Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins
Abstract
1. Introduction
2. Bioactivity of BBs on Brain Health Protection—From In Vitro to Animal Studies
3. Bioavailability and Different Forms of BB Consumption from Animal to Human Studies
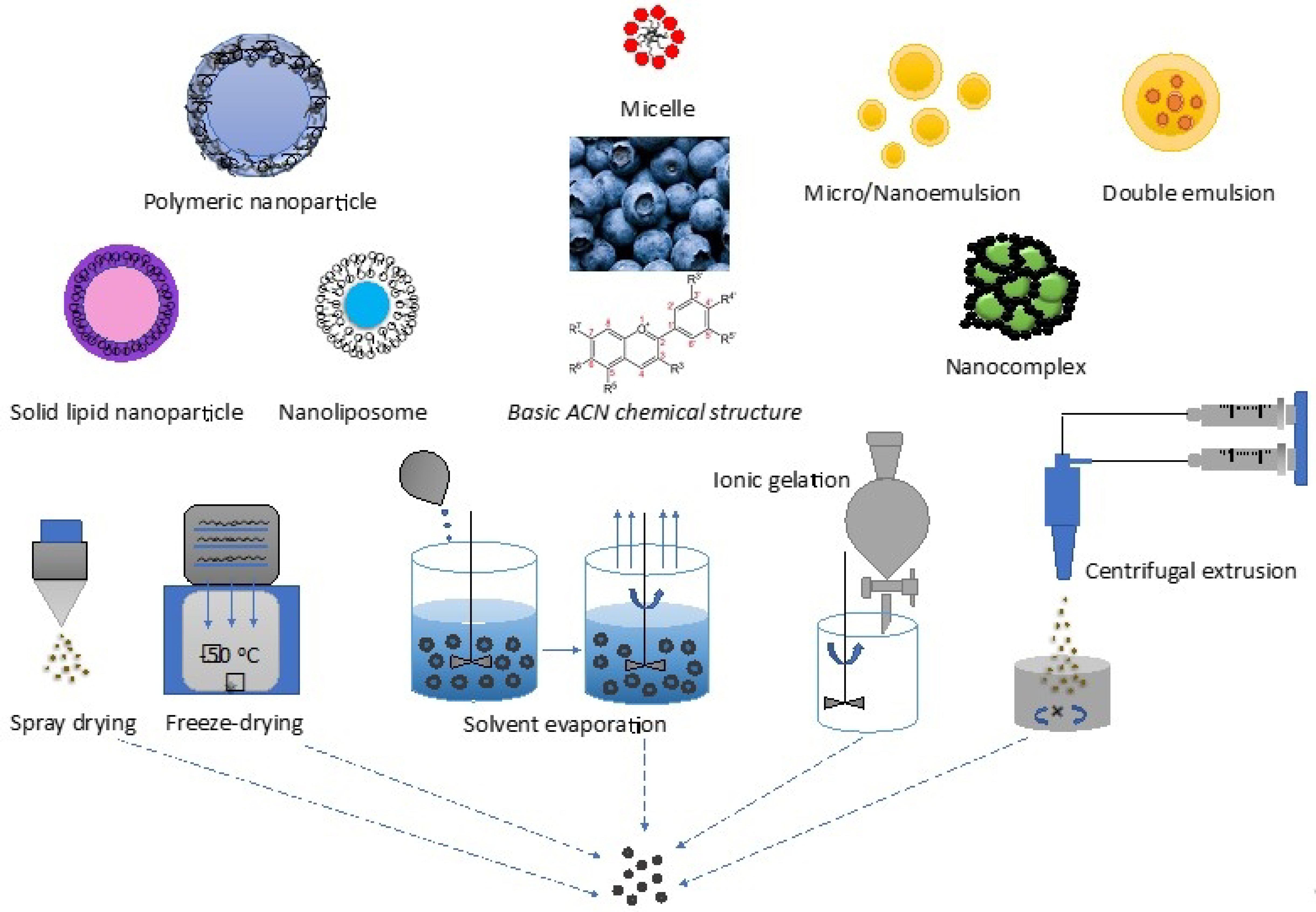
4. Technology in Fabricating ACN-rich BBs into Delivery Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medeiros, J.G.S.; De Bona, C.M.; Cuquel, F.L.; Biasi, L.A. Performance of blueberry cultivars under mild winter conditions. Ciência Rural. 2017, 47. [Google Scholar] [CrossRef]
- Si, L.W. Chapter 16—Trending foods and beverages. In Food and Society; Gibson, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 305–321. [Google Scholar]
- Habtemariam, S. Chapter 7—Bilberries and blueberries as potential modulators of type 2 diabetes and associated diseases. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Habtemariam, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 135–175. [Google Scholar]
- Proesto, C. Superfoods: Recent data on their role in the prevention of diseases. Curr. Res. Nutr. Food Sci. J. 2018, 6, 576–593. [Google Scholar] [CrossRef]
- Sarah, A.J.; Bahram, H.A. Evidence for anti-cancer properties of blueberries: A mini-review. Anti-Cancer Agents Med. Chem. 2013, 13, 1142–1148. [Google Scholar]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Lung, P.; Ng, D. Blueberry/bilberry juice and blueberry fruit supplementation protects DNA. Asian Food Sci. J. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef]
- Carey, A.N.; Gomes, S.M.; Shukitt-Hale, B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem. 2014, 62, 3972–3978. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Yamada, M. Anti-amyloidogenic therapies: Strategies for prevention and treatment of Alzheimer’s disease. Cell. Mol. Life Sci. 2006, 63, 1538–1552. [Google Scholar] [CrossRef]
- Youdim, K.A.; Joseph, J.A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free. Radic. Biol. Med. 2001, 30, 583–594. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Perrin, R.J.; Wang, Q.; Wang, Y.; Perlmutter, J.S.; Morris, J.C.; Benzinger, T.L.S.; Xu, J. Neuroinflammation and myelin status in Alzheimer’s disease, Parkinson’s disease, and normal aging brains: A small sample study. Parkinsons Dis. 2019, 2019, 7975407. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-Harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Pop, R.; Ştefănut, M.; Căta, A.; Tănasie, C.; Medeleanu, M. Ab initio study regarding the evaluation of the antioxidant character of cyanidin, delphinidin and malvidin. Open Chem. 2012, 10, 180–186. [Google Scholar] [CrossRef]
- Howell, A.; Kalt, W.; Duy, J.C.; Forney, C.F.; McDonald, J.E. Horticultural factors affecting antioxidant capacity of blueberries and other small fruit. HortTechnology 2001, 11, 523–528. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of juice processing on blueberry anthocyanins and polyphenolics: Comparison of two pretreatments. J. Food Sci. 2002, 67, 1660–1667. [Google Scholar] [CrossRef]
