Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology
Abstract
1. Introduction
2. Literature Search
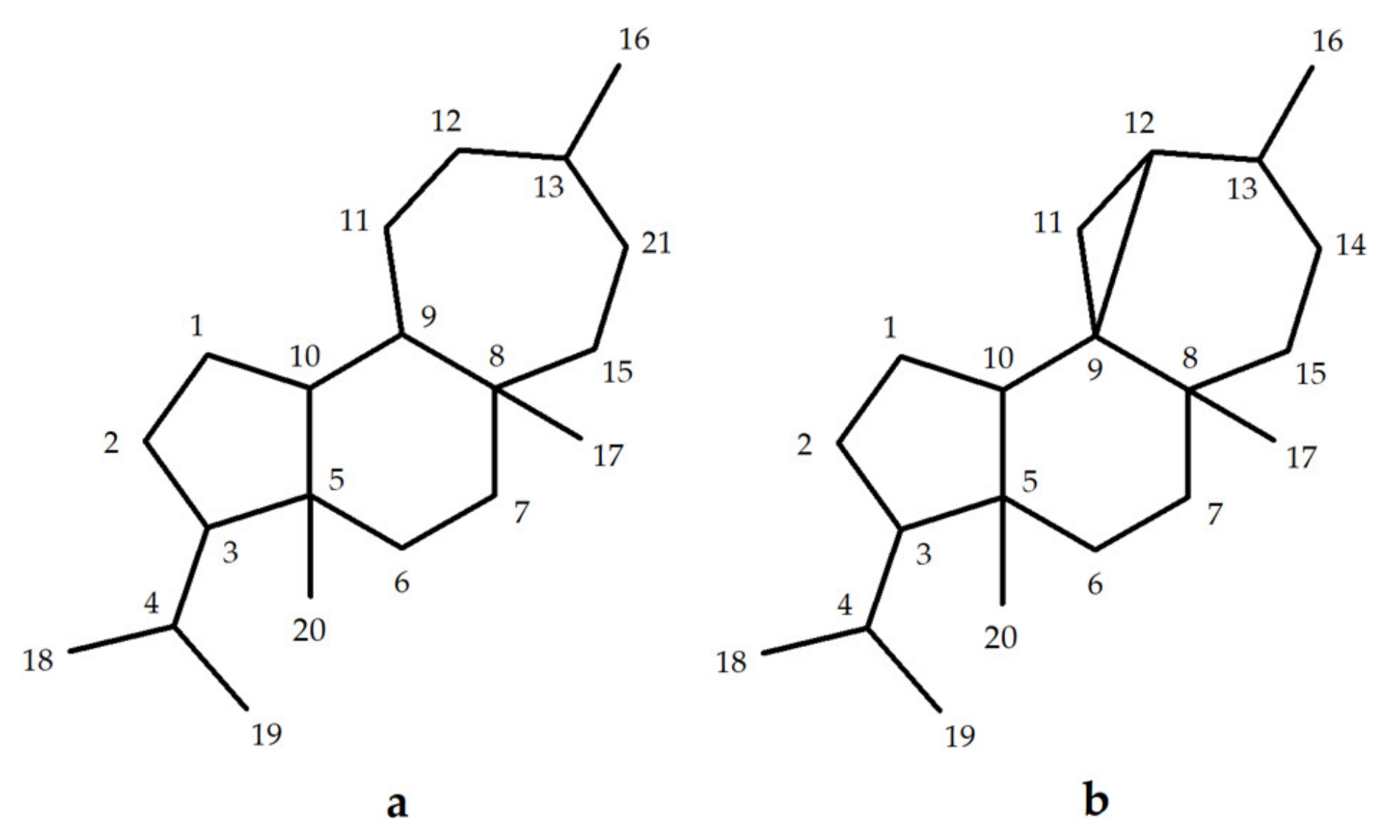
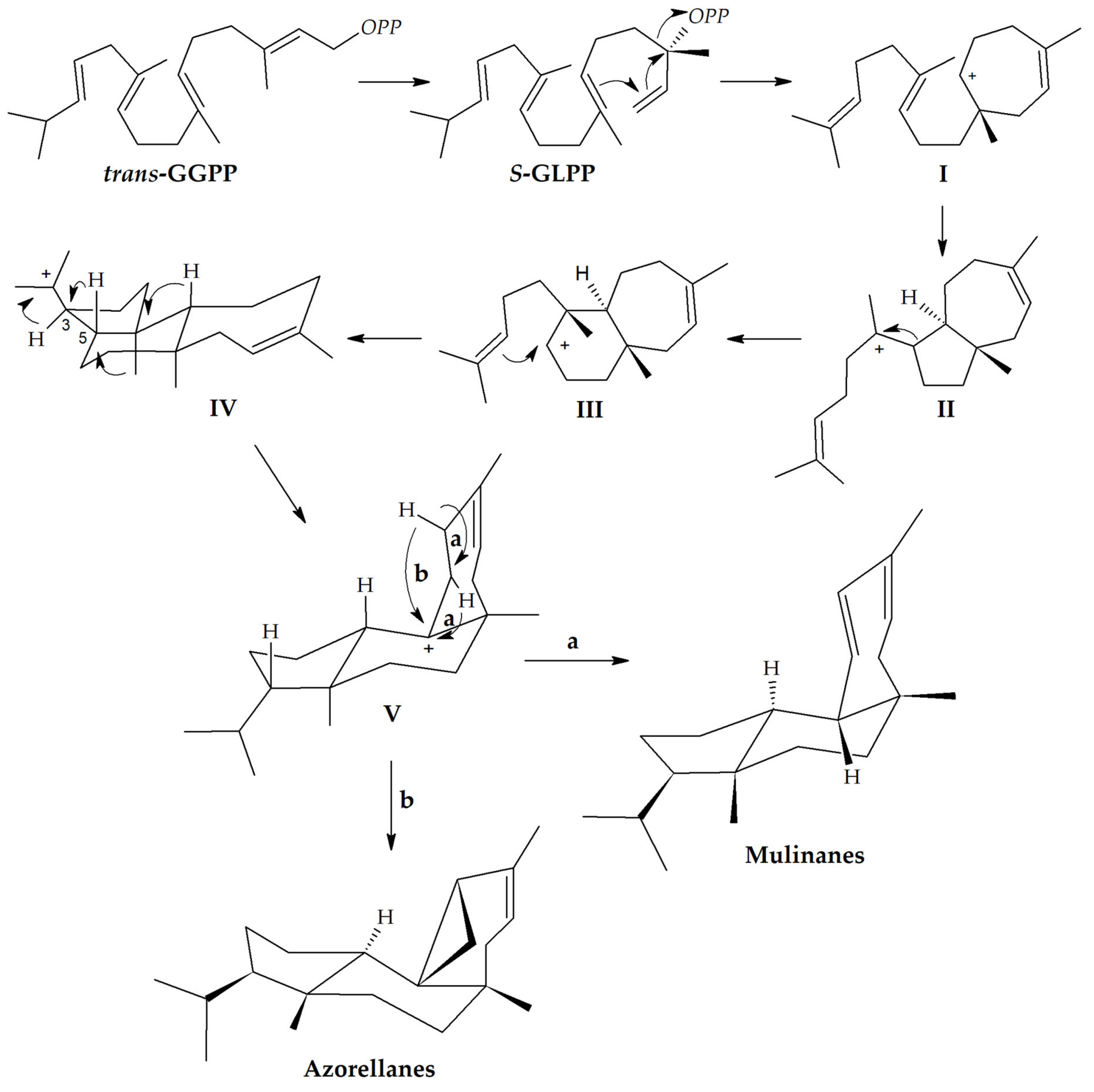
3. Mulinane and Azorellane Biosynthesis
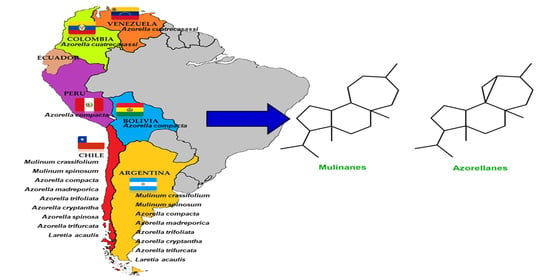
4. Sources and Chemical Structures of Natural Mulinane and Azorellane Diterpenoids
5. Synthetic, Semisynthetic, and Biotransformed Mulinane and Azorellane Diterpenoids
6. Pharmacological Activities of Mulinane and Azorellane Diterpenoids
6.1. Antimicrobial Activity
6.2. Antiprotozoal Activity
6.3. Spermicidal/Spermatostatic Activity
6.4. Antidiabetic
6.5. Antiulcer
6.6. Anti-Inflammatory
6.7. Cytotoxic
6.8. Anti-Alzheimer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Engin./Biotechnol. 2014, 123, 127–141. [Google Scholar]
- Solecki, R. Shanidar IV, a Neanderthal flower burial in northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Medicines Situation 2011—Traditional Medicines: Global Situation, Issues and Challenges. Available online: http://digicollection.org/hss/documents/s180 (accessed on 4 May 2012).
