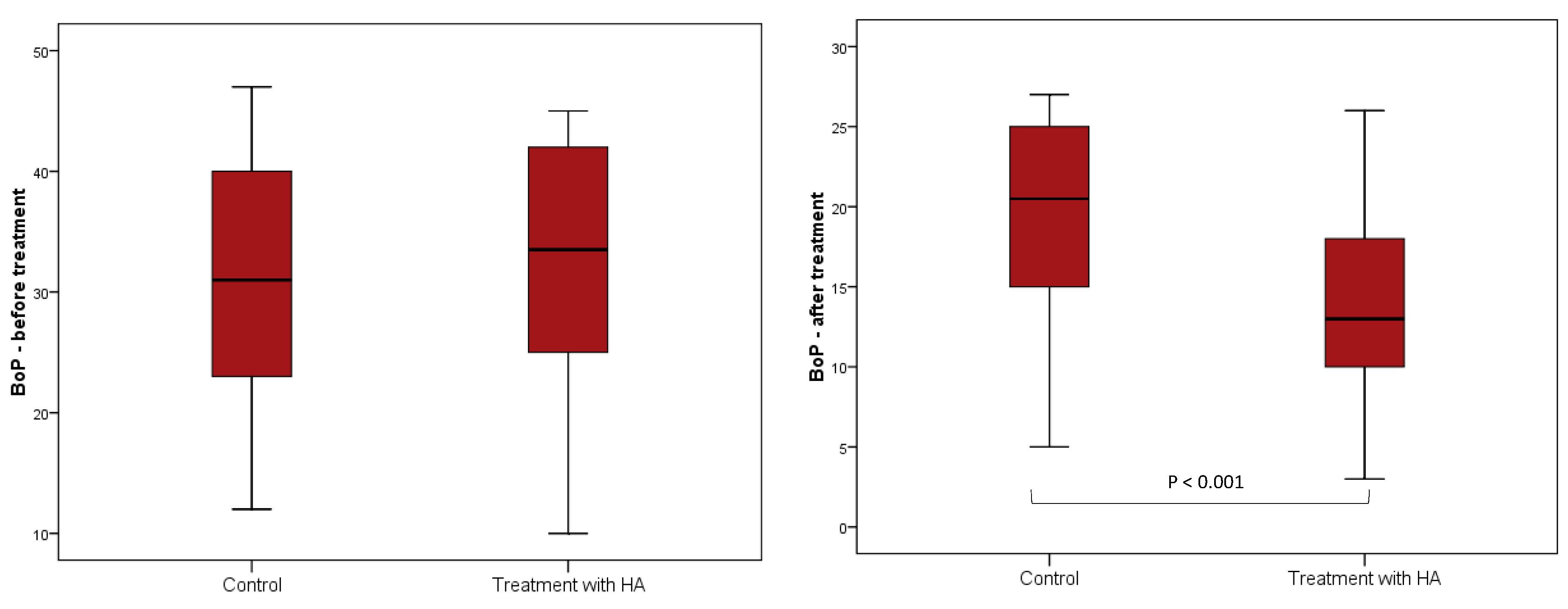
| BoP (%) | 20.5 (15–25) | 13 (9.5–18.25) | −6 (−10 to −3) | <0.001 |
| CAL (mm) | 3 (2–3) | 1.63 (1–2) | −1 (−1.25 to −1) | <0.001 |
| PPD (mm) | 3.5 (2.8–3.8) | 3.5 (2.75–3.75) | 0 (−0.25 to 0.25) | 0.70 |
| Median (Interquartile Range) | Difference † (95% CI) | p * | ||
|---|---|---|---|---|
| Before Treatment | After Treatment | |||
| Control Group | ||||
| BoP (%) | 31 (22.8–40.3) | 20.5 (15–25) | −12 (−14 to −9.5) | <0.001 |
| CAL (mm) | 4 (3–4) | 3 (2–3) | −1 (−1.13 to −1) | <0.001 |
| PPD (mm) | 4.25 (4–4.5) | 3.5 (2.8–3.8) | −1 (−1.13 to −0.88) | <0.001 |
| Study HA Group | ||||
| BoP (%) | 33.5 (23.8–42) | 13 (9.5–18.25) | −18 (−21 to −14.5) | <0.001 |
| CAL (mm) | 4 (3.5–4) | 1.63 (1–2) | −2.25 (−2.5 to −2) | <0.001 |
| PPD (mm) | 4.75 (4.4–5) | 3.5 (2.75–3.75) | −1.5 (−1.63 to −1.25) | <0.001 |
| Median (Interquartile Range) of Difference Before–After Therapy | Difference † (95% CI) | p * | ||
|---|---|---|---|---|
| Control Group | Study HA Group | |||
| BoP (%) | −11.5 (−18 to −6) | −17 (−26 to −10) | −6 (−10 to −2) | 0.003 |
| CAL (mm) | −1 (−1.25 to −0.5) | −2 (−3 to −2) | −1 (−1.5 to −1) | <0.001 |
| PPD (mm) | −1 (−1.25 to −0.75) | −1.5 (−1.75 to −1) | −0.5 (−0.5 to −0.25) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. https://doi.org/10.3390/biom11101491
Olszewska-Czyz I, Kralik K, Prpic J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules. 2021; 11(10):1491. https://doi.org/10.3390/biom11101491
Chicago/Turabian StyleOlszewska-Czyz, Iwona, Kristina Kralik, and Jelena Prpic. 2021. "Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis" Biomolecules 11, no. 10: 1491. https://doi.org/10.3390/biom11101491
APA StyleOlszewska-Czyz, I., Kralik, K., & Prpic, J. (2021). Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules, 11(10), 1491. https://doi.org/10.3390/biom11101491