- Jang, Y.P.; Zhou, J.; Nakanishi, K.; Sparrow, J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; McDonald, J.E.; Fillmore, S.A.E.; Tremblay, F. Blueberry effects on dark vision and recovery after photobleaching: Placebo-Controlled crossover studies. J. Agric. Food Chem. 2014, 62, 11180–11189. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Glovinsky, Y. The effect of anthocyanosides on night vision. Eye 1998, 12, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Vyas, P.; Weber, J.T. Biochemical properties and neuroprotective effects of compounds in various species of berries. Molecules 2017, 23, 26. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Liu, J.; Li, T.; Fu, X.; Liu, R.H. Comparative suppression of NLRP3 inflammasome activation with LPS-induced inflammation by blueberry extracts (Vaccinium spp.). RSC Adv. 2017, 7, 28931–28939. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar]
- Carey, A.N.; Fisher, D.R.; Rimando, A.M.; Gomes, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J. Agric. Food Chem. 2013, 61, 5979–5986. [Google Scholar] [CrossRef]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Liu, Y.-M.; Wang, J.; Wang, X.-N.; Li, C.-Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Alessio, H.M.; Vider, J.; Bagchi, D.; Bagchi, M.; Sen, C.K. Anti-angiogenic property of edible berries. Free. Radic. Res. 2002, 36, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Wang, J.; Liu, Y.-M.; Zheng, Q.-S.; Li, C.-Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef]
- Speciale, A.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside protection against TNF-α-induced endothelial dysfunction: Involvement of nuclear factor-κB signaling. J. Agric. Food Chem. 2010, 58, 12048–12054. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Freeman, L.; Bickford, P.C.; Quintero, E.M.; Umphlet, C.D.; Moore, A.B.; Goetzl, L.; Granholm, A.-C. Blueberry supplementation attenuates microglial activation in hippocampal intraocular grafts to aged hosts. Glia 2010, 58, 679–690. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.-G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef]
- Ebenezer, P.J.; Wilson, C.B.; Wilson, L.D.; Nair, A.R.; Francis, J. The anti-inflammatory effects of blueberries in an animal model of post-traumatic stress disorder (PTSD). PLoS ONE 2016, 11, e0160923–e0160923. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Gildawie, K.R.; Rovnak, A.; Thangthaeng, N.; Fisher, D.R.; Shukitt-Hale, B. Blueberry supplementation attenuates microglia activation and increases neuroplasticity in mice consuming a high-fat diet. Nutr. Neurosci. 2019, 22, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Mitra, S.; Sweeney, M.; Lu, B.; Taylor, B.E.; Bult-Ito, A. Complex interaction of dietary fat and Alaskan bog blueberry supplementation influences manganese mediated neurotoxicity and behavioral impairments. J. Funct. Foods 2019, 53, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D.; Denisova, N.A. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 2003, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Tan, L.; Yang, H.-P.; Pang, W.; Xu, T.; Jiang, Y.-G. Changes of hippocampus proteomic profiles after blueberry extracts supplementation in APP/PS1 transgenic mice. Nutr. Neurosci. 2020, 23, 75–84. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef]
- Joseph, J.A.; Fisher, D.R.; Bielinski, D. Blueberry extract alters oxidative stress-mediated signaling in COS-7 cells transfected with selectively vulnerable muscarinic receptor subtypes. J. Alzheimers Dis. 2006, 9, 35–42. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. Serotonin and serotonin transporters in the adrenal medulla: A potential hub for modulation of the sympathetic stress response. ACS Chem. Neurosci. 2017, 8, 943–954. [Google Scholar] [CrossRef]