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef]
- Nwokeji, P.A.; Enodiana, I.O.; Raymond, E.S.; Osasere, O.-I.; Abiola, A.H. The Chemistry of natural product: Plant secondary metabolites. Int. J. Technol. Enhanc. Merg. Eng. Res. 2016, 4, 1–9. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Li, G.; Low, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2017, 38, 1255–1294. [Google Scholar] [CrossRef]
- Bórquez, J.; Ardiles, A.; Loyola, L.A.; Peña-Rodriguez, L.M.; Molina-salinas, G.M.; Vallejos, J.; Collado, I.G.; Simirgiotis, M.J. Further mulinane and azorellane diterpenoids isolated from Mulinum crassifolium and Azorella compacta. Molecules 2014, 19, 3898–3908. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Gil-Mesón, A.; Díez, D. 7-6-5 Tricarbocyclic diterpenes: Valparanes, mulinanes, cyathanes, homoverrucosanes, and related ones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 137–207. [Google Scholar]
- Reber, K.P.; Xu, J.; Guerrero, C.A. Synthesis of mulinane diterpenoids. J. Org. Chem. 2015, 80, 2397–2406. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Oros, D.R.; Jaffé, R.; Didyk-Peña, A.; Areche, C.; Sepúlveda, B.; Didyk, B.M. Mulinane and azorellane diterpenoid biomarkers by GC-MS from a representative Apiaceae (Umbelliferae) species of the Andes. Molecules 2019, 24, 684. [Google Scholar] [CrossRef] [PubMed]
- Caceres De Baldarrago, F.; Poma, I.; Spadaro, V. Evaluación etnobotanica de la Yareta (Azorella compacta) en Arequipa (Perú) y sus posibles aplicaciones. Quad. Bot. Amb. Appl. 2012, 23, 15–30. [Google Scholar]
- Wickens, G.E. Llareta (Azorella compacta, Umbelliferae): A Review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Areche, C.; Fernandez-Burgos, R.; Cano de Terrones, T.; Simirgiotis, M.; García-Beltran, O.; Borquez, J.; Sepulveda, B. Mulinum crassifolium Phil; two new mulinanes, gastroprotective activity and metabolomic analysis by UHPLC-orbitrap mass spectrometry. Molecules 2019, 24, 1673. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Morales, G.; Rodríguez, B.; Jiménez-Barbero, J.; De la Torre, M.C.; Perales, A.; Torres, M.R. Mulinic and isomulinic acids. Rearranged diterpenes with a new carbon skeleton from Mulinum crassifolium. Tetrahedron 1990, 46, 5413–5420. [Google Scholar] [CrossRef]
- Nicolas, A.N.; Plunkett, G.M. Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): Insights from phylogenetics and biogeography. Taxon 2012, 61, 826–840. [Google Scholar] [CrossRef]
- Luteyn, J.L. Paramos: A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature, 1st ed.; Luteyn, J., Ed.; The New York Botanical Garden Press: New York, NY, USA, 1999; pp. 1–258. [Google Scholar]
- Loyola, L.A.; Borquez, J.; Morales, G. Azorellanol: A diterpenoid with a new carbon skeleton from Azorella compacta. Tetrahedron 1998, 54, 15533–15540. [Google Scholar] [CrossRef]
- Araya, J.E.; Neira, I.; Da Silva, S.; Mortara, R.A.; Manque, P.; Cordero, E.; Sagua, H.; Loyola, A.; Bórquez, J.; Morales, G.; et al. Diterpenoids from Azorella compacta (Umbelliferae) active on Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 2003, 98, 413–418. [Google Scholar] [CrossRef]
- Donoso, V.; Bacho, M.; Nuñez, S.; Rovirosa, J.; San-Martín, A.; Leiva, S. Antimicrobial diterpenes from Azorella species against gram-positive bacteria. Nat. prod. Commun. 2015, 10, 1915–1916. [Google Scholar] [CrossRef]
- Fuentes, N.L.; Sagua, H.; Morales, G.; Borquez, J.; San-Martin, A.; Soto, J.; Loyola, L.A. Experimental antihyperglycemic effect of diterpenoids of llareta Azorella compacta (umbelliferae) Phil in rats. Phytother. Res. 2005, 19, 713–716. [Google Scholar] [CrossRef]
- Areche, C.; Rojas-Alvarez, F.; Campos-Briones, C.; Lima, C.; Pérez, E.G.; Sepúlveda, B. Further mulinane diterpenoids from Azorella compacta. J. Pharm. Pharmacol. 2013, 65, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Borquez, J.B.; Morales, G.; San-Martin, A. Mulinol, a diterpenoid from Azorella compacta. Phytochemistry 1997, 45, 1465–1467. [Google Scholar] [CrossRef]
- Salgado, F.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J.; Cáceres, F.; Quispe, C.; Quispe, L.; Cano, T. A new mulinane diterpenoid from the cushion shrub Azorella compacta growing in Perú. Pharmacogn. Mag. 2014, 10, S543–S548. [Google Scholar] [PubMed]
- Loyola, L.A.; Morales, G.; de la Torre, M.C.; Pedreros, S.; Rodriguez, B. 17-acetoxymulinic acid, a rearranged diterpenoid from Mulinum crassifolim. Phytochemistry 1990, 29, 3950–3951. [Google Scholar] [CrossRef]
