Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Transgenic Fish Lines
2.2. Cloning
2.3. Capped Sense mRNA Synthesis, Microinjection and Whole-Mount in situ Hybridization (WMISH)
2.4. Quantitative PCR (qPCR)
2.5. Cell Culture
2.6. Transfection and Luciferase Assay
2.7. Subcellular Localization
2.8. Isolation of Giant Plasma Membrane Vesicles (GPMVs)
2.9. Co-Immunoprecipitation and Western Blotting
2.10. Fluorescence Cross-Correlation Spectroscopy (FCCS)
2.11. Immunofluorescence Staining
2.12. Annexin V-FITC Apoptosis Assay
3. Results
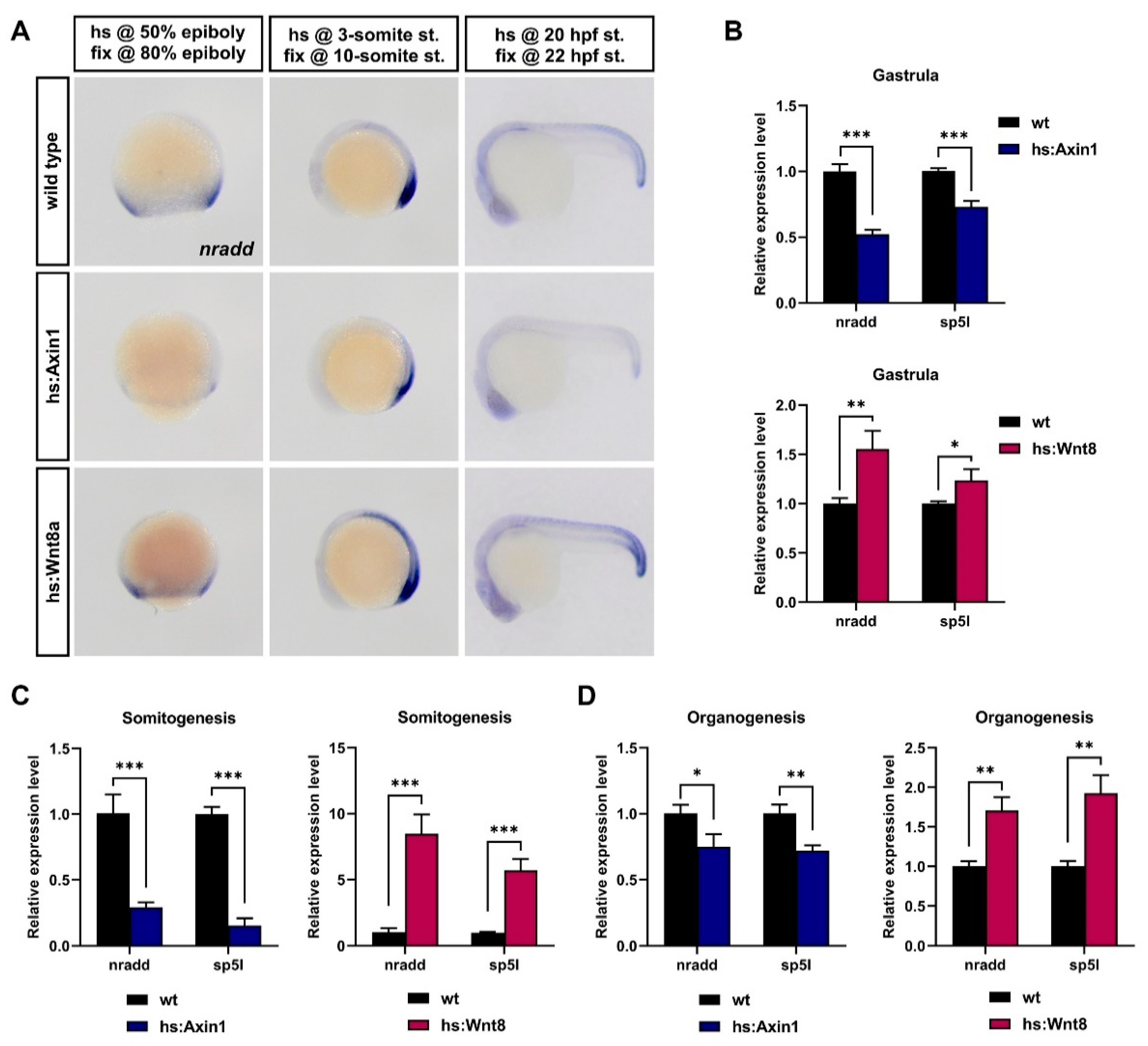
3.1. Nradd is Transcriptionally Regulated by Wnt/β-Catenin Signaling during Development
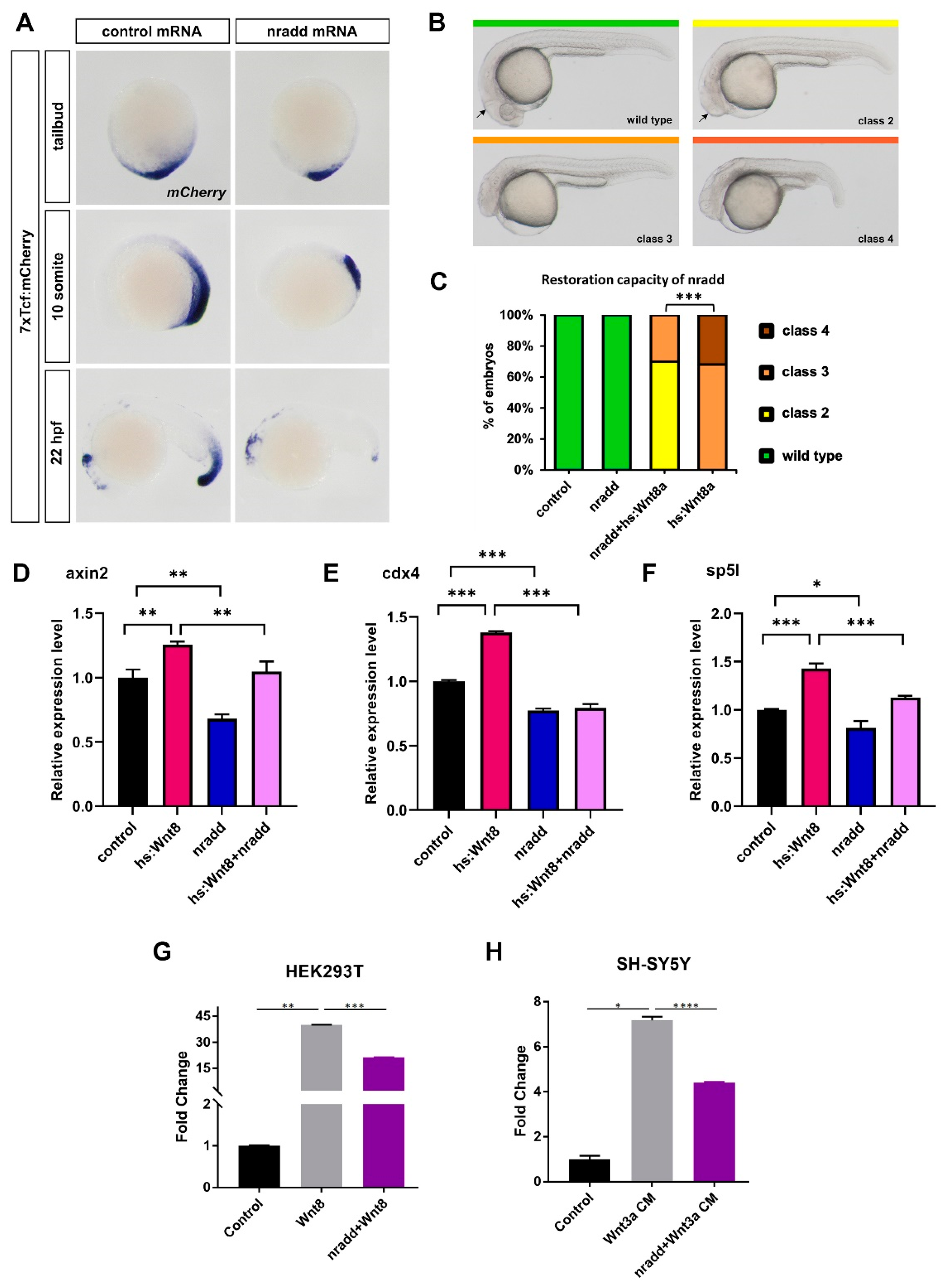
3.2. Nradd Acts as an Inhibitor of Wnt/β-Catenin Signaling
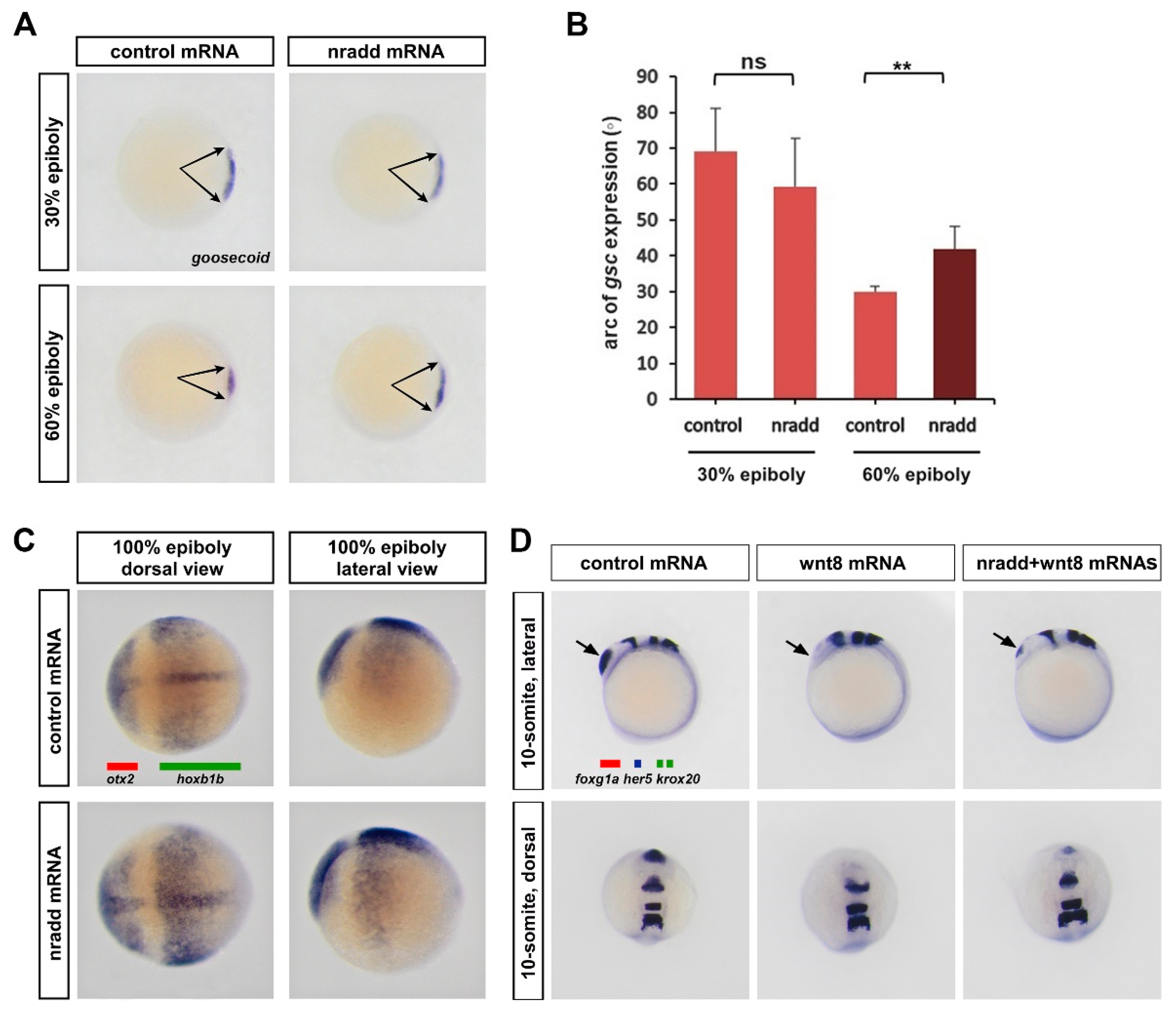
3.3. Nradd Suppresses Wnt-Mediated Patterning of the Mesoderm and the Neuroectoderm
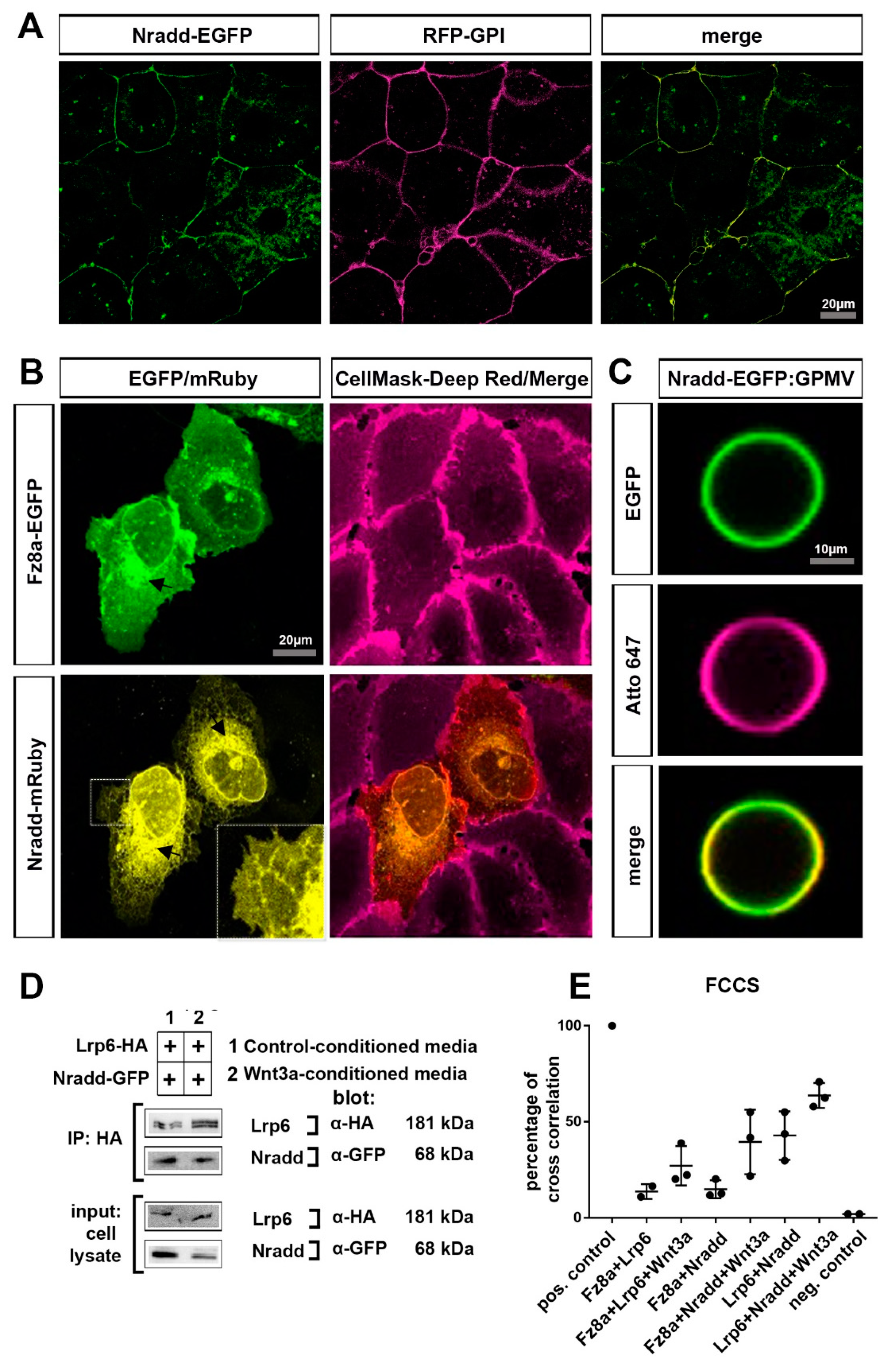
3.4. Nradd Localizes to the Plasma Membrane and Interacts with the Wnt–Receptor Complex
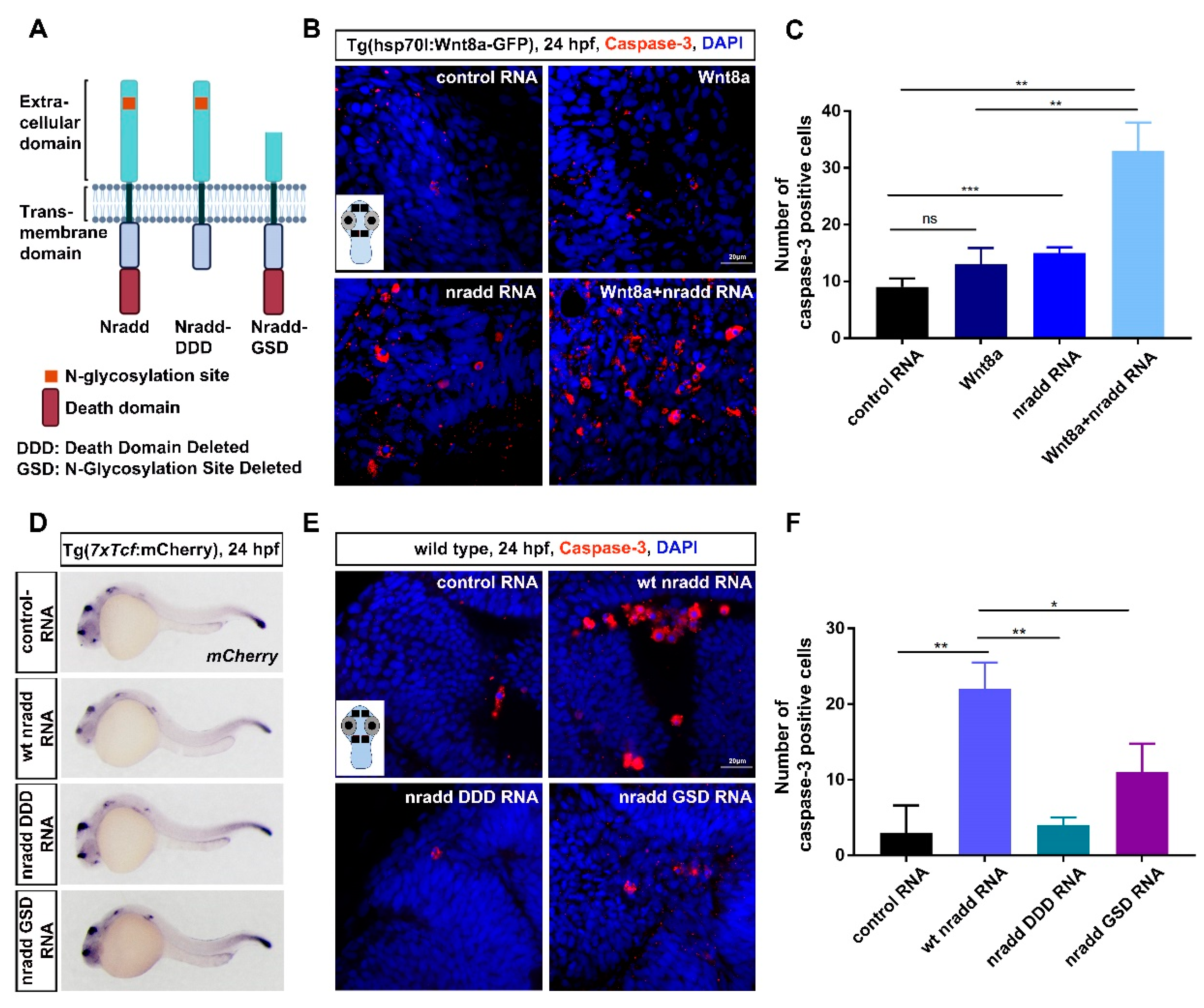
3.5. Nradd Acts Together with Wnt/β-Catenin Signaling to Promote Apoptosis during Development
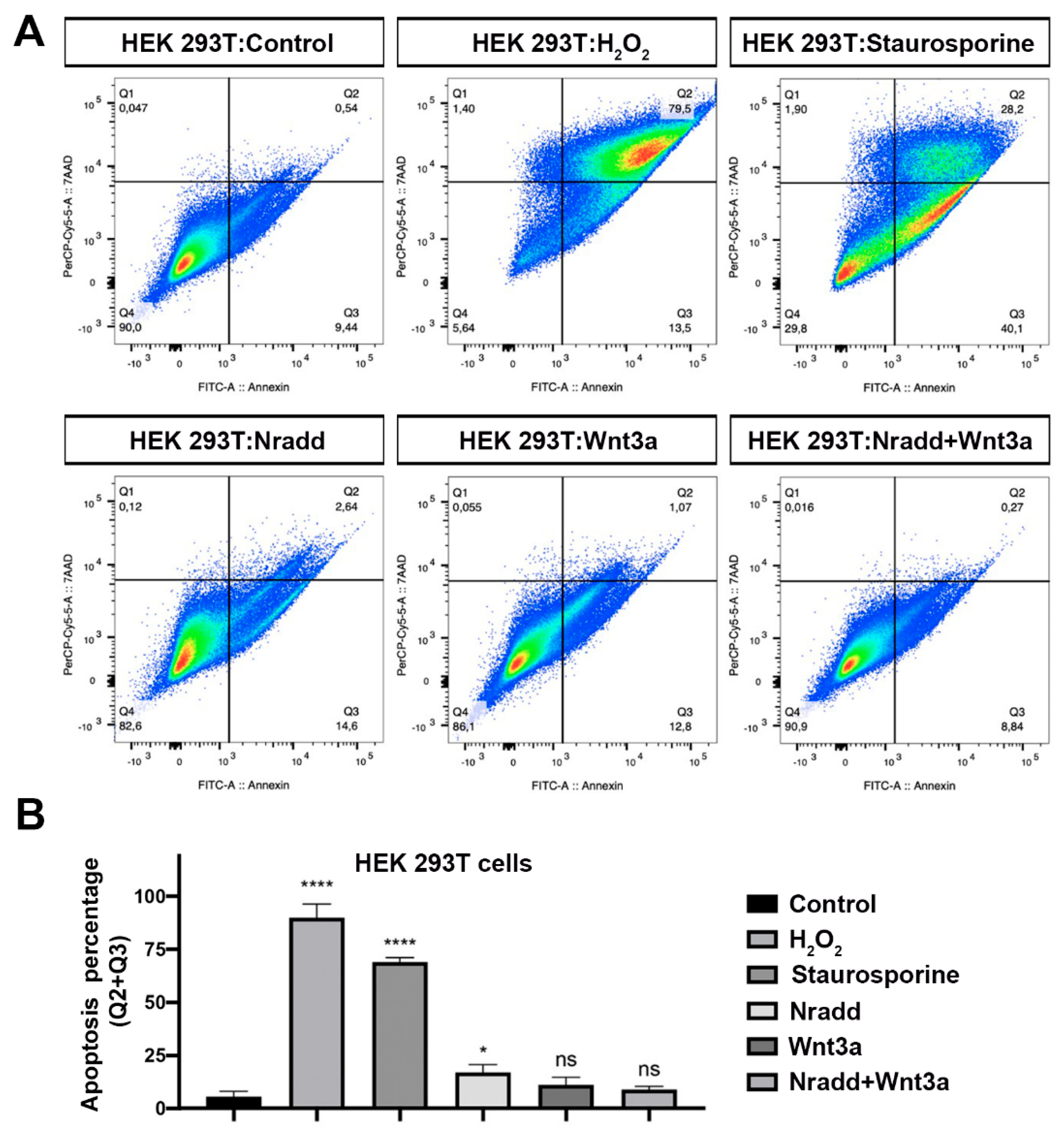
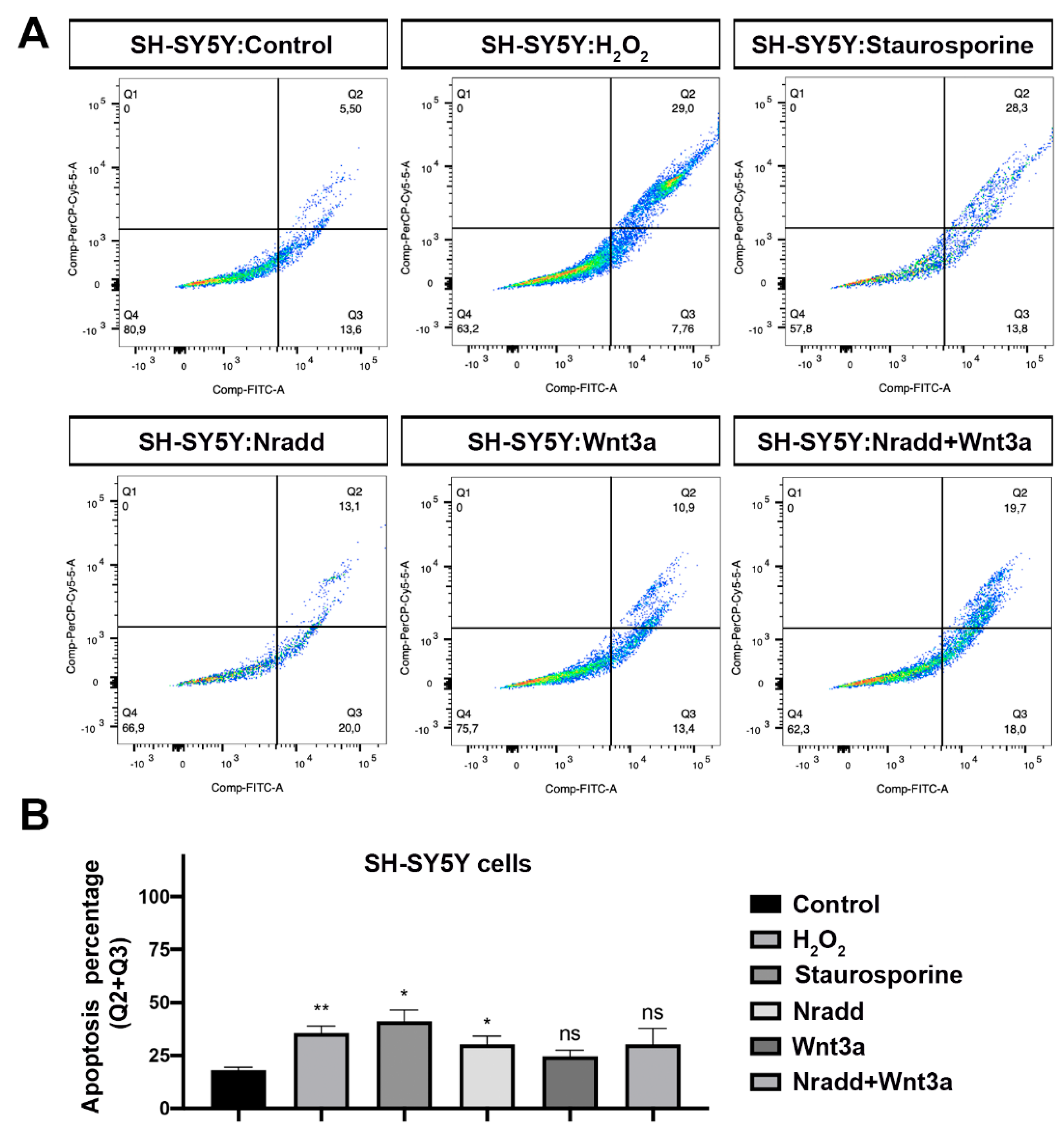
3.6. Zebrafish Nradd Promotes Apoptosis in Human Embryonic and Neuroblastoma Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Pond, K.W.; Doubrovinski, K.; Thorne, C.A. Wnt/β-catenin Signaling in Tissue Self-Organization. Genes 2020, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef] [PubMed]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 2020, 21, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.F.; Kaur, P.; Bunnag, N.; Suresh, J.; Sung, I.C.H.; Tan, Q.H.; Gruber, J.; Tolwinski, N.S. WNT Signaling in Disease. Cells 2019, 8, 826. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, 1461–1473. [Google Scholar] [CrossRef]
- Bajar, B.T.; Wang, E.S.; Lam, A.J.; Kim, B.B.; Jacobs, C.L.; Howe, E.S.; Davidson, M.W.; Lin, M.Z.; Chu, J. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci. Rep. 2016, 6, 20889. [Google Scholar] [CrossRef]
- Foltz, G.; Yoon, J.G.; Lee, H.; Ma, L.; Tian, Q.; Hood, L.; Madan, A. Epigenetic regulation of wnt pathway antagonists in human glioblastoma multiforme. Genes Cancer 2010, 1, 81–90. [Google Scholar] [CrossRef]
- Klarmann, G.J.; Decker, A.; Farrar, W.L. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics 2008, 3, 59–63. [Google Scholar] [CrossRef]