- Song, H.-N.; Ji, S.-A.; Park, H.-R.; Kim, H.-H.; Hogstrand, C. Impact of various factors on color stability of fresh blueberry juice during storage. Prev. Nutr. Food Sci. 2018, 23, 46–51. [Google Scholar] [CrossRef]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent advances in anthocyanin analysis and characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blackberry products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Howard, L.R.; Brownmiller, C.; Mauromoustakos, A.; Prior, R.L. Improved stability of blueberry juice anthocyanins by acidification and refrigeration. J. Berry Res. 2016, 6, 189–201. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2014, 65, 440–448. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Bò, C.D.; Ciappellano, S.; Klimis-Zacas, D.; Martini, D.; Gardana, C.; Riso, P.; Porrini, M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the sprague−dawley rat. J. Agric. Food Chem. 2010, 58, 2491–2497. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Mateus, N.; Calhau, C. Bioavailability of anthocyanins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2465–2487. [Google Scholar]
- Brás, N.F.; Gonçalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; de Freitas, V. Inhibition of pancreatic elastase by polyphenolic compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue™) in maintenance of episodic and working memory in older adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.-L.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Stull, J.A.; Cash, C.K.; Champagne, M.C.; Gupta, K.A.; Boston, R.; Beyl, A.R.; Johnson, D.W.; Cefalu, T.W. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Whyte, A.R.; Schafer, G.; Williams, C.M. Cognitive effects following acute wild blueberry supplementation in 7- to 10-year-old children. Eur. J. Nutr. 2016, 55, 2151–2162. [Google Scholar] [CrossRef]
- Barfoot, K.L.; May, G.; Lamport, D.J.; Ricketts, J.; Riddell, P.M.; Williams, C.M. The effects of acute wild blueberry supplementation on the cognition of 7-10-year-old schoolchildren. Eur. J. Nutr. 2019, 58, 2911–2920. [Google Scholar] [CrossRef]
- Whyte, A.R.; Lamport, D.J.; Schafer, G.; Williams, C.M. The cognitive effects of an acute wild blueberry intervention on 7- to 10-year-olds using extended memory and executive function task batteries. Food Funct. 2020, 11, 4793–4801. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Schrager, M.A.; Hilton, J.; Gould, R.; Kelly, V.E. Effects of blueberry supplementation on measures of functional mobility in older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P.E. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363–363. [Google Scholar] [CrossRef]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef]
- Whyte, A.R.; Williams, C.M. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 2015, 31, 531–534. [Google Scholar] [CrossRef]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Ho-Jun, S.; Won-Myong, B.; Tae-Yun, J.; Jeong-Ho, C. The effect of oxygen inhalation on cognitive function and EEG in healthy adults. Clin. Psychopharmacol. Neurosci. 2007, 5, 25–30. [Google Scholar]