- Bórquez, J.; López-Bartolucci, N.; Echiburú-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food. Agric. 2016, 96, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Bórquez, J.; Morales, G.; San-Martín, A.; Darias, J.; Flores, N.; Giménez, A. Mulinane-type diterpenoids from Azorella compacta display antiplasmodial activity. Phytochemistry 2004, 65, 1931–1935. [Google Scholar] [CrossRef]
- Loyola, L.A.; Morales, G. Mulinenic acid, a rearranged diterpenoid from Mulinum crassifolium. J. Nat. Prod. 1991, 54, 1404–1408. [Google Scholar] [CrossRef]
- Chiaramello, A.I.; Yunes, R.; Rossomando, P.C.; Cifuentes, D.A. In vitro spermatostatic activity of mulinane- and azorellane-type diterpenes on human spermatozoa. Lat. Am. J. Pharm. 2011, 30, 1325–1329. [Google Scholar]
- Chiaramello, A.I.; Ardanaz, C.E.; García, E.E.; Rossomando, P.C. Mulinane-type diterpenoids from Mulinum spinosum. Phytochemistry 2003, 63, 883–886. [Google Scholar] [CrossRef]
- Loyola, L.A.; Borquez, J.; Morales, G.; San-Martin, A. Diterpenoids from Azorella compacta. Phytochemistry 1997, 44, 649–651. [Google Scholar] [CrossRef]
- Loyola, L.A.; Borquez, J.; Morales, G.; San-Martin, A. Mulinolic acid, a diterpenoid from Mulinum crassifolium. Phytochemistry 1996, 43, 165–168. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; Araya, J.; González, J.; Neira, I.; Sagua, H.; San-Martín, A. Diterpenoids from Azorella yareta and their trichomonicidal activities. Phytochemistry 2001, 56, 177–180. [Google Scholar] [CrossRef]
- Areche, C.; Sepulveda, B.; San-Martin, A.; Garcia-Beltrán, O.; Simirgiotis, M.; Cañete, A. An unusual mulinane diterpenoid from the Chilean plant Azorella trifurcata (Gaertn) Pers. Org. Biomol. Chem. 2014, 12, 6406–6413. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Di Fabio, A.; D´Andrea, A.; Salvatore, G.; Van Baren, C.; Coussio, J.D. Diterpenoid acids from Mulinum spinosum. Phytochemistry 1996, 43, 1065–1067. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; San-Martín, A. Mulinane-type diterpenoids from Laretia acaulis. Phytochemistry 2000, 53, 961–963. [Google Scholar] [CrossRef]
- Areche, C.; Vaca, I.; Loyola, L.A.; Borquez, J.; Rovirosa, J.; San-Martín, A. Diterpenoids from Azorella madreporica and their antibacterial activity. Planta Med. 2010, 76, 1749–1751. [Google Scholar] [CrossRef]
- Wächter, G.A.; Matooq, G.; Hoffmann, J.J.; Maiese, W.M.; Singh, M.P.; Montenegro, G.; Timmennann, B.N. Antibacterial diterpenoid acids from Azorella compacta. J. Nat. Prod. 1999, 62, 1319–1321. [Google Scholar] [CrossRef]
- Loyola, L.A.; Borquez, J.; Morales, G.l.; Araya, J.; Gonzalez, J.; Neira, I.; Sagua, H.; San-Martín, A. Azorellane diterpenoids from Laretia acaulis, and its toxoplasmacidal activity. Bol. Soc. Chil. Quím. 2001, 46, 9–13. [Google Scholar] [CrossRef]
- Delporte, C.; Backhouse, N.; Salinas, P.; San-Martín, A.; Bórquez, J.; Loyola, A. Pharmaco-toxicological study of diterpenoids. Bioorg. Med. Chem. 2003, 11, 1187–1190. [Google Scholar] [CrossRef]
- Lima, B.; Sanchez, M.; Agüero, M.B.; Tapia, A.; Palermo, J.A.; Feresin, G.E. Antibacterial activity of extracts and compounds isolated from the Andean medicinal plant Azorella cryptantha (Clos) Reiche, Apiaceae. Ind. Crops Prod. 2015, 64, 152–157. [Google Scholar] [CrossRef]
- Borquez, J.; Loyola, L.A.; Morales, G.; San-Martín, A.; Roldan, R.; Marquez, N.; Muñoz, E. Azorellane diterpenoids from Laretia acaulis inhibit Nuclear Factor-kappa B activity. Phytother. Res. 2007, 1086, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Borquez, J.; Morales, G.; San-Martín, A. 11,12-epoxy-mulin-13-en-20-oic acid, a diterpenoid from Azorella compacta. Phytochemistry 1998, 49, 1091–1093. [Google Scholar] [CrossRef]
- Wächter, G.A.; Franzblau, S.G.; Montenegro, G.; Suarez, E.; Fortunato, R.H.; Saavedra, E.; Timmermann, B.N. A new antitubercular mulinane diterpenoid from Azorella madreporica Clos. J. Nat. Prod. 1998, 61, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Bórquez, J.; Molina-Salinas, G.M.; Loyola, L.A.; San-Martin, A.; Peña-Rodríguez, L.M.; Said-Fernández, S. A new azorellane diterpenoid from Azorella madreporica. Nat. Prod. Res. 2011, 25, 653–657. [Google Scholar] [CrossRef]
- Colloca, C.B.; Pappano, D.B.; Bustos, D.A.; Sosa, V.E.; Baggio, R.F.; Garland, M.T.; Gil, R.R. Azorellane diterpenes from Azorella cryptantha. Phytochemistry 2004, 65, 2085–2089. [Google Scholar] [CrossRef]
- Colloca, C.B.; Bustos, D.A.; Espinar, L.A.; Sosa, V.E. Terpenoid from Azorella Cryptantha. Nat. Prod. Indian J. 2014, 10, 8–11. [Google Scholar]