- Tai, D.; Wells, K.; Arcaroli, J.; Vanderbilt, C.; Aisner, D.L.; Messersmith, W.A.; Lieu, C.H. Targeting the WNT Signaling Pathway in Cancer Therapeutics. Oncologist 2015, 20, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Moon, R.T. Proximal events in Wnt signal transduction. Nat. Rev. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sakane, H.; Yamamoto, H.; Michiue, T.; Kikuchi, A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev. Cell 2008, 15, 37–48. [Google Scholar] [CrossRef]
- Piao, S.; Lee, S.-H.; Kim, H.; Yum, S.; Stamos, J.L.; Xu, Y.; Lee, S.-J.; Lee, J.; Oh, S.; Han, J.-K.; et al. Direct Inhibition of GSK3β by the Phosphorylated Cytoplasmic Domain of LRP6 in Wnt/β-Catenin Signaling. PLoS ONE 2008, 3, e4046. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef]
- Jiang, X.; Cong, F. Novel Regulation of Wnt Signaling at the Proximal Membrane Level. Trends Biochem. Sci. 2016, 41, 773–783. [Google Scholar] [CrossRef]
- Malinauskas, T.; Jones, E.Y. Extracellular modulators of Wnt signalling. Curr. Opin. Struct. Biol. 2014, 29, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kagermeier-Schenk, B.; Wehner, D.; Ozhan-Kizil, G.; Yamamoto, H.; Li, J.; Kirchner, K.; Hoffmann, C.; Stern, P.; Kikuchi, A.; Schambony, A.; et al. Waif1/5T4 inhibits Wnt/beta-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev. Cell 2011, 21, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Ozhan, G.; Sezgin, E.; Wehner, D.; Pfister, A.S.; Kuhl, S.J.; Kagermeier-Schenk, B.; Kuhl, M.; Schwille, P.; Weidinger, G. Lypd6 enhances Wnt/beta-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev. Cell 2013, 26, 331–345. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Z.; Zetoune, F.S.; Zeidler, M.G.; Gowrishankar, K.; Vincenz, C. NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ. 2003, 10, 580–591. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Goncharuk, S.A.; Arseniev, A.S.; Mineev, K.S. NMR structure of a full-length single-pass membrane protein NRADD. Proteins 2019, 87, 786–790. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic Potential of Neurotrophins for Repair After Brain Injury: A Helping Hand From Biomaterials. Front. Neurosci. 2019, 13, 790. [Google Scholar] [CrossRef]
- Hallböök, F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opin. Neurobiol. 1999, 9, 616–621. [Google Scholar] [CrossRef]