- Matthew, T.; Sherwin, R.S.; Murphy, J.; Kerr, D. Importance of cerebral blood flow to the recognition of and physiological responses to hypoglycemia. Diabetes 1997, 46, 829. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Hetz, C. Glucose metabolism: A sweet relief of Alzheimer’s disease. Curr. Biol. 2016, 26, R806–R809. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Paixão, J.; Dinis, T.C.P.; Almeida, L.M. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: An in vitro approach. Apoptosis 2011, 16, 976. [Google Scholar] [CrossRef]
- Tan, L.; Yang, H.P.; Pang, W.; Lu, H.; Hu, Y.D.; Li, J.; Lu, S.J.; Zhang, W.Q.; Jiang, Y.G. Cyanidin-3-O-galactoside and blueberry extracts supplementation improves spatial memory and regulates hippocampal ERK expression in senescence-accelerated mice. Biomed. Environ. Sci. 2014, 27, 186. [Google Scholar]
- Brewer, G.J.; Torricelli, J.R.; Lindsey, A.L.; Kunz, E.Z.; Neuman, A.; Fisher, D.R.; Joseph, J.A. Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: Reversal by blueberry extract. J. Nutr. Biochem. 2010, 21, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free. Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef]
- Rendeiro, C.; Vauzour, D.; Kean, R.J.; Butler, L.T.; Rattray, M.; Spencer, J.P.E.; Williams, C.M. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology 2012, 223, 319–330. [Google Scholar] [CrossRef]
- Kuperstein, F.; Yavin, E. ERK activation and nuclear translocation in amyloid-β peptide- and iron-stressed neuronal cell cultures. Eur. J. Neurosci. 2002, 16, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, O.V.; Sant’Angelo, A.; Costanzo, V.; Battaglia, F.; Arancio, O.; Shelanski, M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13217–13221. [Google Scholar] [CrossRef]
- Li, J.; Ruzhi, D.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.-Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef]
- Barros, D.; Amaral, O.B.; Izquierdo, I.; Geracitano, L.; do Carmo Bassols Raseira, M.; Henriques, A.T.; Ramirez, M.R. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol. Biochem. Behav. 2006, 84, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins Inhibit Nuclear Factor-κB Activation in Monocytes and Reduce Plasma Concentrations of Pro-Inflammatory Mediators in Healthy Adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.L.; Bielinski, D.F.; Szprengiel, A.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol. Aging 2006, 27, 344–350. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Brewer, G.J.; Weikel, K.A.; Kalt, W.; Fisher, D.R. Differential protection among fractionated blueberry polyphenolic families against DA-, Abeta(42)- and LPS-induced decrements in Ca(2+) buffering in primary hippocampal cells. J. Agric. Food Chem. 2010, 58, 8196–8204. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen Res. 2014, 9, 1557–1566. [Google Scholar]
- Shukitt-Hale, B. Blueberries and neuronal aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef]
- Miller, M.G.; Shukitt-Hale, B. Berry fruit enhances beneficial signaling in the brain. J. Agric. Food Chem. 2012, 60, 5709–5715. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.Á.; Zafrilla, M.P.; Marhuenda, J. Cognitive function and consumption of fruit and vegetable polyphenols in a young population: Is there a relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef]
- NCT02446314. ClinicalTrials.gov Identifier. An Investigation Into the Effects of a Wild Blueberry Powder and a Wild Blueberry Extract on Cognition in Older Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT02446314 (accessed on 24 November 2020).