- Astudillo, L.; Gutiérrez, M.; Quesada, L.; San-Martín, A.; Espinoza, L.; Peñailillo, P. New Diterpenes from Azorella spinosa. Nat. Prod. Commun. 2014, 9, 9–12. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Quispe, C.; Simirgiotis, M.; García-Beltrán, O.; Areche, C. Gastroprotective effects of new diterpenoid derivatives from Azorella cuatrecasasii Mathias & Constance obtained using a β-cyclodextrin complex with microbial and chemical transformations. Bioorg. Med. Chem. Lett. 2016, 26, 3220–3222. [Google Scholar]
- San-Martín, A.; Bacho, M.; Nuñez, S.; Rovirosa, J.; Soler, A.; Blanc, V.; Leon, R.; Olea, A.F. A novel normulinane isolated from Azorella compacta and assessment of its antibacterial activity. J. Chil. Chem. Soc. 2018, 63, 3–6. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Li, L.-P.; Xie, J.-H.; Zhou, Q.-L. Divergent asymmetric total synthesis of mulinane diterpenoids. Angew. Chem. Int. Ed. 2017, 56, 12708–12711. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Bórquez, J.; Ardiles, A.; Said-Fernández, S.; Loyola, L.A.; Yam-Puc, A.; Becerril-Montes, P.; Escalante-Erosa, F.; San-Martin, A.; González-Collado, I.; et al. Bioactive metabolites from the Andean flora. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Phytochem. Rev. 2010, 9, 271–278. [Google Scholar] [CrossRef]
- Herrera-Canché, S.G.; Sánchez-González, M.; Loyola, L.A.; Bórquez, J.; García-Sosa, K.; Peña-Rodríguez, L.M. Biotransformation of a mulinane diterpenoid by Aspergillus alliaceus and Mucor circinelloides. Biocatal. Biotransfor. 2019, 38, 1–6. [Google Scholar] [CrossRef]
- Areche, C.; Loyola, L.A.; Borquez, J.; Rovirosa, J.; San-Martin, A. Microbial transformation of the diterpene mulin-11, 13-dien-20-oic acid by Mucor plumbeus. Magn. Reson. Chem. 2008, 46, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Molina-Salinas, G.M.; Bórquez, J.; Ardiles, A.; Said-Fernández, S.; Loyola, L.A.; San-Martín, A.; González-Collado, I.; Peña-Rodríguez, L.M. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Fitoterapia 2010, 81, 50–54. [Google Scholar] [CrossRef]
- Nuñez, S.; San-Martin, A.; Corsini, G. Antimicrobial activities of diterpenoids and semisynthetic derivatives from Azorella compacta. J. Chil. Chem. Soc. 2018, 63, 4200–4204. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Bórquez, J.; Said-Fernández, S.; Loyola, L.A.; Yam-Puc, A.; Becerril-Montes, P.; Escalante-Erosa, F.; Peña-Rodríguez, L.M. Antituberculosis activity of alkylated mulinane diterpenoids. Fitoterapia 2010, 81, 219–222. [Google Scholar] [CrossRef]
- Brito, I.; Borquez, J.; Muñoz, R.; Cárdenas, A. Crystal and molecular structure of 14α-acetoxy-13α-hydroxymulin-11-en-20-oic acid monohydrate. J. Chil. Chem. Soc. 2018, 1, 17–19. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Zinner, S.H. The search for new antimicrobials: Why we need new options. Expert Rev. Anti Infect. Ther. 2005, 3, 907–913. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2016, 12, 121–140. [Google Scholar] [CrossRef]
- Dzul-Beh, A.d.J.; García-Sosa, K.; Uc-Cachón, A.H.; Bórquez, J.; Loyola, L.A.; Barrios-García, H.B.; Peña-Rodríguez, L.M.; Molina-Salinas, G.M. In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Rev. Bras. Farmacogn. 2019, 29, 798–800. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, M.S.; Hayat, F.; Shin, D. Recent advances in the discovery of novel antiprotozoal agents. Molecules 2019, 24, 3886. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.B.; Mäser, P.; Kaiser, M.; Hamburger, M.; Khalid, S. Mining sudanese medicinal plants for antiprotozoal agents. Front. Pharmacol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Herrera, A.; Cortez-Maya, S.; Bocanegra-Garcia, V.; Banik, B.K.; Rivera, G. Recent advances in the development of broad-spectrum antiprotozoal agents. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Morales, P.; Kong, M.; Pizarro, E.; Pasten, C.; Morales, G.; Borquez, J.; Loyola, L.A. Effect of azorellanone, a diterpene from Azorella yareta Hauman, on human sperm physiology. J. Androl. 2003, 24, 364–370. [Google Scholar] [CrossRef]
- Areche, C.; Cejas, P.; Thomas, P.; San-Martin, A.; Astudillo, L.; Gutiérrez, M.; Loyola, L.A. Triterpenoids from Azorella trifurcata (Gaertn.) Pers and their effect against the enzyme acetylcholinesterase. Quim. Nova 2009, 32, 2023–2025. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κ B signaling in inflammation. Signal Transduct Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
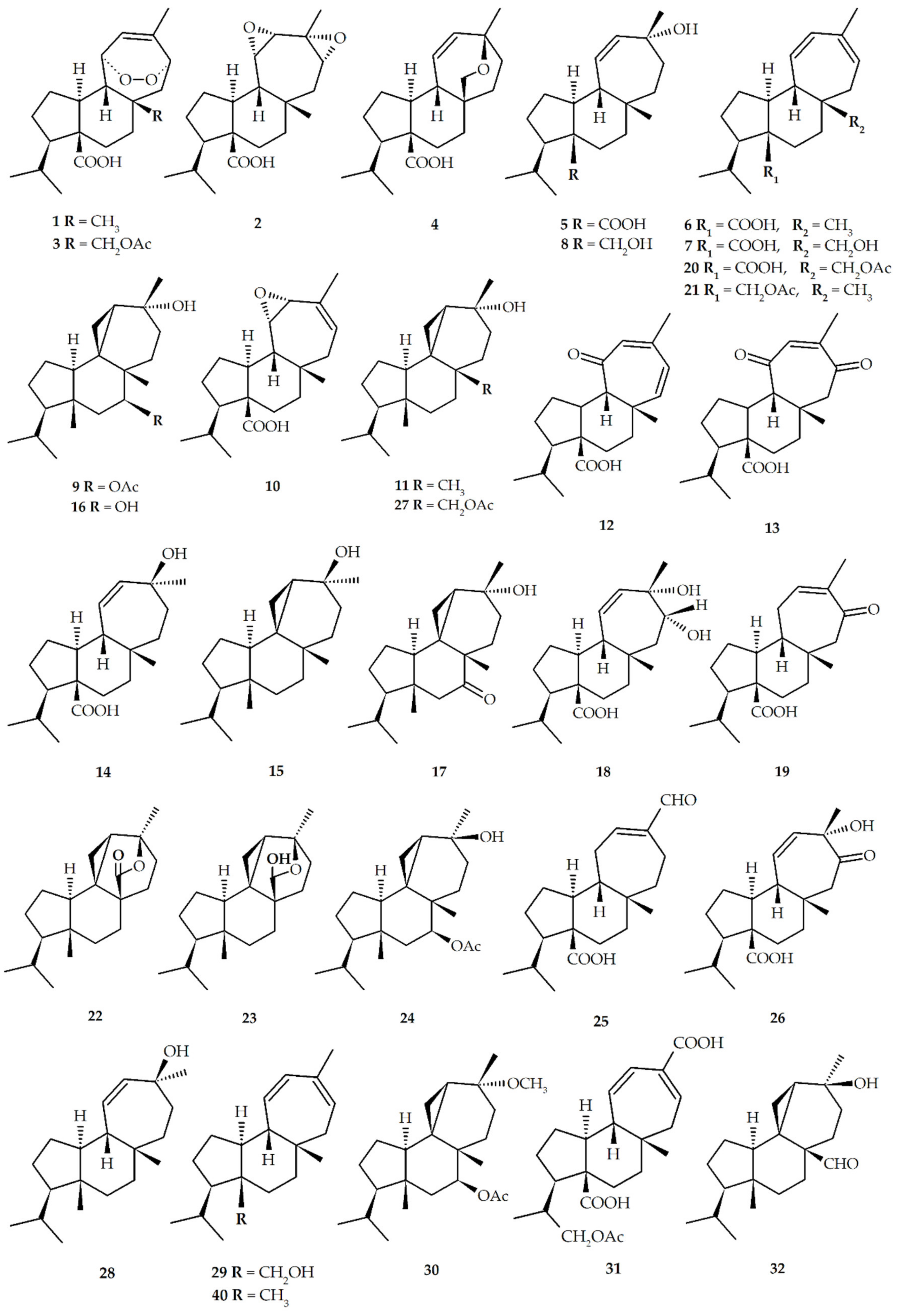
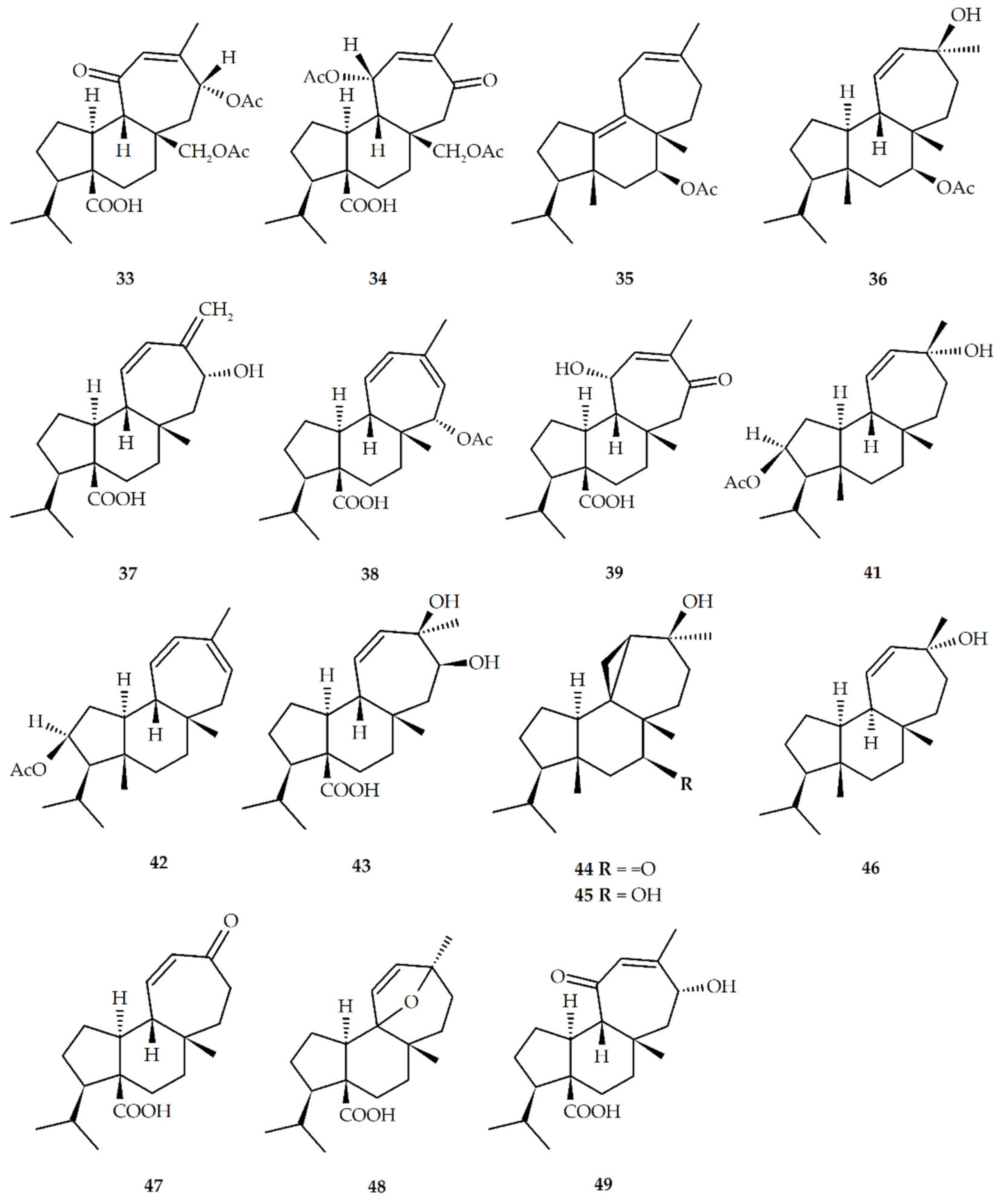
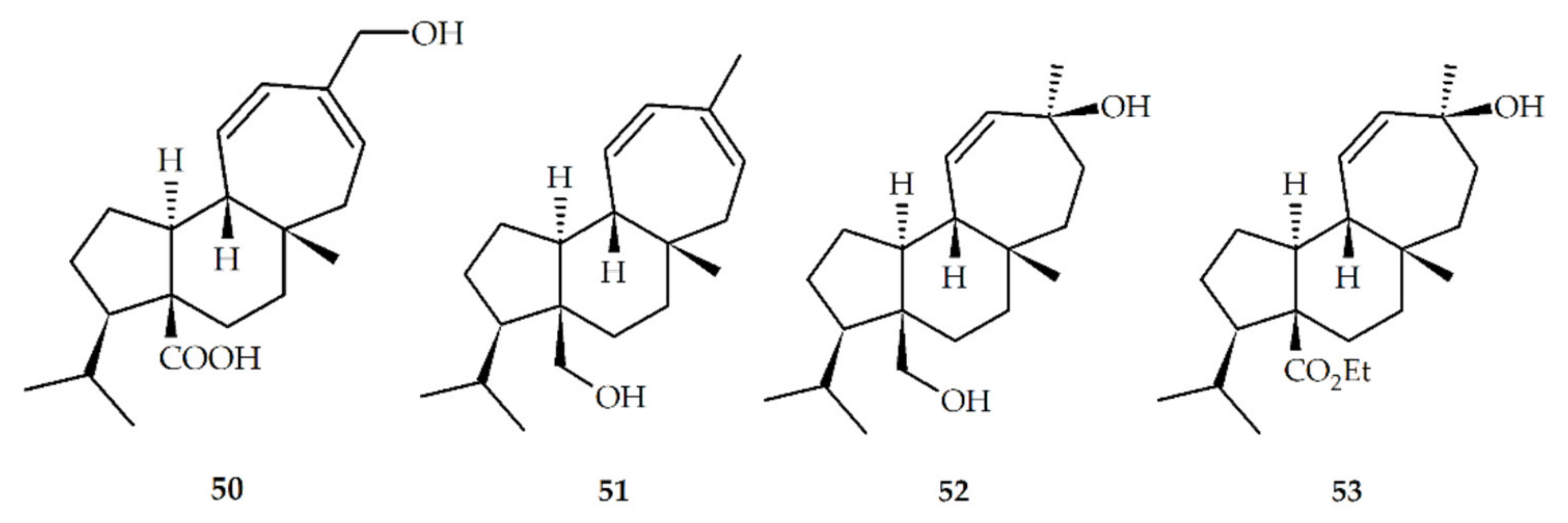
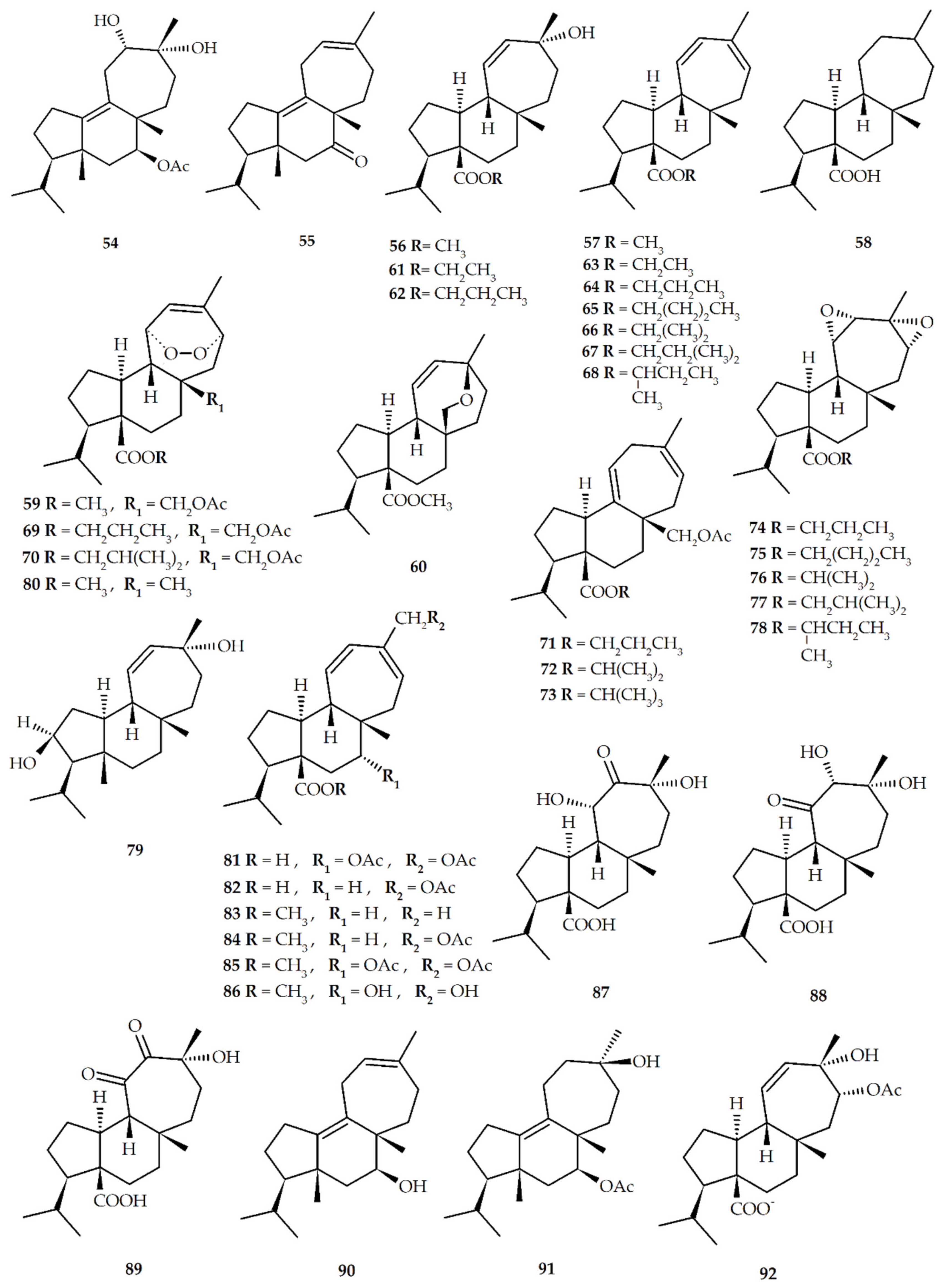