- Barford, K.; Deppmann, C.; Winckler, B. The neurotrophin receptor signaling endosome: Where trafficking meets signaling. Dev. Neurobiol. 2017, 77, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Bassili, M.; Birman, E.; Schor, N.F.; Saragovi, H.U. Differential roles of Trk and p75 neurotrophin receptors in tumorigenesis and chemoresistance ex vivo and in vivo. Cancer Chemother. Pharm. 2010, 65, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J. Neurobiol. 2001, 49, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.K.; Felice, S.; Kim, T.; Hempstead, B.L. Understanding proneurotrophin actions: Recent advances and challenges. Dev. Neurobiol. 2010, 70, 350–359. [Google Scholar] [CrossRef]
- Feng, D.; Kim, T.; Ozkan, E.; Light, M.; Torkin, R.; Teng, K.K.; Hempstead, B.L.; Garcia, K.C. Molecular and structural insight into proNGF engagement of p75NTR and sortilin. J. Mol. Biol. 2010, 396, 967–984. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef]
- Gibon, J.; Barker, P.A. Neurotrophins and Proneurotrophins: Focus on Synaptic Activity and Plasticity in the Brain. Neurosci.: A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2017, 23, 587–604. [Google Scholar] [CrossRef]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef]
- Casaccia-Bonnefil, P.; Carter, B.D.; Dobrowsky, R.T.; Chao, M.V. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature 1996, 383, 716–719. [Google Scholar] [CrossRef]
- Roux, P.P.; Bhakar, A.L.; Kennedy, T.E.; Barker, P.A. The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 2001, 276, 23097–23104. [Google Scholar] [CrossRef] [PubMed]
- Volonte, C.; Angelastro, J.M.; Greene, L.A. Association of protein kinases ERK1 and ERK2 with p75 nerve growth factor receptors. J. Biol. Chem. 1993, 268, 21410–21415. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef]
- Yoon, S.O.; Casaccia-Bonnefil, P.; Carter, B.; Chao, M.V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998, 18, 3273–3281. [Google Scholar] [CrossRef]
- Casaccia-Bonnefil, P.; Kong, H.; Chao, M.V. Neurotrophins: The biological paradox of survival factors eliciting apoptosis. Cell Death Differ. 1998, 5, 357–364. [Google Scholar] [CrossRef][Green Version]
- Weidinger, G.; Thorpe, C.J.; Wuennenberg-Stapleton, K.; Ngai, J.; Moon, R.T. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr. Biol. 2005, 15, 489–500. [Google Scholar] [CrossRef]
- Moro, E.; Ozhan-Kizil, G.; Mongera, A.; Beis, D.; Wierzbicki, C.; Young, R.M.; Bournele, D.; Domenichini, A.; Valdivia, L.E.; Lum, L.; et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 2012, 366, 327–340. [Google Scholar] [CrossRef]