- NCT04049162. ClinicalTrials.gov Identifier. Blueberry Enhances Activity and Cognition Through Increased Vascular Efficiency (BEACTIVE). Available online: https://clinicaltrials.gov/ct2/show/NCT04049162 (accessed on 24 November 2020).
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- De Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Da Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; de Menezes, C.R.; da Rosa, C.S. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Xu, Q.; Li, B.; Wang, D.; Luo, L.; Liu, G.; Zhou, Y. Microencapsulation and stability analysis of blueberry anthocyanins. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Xi’an, China, 15–16 December 2018; Volume 252, p. 52133. [Google Scholar]
- Chen, J.; Ma, X.-h.; Yao, G.-l.; Zhang, W.-t.; Zhao, Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M.A.B. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.D.; Tran, P.H.L. Nanoconjugation and encapsulation strategies for improving drug delivery and therapeutic efficacy of poorly water-soluble drugs. Pharmaceutics 2019, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. 13-Nanoencapsulation strategies applied to maximize target delivery of intact polyphenols. In Encapsulations; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 559–595. [Google Scholar]
- Ha-Lien Tran, P.; Wang, T.; Yang, C.; Tran, T.T.D.; Duan, W. Development of conjugate-by-conjugate structured nanoparticles for oral delivery of docetaxel. Mater. Sci. Eng. C 2020, 107, 110346. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2020, 575, 118956. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Duan, W.; Lee, B.J.; Tran, T.T.D. Drug stabilization in the gastrointestinal tract and potential applications in the colonic delivery of oral zein-based formulations. Int. J. Pharm. 2019, 569, 118614. [Google Scholar] [CrossRef]
- Pham, D.T.T.; Tran, P.H.L.; Tran, T.T.D. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharm. J. 2019, 27, 1019–1024. [Google Scholar] [CrossRef]
- Le, N.D.T.; Tran, P.H.L.; Lee, B.J.; Tran, T.T.D. Solid lipid particle-based tablets for buccal delivery: The role of solid lipid particles in drug release. J. Drug Deliv. Sci. Technol. 2019, 52, 96–102. [Google Scholar] [CrossRef]
- Ge, J.; Yue, X.; Wang, S.; Chi, J.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Nanocomplexes composed of chitosan derivatives and β-Lactoglobulin as a carrier for anthocyanins: Preparation, stability and bioavailability in vitro. Food Res. Int. 2019, 116, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, Q.; Ying, R.; Wang, Y.; Wang, Z.; Huang, M. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocoll. 2020, 100, 105448. [Google Scholar] [CrossRef]
- Guo, J.; Giusti, M.M.; Kaletunç, G. Encapsulation of purple corn and blueberry extracts in alginate-pectin hydrogel particles: Impact of processing and storage parameters on encapsulation efficiency. Food Res. Int. 2018, 107, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.-T.; Xu, W.; Xu, G. Coencapsulation of polyphenols and anthocyanins from blueberry pomace by double emulsion stabilized by whey proteins: Effect of homogenization parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Chen, B.-H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Garcia, V.A.d.S.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; Lapa-Guimaraes, J.d.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]

| Consumption Type | Key Results | References |
|---|---|---|
| BB extracts | Lowering the gene expression of MusIL-1b, MusNLRP3, MusCaspase-1, MusASC, MusTNF-a, MusIL-6, and MusiNOS and the protein expression of NLRP3 and Caspase-1 | [30] |
| BB fruit or leaf extracts | Inhibiting cell death and decreased inflammatory conditions | [31,32] |
| BB extract | Protective ability of BB ACNs for microglia undergoing inflammatory stress | [33] |
| BB extracts | Protecting microglia from inflammation-induced stress signaling | [34] |
| BB extracts | Human microvascular endothelial cells were protected from TNFα-induced damage | [35] |