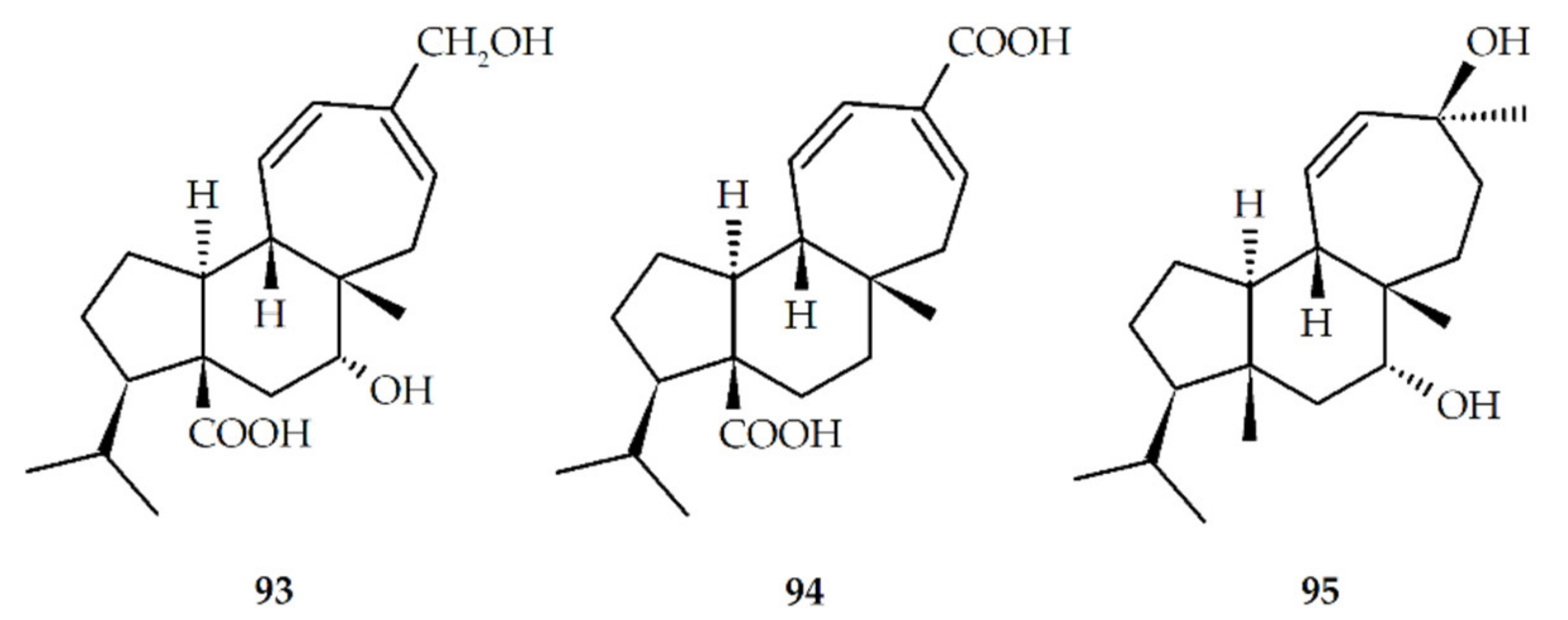
| Structure No. | Diterpenoid | Skeleton Type | First Report | Species | References |
|---|---|---|---|---|---|
| 1 | Mulinic acid (mulin-11,14-peroxi-12-en-20-oic acid) | M | 1990 | M. crassifolium A. compacta A. trifoliata | [17,21,22,23,24,25,26] |
| 2 | Isomulinic acid (mulin-11,12,13,14-diepoxy-20-oic acid) | M | 1990 | M. crassifolium | [17] |
| 3 | 17-acetoxymulinic acid (mulin-17-acetoxy-11,14-peroxi-12-en-20-oic acid) | M | 1990 | M. crassifolium A. compacta | [10,16,27,28,29] |
| 4 | Mulinenic acid (mulin-13α-hydroxy-11-en-20-oic acid | M | 1991 | M. crassifolium A. compacta M. spinosum | [30,31,32] |
| 5 | Mulinolic acid (mulin-13α-hydroxy-11-en-20-oic acid) | M | 1996 | M. crassifolium L. acualis A. yareta A. compacta A. madreporica M. spinosum A. trifoliata | [10,16,21,23,24,26,28,29,31,32,33,34,35,36,37,38,39,40] |
| 6 | Mulin-11,13-dien-20-oic acid | M | 1996 | M. spinosum A. compacta A. yareta M. crassifolium L. acaulis A. trifurcata A. cuatrecasasii M. spinosum | [16,21,23,24,26,28,33,35,36,37,38] |
| 7 | 17-hydroxy-mulin-11,13-dien-20-oic acid (mulin-17-hydroxy-11,13-dien-20-oic acid) | M | 1996 | M. spinosum | [37] |
| 8 | Mulinol (mulin-11-en-13α,20-diol) | M | 1997 | A. compacta A. criptantha | [25,41,42,43] |
| 9 | Azorellanol (azorellan-13α-hydroxy-7β-yl acetate) | A | 1998 | A. compacta A. yareta L. acaulis A. trifurcata A. criptantha | [10,20,21,22,23,24,26,28,35,36,37,41,42,44] |
| 10 | 11,12-epoxy-mulin-13-en-20-oic acid (mulin-11,12-epoxy-13-en-20-oic acid) | M | 1998 | A. compacta | [45] |
| 11 | 13α-hydroxy-azorellane (azorellan-13α-ol) | A | 1998 | A. madreporica A. yareta A. compacta A. trifurcata A. trifoliata | [22,24,26,35,36,39,46,47] |
| 12 | Mulin-12,14-dien-11-on-20-oic acid | M | 1999 | A. compacta | [40] |
| 13 | Mulin-12-ene-11,14-dion-20oic acid | M | 1999 | A. compacta | [40] |
| 14 | 13β-epimulinolic acid (mulin-13β-hydroxy-11-en-20-oic acid) | M | 2000 | L. acaulis | [38] |
| 15 | 13β-hydroxyazorellane (azorellan-13β-ol) | A | 2001 | A. yareta A. compacta L. acaulis A. trifurcata | [24,35,36,44,46] |
| 16 | 7β-deacetylazorellanol (azorellan-13α,7β-diol) | A | 2001 | L. acaulis A. compacta A. trifoliata | [21,22,23,28,41,42,44] |
| 17 | Azorellanone (azorellan-13α-hydroxy-7-one) | A | 2003 | A. yareta A. trifurcata L. acaulis | [42] |
| 18 | 13α,14α-dihydroxymulin-11-en-20-oic acid (mulin-13α,14α-dihydroxy-11-ene-20-oic acid) | M | 2003 | M. spinosum A. compacta | [28,29,32] |
| 19 | 14-oxo-mulin-12-en-20-oic acid (mulin-12-en-14-oxo-20-oic acid) | M | 2003 | M. spinosum | [32] |
| 20 | 17-acetoxy-mulin-11,13-dien-20-oic acid (mulin-17-acetoxy-11,13-dien-20-oic acid) | M | 2004 | A. compacta | [28,29] |
| 21 | 20-hydroxymulin-11,13-dienyl acetate (mulin-11,13-dien-20-yl acetate) | M | 2004 | A. compacta | [29] |
| 22 | Azorellolide (azorellan-17,13-(β)olide) | A | 2004 | A. cryptantha M. spinosum L. acaulis | [31,43,48] |
| 23 | Dyhydroazorellolide (azorellan-17,13-(β)hemiacetal) | A | 2004 | A. cryptantha | [48] |
| 24 | 13β-epiazorellanol (azorellan-13β-hydroxy-7β-yl acetate) | A | 2007 | L. acaulis A. compacta | [28,44] |
| 25 | Mulin-12-en-16-al-20-oic acid | M | 2010 | A. madreporica | [39] |
| 26 | 13α-hydroxy-mulin-11-en-14-one-20-oic acid (mulin-13α-hydroxy-14-oxo-11-en-20 oic acid) | M | 2010 | A. madreporica | [39] |
| 27 | 17-acetoxy-13α-hydroxyazorellane (azorellan-13α-hydroxy-17-yl acetate) | A | 2011 | A. madreporica | [47] |
| 28 | 13β-hydroxymulinane (mulin-13β-ol) | M | 2013 | A. compacta | [24] |
| 29 | Mulin-11,13-dien-20-ol | M | 2013 | A. compacta | [24] |
| 30 | 13α-methoxyazorellanol (azorellan-13α-methoxy-7β-yl acetate) | M | 2013 | A. compacta | [24] |
| 31 | Mulin-11,13-dien-18-acetoxy-16,20-dioic acid | M | 2013 | A. compacta A. trifurcata | [24,36] |
| 32 | Azorelaldehyde (azorellan-13α-hydroxy-17-al) | A | 2014 | A. cryptantha | [49] |
| 33 | Mulinone A (mulin-14α,17-diacetoxy-12-en-11-oxo-20-oic acid) | M | 2014 | M. crassifolium | [10] |
| 34 | Mulinone B (mulin-11α,17-diacetoxy-12-en-14-oxo-20-oic acid) | M | 2014 | M. crassifolium | [10] |
| 35 | 7-acetoxy-mulin-9,12-diene (mulin-9,12-dien-7β-yl acetate) | M | 2014 | M. crassifolium A. compacta | [10,28] |
| 36 | 7α-acetoxy-9-epi-13β-hydroxymulinane (mulin-9-epi-13β-hydroxy-11-en-7β-yl acetate) | M | 2014 | A. trifurcata | [36] |
| 37 | 14α-hydroxymulin-11,13(16)-dien-20-oic acid (mulin-14α-hydroxy-11,13(16)-dien-20-oic acid) | M | 2014 | A. trifurcata | [36] |
| 38 | 15α-acetoxymulin-11,13-dien-20-oic acid (mulin-15α-acetoxy-11,13-dien-20-oic acid) | M | 2014 | A. trifurcata | [36] |