- Jowett, T.; Lettice, L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 1994, 10, 73–74. [Google Scholar] [CrossRef]
- Biechele, T.L.; Moon, R.T. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol. Biol. Clifton N.J. 2008, 468, 99–110. [Google Scholar]
- Waithe, D.; Clausen, M.P.; Sezgin, E.; Eggeling, C. FoCuS-point: Software for STED fluorescence correlation and time-gated single photon counting. Bioinformatics 2015, 32, 958–960. [Google Scholar] [CrossRef]
- Lekven, A.C.; Thorpe, C.J.; Waxman, J.S.; Moon, R.T. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell 2001, 1, 103–114. [Google Scholar] [CrossRef]
- Pilon, N.; Oh, K.; Sylvestre, J.R.; Bouchard, N.; Savory, J.; Lohnes, D. Cdx4 is a direct target of the canonical Wnt pathway. Dev. Biol. 2006, 289, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Kessler, D.S. Goosecoid promotes head organizer activity by direct repression of Xwnt8 in Spemann’s organizer. Dev. (Camb. Engl.) 2001, 128, 2975–2987. [Google Scholar]
- Boyl, P.P.; Signore, M.; Acampora, D.; Martinez-Barbera, J.P.; Ilengo, C.; Annino, A.; Corte, G.; Simeone, A. Forebrain and midbrain development requires epiblast-restricted Otx2 translational control mediated by its 3′ UTR. Dev. (Camb. Engl.) 2001, 128, 2989–3000. [Google Scholar]
- Alexandre, D.; Clarke, J.D.; Oxtoby, E.; Yan, Y.L.; Jowett, T.; Holder, N. Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Dev. (Camb. Engl.) 1996, 122, 735–746. [Google Scholar]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Sezgin, E.; Schneider, F.; Galiani, S.; Urbančič, I.; Waithe, D.; Lagerholm, B.C.; Eggeling, C. Measuring nanoscale diffusion dynamics in cellular membranes with super-resolution STED-FCS. Nat. Protoc. 2019, 14, 1054–1083. [Google Scholar] [CrossRef]
- Tovar, Y.R.L.B.; Ramírez-Jarquín, U.N.; Lazo-Gómez, R.; Tapia, R. Trophic factors as modulators of motor neuron physiology and survival: Implications for ALS therapy. Front. Cell. Neurosci. 2014, 8, 61. [Google Scholar] [CrossRef]
- Cavallaro, S. Cracking the code of neuronal apoptosis and survival. Cell Death Dis. 2015, 6, e1963. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Pfisterer, U.; Khodosevich, K. Neuronal survival in the brain: Neuron type-specific mechanisms. Cell Death Dis. 2017, 8, e2643. [Google Scholar] [CrossRef] [PubMed]