| Consumption Type/Animal Models | Key Results | References |
|---|---|---|
| BB supplementation/Fischer 344 rats | A decrease in the activation of microglia | [42] |
| Dried aqueous extract/Fischer 344 rats | Reversing age-related deficits in several neuronal signaling pathways and behaviors | [43] |
| BB extract/Fischer 344 rats | Increasing hippocampal plasticity parameters, IGF-1 and IGF-1R levels | [44] |
| BB diet/Sprague-Dawley rats | Anti-inflammatory activity in post-traumatic stress disorder | [45] |
| Freeze-dried BBs/C57Bl/6 mice | Fewer microglia and increased neuroplasticity in the brains of high-fat diet mice than in those of high-fat diet mice who did not consume BBs and of low-fat diet mice | [46] |
| Dietary supplementation with BBs and fat diets/C57BL6/J mice | Greatest benefits for age-related neurodegenerative disorders when consumed with a low-fat diet | [47] |
| BB extract/a mixture of C57BL/6, DBA, SJL and Swiss Webster mice | Enhancing the memory-associated neuronal signaling pathway and modifying neutral sphingomyelin-specific phospholipase C activity | [48] |
| BB extract/APP/PS1 transgenic mice | A connection between the expression of proteins involved in cognitive dysfunction and the improvement of this impairment | [49] |
| Consumption Type | Quantity (Daily Intake) | Equivalent Anthocyanins (ACNs) | Age Group | Number of Participants | Effects | Reference |
|---|---|---|---|---|---|---|
| Extract vs. powder (encapsulated in transparent capsules) | Extract: 100 mg Powder: 500 or 1000 mg | 7 mg 1.35 or 2.7 mg | 65–80 | 122 | 3-month intervention: extract facilitated better episodic memory performance (improved cardiovascular function over 6 months) | [67] |
| Concentrate (30 mL, diluted to 240 mL with water) | 230 g | 387 mg | 65–77 | 26 | 12-week ingestion: improved cognitive function and working memory | [68] |
| Powder (whole frozen BBs freeze-dried and powdered to 20 mesh/consumed with morning and evening meals) | 25 g (12.5 g per packet, twice a day) | 269 mg | 62–80 | 94 | 24-week intervention: cognitive enhancement | [69] |
| Powder (freeze-dried/mixed with 30 mL of low-energy fruit squash, Rocks® UK and 170 mL of water) | 15 or 30 g (equivalent to ~ 120 or 240 g fresh BBs) | 127 or 253 mg | 7–10 | 24 | 30 g dose showed the better effect. Consumption improved cognitive performance and immediate recall 1.15 h following the intervention; improved word recognition and accuracy on cognitively demanding incongruent trials in the interference task 3 h following the intervention; improved epi- sodic memory at 75 min and executive function 3 h post-consumption. | [71,72,73] |
| Juice | Individuals 54–64 kg: 444 mL/day; 65–76 kg: 532 mL/day; 77–91 kg: 621 mL/day | 0.428 g 0.512 g 0.598 g | 76.2 ± 5.2 | 9 | Moderate-term BB supplementation provided a preventive effect on neurocognitive function. | [74] |
| Fruit (frozen BBs) | ~0.46 kg (2 cups daily) | N/A | 61–81 | 20 | 6-week consumption: Positive impacts on age-related declines in functional mobility. | [75] |
| Freeze-dried BBs | 24 g | N/A | 60–75 | 40 | 3-month intervention: improved cognitive function (significantly fewer repetition errors in verbal learning tests and reduced switching costs on a task-switching test) without improvement in gait or balance. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, P.H.L.; Tran, T.T.D. Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules 2021, 11, 102. https://doi.org/10.3390/biom11010102
Tran PHL, Tran TTD. Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules. 2021; 11(1):102. https://doi.org/10.3390/biom11010102
Chicago/Turabian StyleTran, Phuong H.L., and Thao T.D. Tran. 2021. "Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins" Biomolecules 11, no. 1: 102. https://doi.org/10.3390/biom11010102
APA StyleTran, P. H. L., & Tran, T. T. D. (2021). Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules, 11(1), 102. https://doi.org/10.3390/biom11010102