| 39 | 11α-hydroxymulin12-en-14-one-20-oic acid (mulin-11α-hydroxy-12-en-14-oxo-20-oic-acid) | M | 2014 | A. trifurcata | [36] |
| 40 | Mulin-11,13-diene | M | 2014 | A. compacta | [26] |
| 41 | 2-acetoxy-13α-hydroxy-mulin-11-ene (mulin-13α-hydroxy-11-en-2-yl acetate) | M | 2014 | A. spinosa | [50] |
| 42 | 2-acetoxy-mulin-11,13-diene (mulin-11,13-dien-2-yl acetate) | M | 2014 | A. spinosa | [50] |
| 43 | 13β,14β-dihydroxymulin-11-en-20-oic acid (mulin-13β,14β-dihydroxy-11-en-20-oic acid) | M | 2015 | A. compacta | [28] |
| 44 | 13β-epiazorellanone (azorellan-13β-hydroxy-7-one) | A | 2015 | A. compacta | [28] |
| 45 | 13β-epi-7β-deacetyl-azorellanol (azorellan-13β,7β-diol) | A | 2015 | A. compacta | [28] |
| 46 | 9-epi-13α-hydroxymulinene (mulin-9-epi-13α-ol) | M | 2016 | A. cuatrecasassi | [51] |
| 47 | Normulin-11-en-13-oxo-20-oic acid | M | 2018 | A. compacta | [52] |
| 48 | 9,13-epoxymulin-11-en-20-oic acid (mulin-9,13-epoxy-11-en-20-oic acid) | M | 2019 | M. crassifolium | [16] |
| 49 | 14α-hydroxymulin-12-en-11-one-20-oic acid (mulin-14α-hydroxy-12-en-11-oxo-20-oic acid) | M | 2019 | M. crassifolium | [16] |
| No | Synthetic Mulinanes |
|---|---|
| 1 * | Mulinic acid (mulin-11,14-peroxi-12-en-20-oic acid) |
| 2 * | Isomulinic acid (mulin-11,12,13,14-diepoxy-20-oic acid) |
| 6 * | mulin11,13-dien-20-oic acid |
| 14 * | 13β-epimulinolic acid (mulin-13β-hydroxy-11-en-20-oic acid) |
| 21 * | 20-hydroxymulin-11,13-dienyl acetate (mulin-11,13-dien-20-yl acetate) |
| 50 | 16-hydroxy-mulin-11,13-dien-20-oic acid (mulin-16-hydroxy-11,13-dien-20-oic acid) |
| 51 | Mulin-11,13-dien-20-ol |
| 52 | 13β-epi-mulinol (mulin-11-en-13β,20-diol) |
| 53 | 13β-epi-mulinolic acid ethyl ester (mulin-13β-hydroxy-11-en-20-oic acid ethyl ester) |
| No. | Semisynthetic Mulinanes | References |
|---|---|---|
| 35 * | 7β-acetoxy-mulin-9,12-diene (mulin-9,12-dien-7β-yl acetate) | [22,57,58] |
| 54 | 7β-acetoxy-12α,13α-dihydroxy-mulin-9-ene (mulin-12α,13α-dihydroxy-9-en-7β-yl acetate) | [54,57] |
| 55 | 7-oxo-mulin-9,12-diene (mulin-9,12-dien-7-one) | |
| 56 | 13α--hydroxy-mulin-11-en-20-oic-acid methyl ester (mulin-13α-hydroxy-11-en-20-oic acid methyl ester) | |
| 57 | Mulin-11,13-dien-20-oic acid methyl ester | |
| 58 | Mulin-20-oic acid | |
| 59 | 17-acetoxy-mulinic acid methyl ester (mulin-17-acetoxy-11,14-peroxi-12-en-20-oic acid methyl ester) | |
| 60 | Mulinenic acid methyl ester (mulin-13α,17-oxy-11-en-20-oic acid methyl ester) | |
| 61 | 13α--hydroxy-mulin-11-en-20-oic-acid ethyl ester(mulin-13α -hydroxy-11-en-20-oic acid ethyl ester) | [54,59] |
| 62 | 13α--hydroxy-mulin-11-en-20-oic-acid n-propyl ester (mulin-13α -hydroxy-11-en-20-oic acid n-propyl ester) | |
| 63 | Mulin-11,13-dien-20-oic acid ethyl ester | |
| 64 | Mulin-11,13-dien-20-oic acid n-propyl ester | |
| 65 | Mulin-11,13-dien-20-oic acid n-butyl ester | |
| 66 | Mulin-11,13-dien-20-oic acid iso-propyl ester | |
| 67 | Mulin-11,13-dien-20-oic acid iso-butyl ester | |
| 68 | Mulin-11,13-dien-20-oic acid sec-butyl ester | |
| 69 | 17-acetoxy-mulinic acid n-propyl ester (mulin-17-acetoxy-11,14-peroxi-12-en-20-oic acid n-propyl ester) | |
| 70 | 17-acetoxy-mulinic acid iso-butyl ester (mulin-17-acetoxy-11,14-peroxi-12-en-20-oic acid iso-butyl ester) | |
| 71 | 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid n-propyl ester (mulin-17-acetoxy-9(11),13(14)-dien-20-oic acid n-propyl ester) | |
| 72 | 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid iso-propyl ester (mulin-17-acetoxy-9(11),13(14)-dien-20-oic acid iso-propyl ester) | |
| 73 | 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid sec-butyl ester (mulin-17-acetoxy-9(11),13(14)-dien-20-oic acid sec-butyl ester) | |
| 74 | Isomulinic acid n-propyl ester (mulin-11,12,13,14-diepoxy-20-oic acid n-propyl ester) | |
| 75 | Isomulinic acid n-butyl ester (mulin-11,12,13,14-diepoxy-20-oic acid n-butyl ester) | |
| 76 | Isomulinic acid iso-propyl ester (mulin-11,12,13,14-diepoxy-20-oic acid iso-propyl ester) | |
| 77 | Isomulinic acid iso-butyl ester (mulin-11,12,13,14-diepoxy-20-oic acid iso-butyl ester) | |
| 78 | Isomulinic acid sec-butyl ester (mulin-11,12,13,14-diepoxy-20-oic acid sec-butyl ester) | |
| 79 | 2,13α-dihydroxymulin-11-ene (mulin-11-en-2,13α-diol) | [50] |
| 80 | Mulinic acid methyl ester (mulin-11,14-peroxi-12-en-20-oic acid methyl ester) | [22] |
| 81 | 7α,16-diacetoxy-11,13-dien-20-oic acid (mulin-7α,16-diacetoxy-11,13-dien-20-oic acid) | [51] |
| 82 | 16-acetoxymulin-11,13-dien-20-oic acid (mulin-16-acetoxy-11,13-dien-20-oic acid) | |