- Haase, G.; Pettmann, B.; Raoul, C.; Henderson, C.E. Signaling by death receptors in the nervous system. Curr. Opin. Neurobiol. 2008, 18, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hempstead, B.L.; Martin-Zanca, D.; Kaplan, D.R.; Parada, L.F.; Chao, M.V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 1991, 350, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.R.; Yoon, S.O.; Carter, B.D. The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handb. Exp. Pharmacol. 2014, 220, 121–164. [Google Scholar] [CrossRef]
- Pathak, A.; Carter, B.D. Retrograde apoptotic signaling by the p75 neurotrophin receptor. Neuronal Signal. 2017, 1, NS20160007. [Google Scholar] [CrossRef] [PubMed]
- Bamji, S.X.; Majdan, M.; Pozniak, C.D.; Belliveau, D.J.; Aloyz, R.; Kohn, J.; Causing, C.G.; Miller, F.D. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J. Cell Biol. 1998, 140, 911–923. [Google Scholar] [CrossRef]
- Ellies, D.L.; Church, V.; Francis-West, P.; Lumsden, A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Dev. (Camb. Engl.) 2000, 127, 5285–5295. [Google Scholar]
- Grotewold, L.; Rüther, U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. Embo J. 2002, 21, 966–975. [Google Scholar] [CrossRef]
- Li, F.; Chong, Z.Z.; Maiese, K. Winding through the WNT pathway during cellular development and demise. Histol. Histopathol. 2006, 21, 103–124. [Google Scholar] [CrossRef]
- Pećina-Slaus, N. Wnt signal transduction pathway and apoptosis: A review. Cancer Cell Int. 2010, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Gautier, J. Early neural cell death: Dying to become neurons. Dev. Biol 2004, 274, 233–244. [Google Scholar] [CrossRef]
- Hiester, B.G.; Galati, D.F.; Salinas, P.C.; Jones, K.R. Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol. Cell. Neurosci. 2013, 56, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.L.; Deng, W.P.; Lai, W.F.; Chiu, W.T.; Yang, C.B.; Tsai, Y.H.; Hwang, S.M.; Renshaw, P.F. Wnts enhance neurotrophin-induced neuronal differentiation in adult bone-marrow-derived mesenchymal stem cells via canonical and noncanonical signaling pathways. PLoS ONE 2014, 9, e104937. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.J.; Richmond, D.L.; Kaminker, J.S.; Modrusan, Z.; Martin-McNulty, B.; Cao, T.C.; Weimer, R.M.; Carano, R.A.; van Bruggen, N.; Watts, R.J. Death receptors DR6 and TROY regulate brain vascular development. Dev. Cell 2012, 22, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Akieda, Y.; Ogamino, S.; Furuie, H.; Ishitani, S.; Akiyoshi, R.; Nogami, J.; Masuda, T.; Shimizu, N.; Ohkawa, Y.; Ishitani, T. Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Beattie, M.S.; Harrington, A.W.; Lee, R.; Kim, J.Y.; Boyce, S.L.; Longo, F.M.; Bresnahan, J.C.; Hempstead, B.L.; Yoon, S.O. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 2002, 36, 375–386. [Google Scholar] [CrossRef]