| 83 | 16-hydroxymulin-11,13-dien-20-oic acid methyl ester (mulin-16-hydroxy-11,13-dien-20-oic acid methyl ester) | |
| 84 | 16-acetoxymulin-11,13-dien-20-oic acid methyl ester (mulin-16-acetoxy-11,13-dien-20-oic-acid methyl ester) | |
| 85 | 7α,16-dihydroxymulin-11,13-dien-20-oic acid methyl ester (mulin-7α,16-dihydroxy-11,13-dien-20-oic acid methyl ester) | |
| 86 | 7α,16-diacetoxymulin-11,13-dien-20-oic acid methyl ester (mulin-7α,16-acetoxy-11,13-dien-20-oic acid methyl ester) | |
| 87 | 12-oxo-11α,13α-dihydroxymulin-20-oic acid (mulin-11α,13α-dihydroxy-12-oxo-20-oic acid) | [58] |
| 88 | 11-oxo-12α,13α-dihydroxymulin-20-oic acid (mulin-12α,13α-dihydroxy-11-oxo-20-oic acid) | |
| 89 | 11,12-dioxo-13α-hydroxymulin-20-oic acid (mulin-13α-hydroxy-11,12-dioxo-20-oic acid) | |
| 90 | Mulin-9,12-dien-7-ol | |
| 91 | 7β-acetoxy-12,13-dihydroxymulin-9-en (mulin-12,13-dihydroxy-9-en-7β-yl acetate) | |
| 92 | 14α-acetoxy-13α-hydroxymulin-11-en-20-oic acid monohydrate (mulin-14α-acetoxy-13α-hydroxy-11-en-20-oic acid monohydrate) | [60] |
| No. | Biotransformed Mulinanes | References |
|---|---|---|
| 50 * | 16-hydroxy-mulin-11,13-dien-20-oic acid (mulin-16-hydroxy-11,13-dien-20-oic acid) | [51,56] |
| 93 | 7α,16-dihydroxymulin-11,13-dien-20-oic acid (mulin-7α,16-dihydroxy-11,13-dien-20-oic acid) | |
| 94 | Mulin-11,13-dien-16,20-oic acid | [55] |
| 95 | 7α,13β-dihydroxymulin-11-en-dien-20-oic acid (mulin-7α,13β-dihydroxy-11-en-dien-20-oic acid) |
| Activity | Study Model | Compound (Number) | Skeleton Type | Compound Origin | Biological Result | Positive Control | References |
|---|---|---|---|---|---|---|---|
| Antimicrobial | Mycobacterium tuberculosis H37Rv (ATCC 27294) | 13α-hydroxyazorellane (11) | A | N | MIC = 20 µg/mL | Rifampin MIC = 0.125 µg/mL | [46] |
| 13β-hydroxyazorellane (15) | A | N | MIC = 12.5 µg/mL | Rifampin MIC = 0.062 µg/mL Ofloxacin MIC = 0.125 µg/mL | [57] | ||
| Azorellanol (9) | A | N | MIC = 12.5 µg/mL | ||||
| 17-acetoxy-13α-hydroxyazorellane (27) | A | N | MIC = 12.5 µg/mL | ||||
| 7β-deacetylazorellanol (16) | A | N | MIC = 25 µg/mL | ||||
| Azorellanone (17) | A | N | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid (6) | M | M | MIC = 50 µg/mL | ||||
| Mulinic acid (1) | M | N | MIC = 50 µg/mL | ||||
| Mulinol (8) | M | N | MIC = 25 µg/mL | ||||
| 7β-acetoxy-12α,13α-dihydroxy-mulin-9-ene (54) | M | SS | MIC = 25 µg/mL | ||||
| 13α-hydroxy-mulin-11-en-20-oic-acid methyl ester (56) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid methyl ester (57) | M | SS | MIC = 25 µg/mL | ||||
| Mulinenic acid methyl ester (60) | M | SS | MIC = 12.5 µg/mL | ||||
| 13α -hydroxy-mulin-11-en-20-oic-acid ethyl ester (61) | M | SS | MIC = 25 µg/mL | Rifampin MIC = 0.062 µg/mL Ofloxacin MIC = 0.125 µg/mL | [59] | ||
| 13α -hydroxy-mulin-11-en-20-oic-acid n-propyl ester (62) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid ethyl ester (63) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid n-propyl ester (64) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid n-butyl ester (65) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid iso-propyl ester (66) | M | SS | MIC = 50 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid iso-butyl ester (67) | M | SS | MIC = 50 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid sec-butyl ester (68) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulinic acid n-propyl ester (69) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulinic acid iso-butyl ester (70) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid iso-propyl ester (72) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid sec-butyl ester (73) | M | SS | MIC = 50 µg/mL | ||||
| Isomulinic acid n-propyl ester (74) | M | SS | MIC = 25 µg/mL | ||||
| Isomulinic acid n-butyl ester (75) | M | SS | MIC = 25 µg/mL | ||||
| Isomulinic acid iso-propyl ester (76) | M | SS | MIC = 50 µg/mL | ||||
| Isomulinic acid iso-butyl ester (77) | M | SS | MIC = 50 µg/mL | ||||
| M. tuberculosis clinical isolate (MDR) | 13β-hydroxyazorellane (15) | M | N | MIC = 25 µg/mL | Ofloxacin MIC = 0.250 µg/mL | [57] | |
| Azorellanol (9) | A | N | MIC = 12.5 µg/mL | ||||
| 17-acetoxy-13α-hydroxyazorellane (27) | A | N | MIC = 12.5 µg/mL | ||||
| 7β-deacetylazorellanol (16) | A | N | MIC = 25 µg/mL | ||||
| Azorellanone (17) | A | N | MIC = 25 µg/mL | ||||
| 13β-epiazorellanol (24) | A | N | MIC = 50 µg/mL | ||||
| mulin-13α-hydroxy-11-en-20-oic acid (5) | M | N | MIC = 50 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid (6) | M | N | MIC = 25 µg/mL | ||||