- Jansen, P.; Giehl, K.; Nyengaard, J.R.; Teng, K.; Lioubinski, O.; Sjoegaard, S.S.; Breiderhoff, T.; Gotthardt, M.; Lin, F.; Eilers, A.; et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat. Neurosci. 2007, 10, 1449–1457. [Google Scholar] [CrossRef]
- Pedraza, C.E.; Podlesniy, P.; Vidal, N.; Arévalo, J.C.; Lee, R.; Hempstead, B.; Ferrer, I.; Iglesias, M.; Espinet, C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am. J. Pathol. 2005, 166, 533–543. [Google Scholar] [CrossRef]
- Volosin, M.; Trotter, C.; Cragnolini, A.; Kenchappa, R.S.; Light, M.; Hempstead, B.L.; Carter, B.D.; Friedman, W.J. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J. Neurosci. 2008, 28, 9870–9879. [Google Scholar] [CrossRef]
- Lin, Z.; Tann, J.Y.; Goh, E.T.H.; Kelly, C.; Lim, K.B.; Gao, J.F.; Ibanez, C.F. Structural basis of death domain signaling in the p75 neurotrophin receptor. Elife 2015, 4, e11692. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.; Charalampopoulos, I.; Kenchappa, R.S.; Simi, A.; Karaca, E.; Reversi, A.; Choi, S.; Bothwell, M.; Mingarro, I.; Friedman, W.J.; et al. Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron 2009, 62, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ibáñez, C.F.; Lin, Z. Death domain of p75 neurotrophin receptor: A structural perspective on an intracellular signalling hub. Biol. Rev. 2019, 94, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Vilar, M.; Charalampopoulos, I.; Kenchappa, R.S.; Reversi, A.; Klos-Applequist, J.M.; Karaca, E.; Simi, A.; Spuch, C.; Choi, S.; Friedman, W.J.; et al. Ligand-independent signaling by disulfide-crosslinked dimers of the p75 neurotrophin receptor. J. Cell Sci. 2009, 122, 3351–3357. [Google Scholar] [CrossRef]
- Frankowski, H.; Castro-Obregon, S.; del Rio, G.; Rao, R.V.; Bredesen, D.E. PLAIDD, a type II death domain protein that interacts with p75 neurotrophin receptor. Neuromolecular Med. 2002, 1, 153–170. [Google Scholar] [CrossRef]
- Kim, T.; Hempstead, B.L. NRH2 is a trafficking switch to regulate sortilin localization and permit proneurotrophin-induced cell death. Embo J. 2009, 28, 1612–1623. [Google Scholar] [CrossRef]
- Zimmerman, Z.F.; Kulikauskas, R.M.; Bomsztyk, K.; Moon, R.T.; Chien, A.J. Activation of Wnt/β-Catenin Signaling Increases Apoptosis in Melanoma Cells Treated with Trail. PLoS ONE 2013, 8, e69593. [Google Scholar] [CrossRef]
- Takata, A.; Terauchi, M.; Hiramitsu, S.; Uno, M.; Wakana, K.; Kubota, T. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 372–379. [Google Scholar] [CrossRef]
- Li, B.; Cai, S.; Zhao, Y.; He, Q.; Yu, X.; Cheng, L.; Zhang, Y.; Hu, X.; Ke, M.; Chen, S.; et al. Nerve growth factor modulates the tumor cells migration in ovarian cancer through the WNT/β-catenin pathway. Oncotarget 2016, 7, 81026–81048. [Google Scholar] [CrossRef]
- Brafman, D.; Willert, K. Wnt/β-catenin signaling during early vertebrate neural development. Dev. Neurobiol. 2017, 77, 1239–1259. [Google Scholar] [CrossRef]
- Oliva, C.A.; Vargas, J.Y.; Inestrosa, N.C. Wnts in adult brain: From synaptic plasticity to cognitive deficiencies. Front. Cell. Neurosci. 2013, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chew, L.J.; Packer, R.J.; Gallo, V. Modulation of the Wnt/beta-catenin pathway in human oligodendroglioma cells by Sox17 regulates proliferation and differentiation. Cancer Lett. 2013, 335, 361–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Fernandez, C.; González, P.; Rodríguez, F.J. New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: A potential therapeutic target? Neural Regen. Res. 2020, 15, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Manoranjan, B.; Venugopal, C.; Bakhshinyan, D.; Adile, A.A.; Richards, L.; Kameda-Smith, M.M.; Whitley, O.; Dvorkin-Gheva, A.; Subapanditha, M.; Savage, N.; et al. Wnt activation as a therapeutic strategy in medulloblastoma. Nat. Commun. 2020, 11, 4323. [Google Scholar] [CrossRef]
- Parish, C.L.; Thompson, L.H. Modulating Wnt signaling to improve cell replacement therapy for Parkinson’s disease. J. Mol. Cell Biol. 2014, 6, 54–63. [Google Scholar] [CrossRef]
- Suwala, A.K.; Hanaford, A.; Kahlert, U.D.; Maciaczyk, J. Clipping the Wings of Glioblastoma: Modulation of WNT as a Novel Therapeutic Strategy. J. Neuropathol. Exp. Neurol. 2016, 75, 388–396. [Google Scholar] [CrossRef]
- Bier, A.; Oviedo-Landaverde, I.; Zhao, J.; Mamane, Y.; Kandouz, M.; Batist, G. Connexin43 pseudogene in breast cancer cells offers a novel therapeutic target. Mol. Cancer Ther. 2009, 8, 786–793. [Google Scholar] [CrossRef]
- Feenstra, M.; Bakema, J.; Verdaasdonk, M.; Rozemuller, E.; van den Tweel, J.; Slootweg, P.; de Weger, R.; Tilanus, M. Detection of a putative HLA-A*31012 processed (intronless) pseudogene in a laryngeal squamous cell carcinoma. GenesChromosomes Cancer 2000, 27, 26–34. [Google Scholar] [CrossRef]
- Fujii, G.H.; Morimoto, A.M.; Berson, A.E.; Bolen, J.B. Transcriptional analysis of the PTEN/MMAC1 pseudogene, psiPTEN. Oncogene 1999, 18, 1765–1769. [Google Scholar] [CrossRef]
- Han, Y.J.; Ma, S.F.; Yourek, G.; Park, Y.D.; Garcia, J.G. A transcribed pseudogene of MYLK promotes cell proliferation. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Suo, G.; Han, J.; Wang, X.; Zhang, J.; Zhao, Y.; Zhao, Y.; Dai, J. Oct4 pseudogenes are transcribed in cancers. Biochem. Biophys. Res. Commun. 2005, 337, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Baitei, E.Y.; Alzahrani, A.S.; Al-Mohanna, F.; Farid, N.R.; Meyer, B.; Shi, Y. Oncogenic activation of MAP kinase by BRAF pseudogene in thyroid tumors. Neoplasia 2009, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Haimovic, A.; Christos, P.J.; Vega, Y.S.D.M.E.C.; Shapiro, R.; Pavlick, A.; Berman, R.S.; Darvishian, F.; Osman, I. Deletion of PTENP1 pseudogene in human melanoma. J. Invest. Derm. 2011, 131, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Morris, K.V. Not so pseudo anymore: Pseudogenes as therapeutic targets. Pharmacogenomics 2013, 14, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozalp, O.; Cark, O.; Azbazdar, Y.; Haykir, B.; Cucun, G.; Kucukaylak, I.; Alkan-Yesilyurt, G.; Sezgin, E.; Ozhan, G. Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis. Biomolecules 2021, 11, 100. https://doi.org/10.3390/biom11010100
Ozalp O, Cark O, Azbazdar Y, Haykir B, Cucun G, Kucukaylak I, Alkan-Yesilyurt G, Sezgin E, Ozhan G. Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis. Biomolecules. 2021; 11(1):100. https://doi.org/10.3390/biom11010100
Chicago/Turabian StyleOzalp, Ozgun, Ozge Cark, Yagmur Azbazdar, Betul Haykir, Gokhan Cucun, Ismail Kucukaylak, Gozde Alkan-Yesilyurt, Erdinc Sezgin, and Gunes Ozhan. 2021. "Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis" Biomolecules 11, no. 1: 100. https://doi.org/10.3390/biom11010100
APA StyleOzalp, O., Cark, O., Azbazdar, Y., Haykir, B., Cucun, G., Kucukaylak, I., Alkan-Yesilyurt, G., Sezgin, E., & Ozhan, G. (2021). Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis. Biomolecules, 11(1), 100. https://doi.org/10.3390/biom11010100