| 13α,14α-dihydroxy-mulin-11-en-20-oic acid (18) | M | N | MIC = 50 µg/mL | ||||
| Mulinic acid (1) | M | N | MIC = 25 µg/mL | ||||
| 17-acetoxymulinic acid (3) | M | N | MIC = 50 µg/mL | ||||
| Mulinol (8) | M | N | MIC = 12.5 µg/mL | ||||
| 7β-acetoxy-12α,13α-dihydroxy-mulin-9-ene (54) | M | SS | MIC = 25 µg/mL | ||||
| 7-oxo-mulin-9,12-diene (55) | M | SS | MIC = 50 µg/mL | ||||
| 13α-hydroxy-mulin-11-en-20-oic-acid methyl ester (56) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid methyl ester (57) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-20-oic acid (58) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulinic acid methyl ester (59) | M | SS | MIC = 50 µg/mL | ||||
| Mulinenic acid methyl ester (60) | M | SS | MIC = 12.5 µg/mL | ||||
| 13α-hydroxy-mulin-11-en-20-oic-acid ethyl ester (61) | M | SS | MIC = 12.5 µg/mL | Ofloxacin MIC = 0.250 µg/mL | [59] | ||
| 13α-hydroxy-mulin-11-en-20-oic-acid n-propyl ester (62) | M | SS | MIC = 6.25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid ethyl ester (63) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid n-propyl ester (64) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid n-butyl ester (65) | M | SS | MIC = 12.5 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid iso-propyl ester (66) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid iso-butyl ester (67) | M | SS | MIC = 25 µg/mL | ||||
| Mulin-11,13-dien-20-oic acid sec-butyl ester (68) | M | SS | MIC = 25 µg/mL | ||||
| 17-acetoxy-mulinic acid n-propyl ester (69) | M | SS | MIC = 12.5 µg/mL | ||||
| 17-acetoxy-mulinic acid iso-butyl ester (70) | M | SS | MIC = 25 µg/mL | ||||
| 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid n-propyl ester (71) | M | SS | MIC = 50 µg/mL | ||||
| 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid iso-propyl ester (72) | M | SS | MIC = 25 µg/mL | ||||
| 17-acetoxy-mulin-9(11),13(14)-dien-20-oic acid sec-butyl ester (73) | M | SS | MIC = 25 µg/mL | ||||
| Isomulinic acid n-propyl ester (74) | M | SS | MIC = 6.25 µg/mL | ||||
| Isomulinic acid n-butyl ester (75) | M | SS | MIC = 6.25 µg/mL | ||||
| Isomulinic acid iso-propyl ester (76) | M | SS | MIC = 12.5 µg/mL | ||||
| Isomulinic acid iso-butyl ester (77) | M | SS | MIC = 12.5 µg/mL | ||||
| Isomulinic acid sec-butyl ester (78) | M | SS | MIC = 50 µg/mL | ||||
| M. smegmatis (ATCC 14468) | 7β-acetoxy-mulin-9,12-diene (35) | M | SS | MIC = 43.8 µg/mL | No data | [22] | |
| Staphylococcus aureus MSSA (ATCC 25923) | 7β-acetoxy-mulin-9,12-diene (35) | M | SS | 70 µg/dik: ZI = 15 mm | Penicillins/Streptomycin | [22] | |
| S. aureus clinical isolate MSSA | Mulin-12,14-dien-11-on-20-oic acid (12) | M | N | 20 µg/disk: ZI = 8–12 mm | Vancomycin 30 µg/disk: ZI = 16–18 mm | [40] | |
| Mulin-12-ene-11,14-dion-20oic acid (13) | M | N | 20 µg/disk: ZI = 8–12 mm | ||||
| S. aureus clinical isolate MRSA | Mulin-12,14-dien-11-on-20-oic acid (12) | M | N | 20 µg/disk: ZI = 8–12 mm | |||
| Mulin-12-ene-11,14-dion-20-oic acid (13) | M | N | 20 µg/disk ZI = 8–12 mm | ||||
| S. aureus clinical isolate MDR | 7β-acetoxy-mulin-9,12-diene (35) | M | SS | 70 µg/disk: ZI = 13 mm | Penicillin/Streptomycin | [22] | |
| Escherichia coli (ATCC BAS-849) | Mulin-12,14-dien-11-on-20-oic acid (12) | M | N | 20 µg/disk ZI = 8–12 mm | Cefoxitin 30 µg/dik: ZI = 16–18 mm | [40] | |
| Mulin-12-ene-11,14-dion-20-oic acid (13) | M | N | 20 µg/disk: ZI = 8–12 mm | ||||
| Enterococcus faecium clinical isolate Vancomycin resistant | Mulin-12,14-dien-11-on-20-oic acid (12) | M | N | 20 µg/disk: ZI = 8–12 mm | Bacitracin 10 µg/disk: ZI = 16–18 mm | ||
| Mulin-12-ene-11,14-dion-20-oic acid (13) | M | N | 20 µg/disk: ZI = 8–12 mm | ||||
| Antiprotozoal | Trichomonas vaginalis trophozoite as (Ant-1 strain) | 13β-hydroxyazorellane (15) | A | N | LD50 = 100 µM | Metronidazole LD50 = 6.6 µM | [35] |
| 13α-hydroxyazorellane (11) | A | N | LD50 = 119 µM | ||||
| Azorellanol (9) | A | N | LD50 = 40.5 µM | ||||
| Toxoplasma gondii trophozoites | 7β-deacetylazorellanol (16) | A | N | LD50 = 54 µM | Clindamycin LD50 = 84 µM | [41] | |
| Azorellanol (9) | A | N | LD50 = 42 µM | ||||
| Trypanosoma cruzi strains Tula-huen, SPA-14 and CL Brener clone | Mulin-11,3-dien-20-oic acid (6) | M | N | %I = 92–98.4 (IC50 = 41–87 µM) to 10 µM | Gentian violet 1 µM | [21] | |
| Azorellanol (9) | A | N | %I = 88.4–99IC50 = 20–84 µM to 10 µM | ||||
| Plasmodium berghei (NK 65) | 20-hydroxymulin-11,13-dienyl acetate (21) | M | N | 20 mg/kg/day: %I = 29 to | Chloroquine 5 mg/kg/day IC50 = 2.5 mg/kg/day | [29] | |
| 13α,14α-dihydroxymulin-11-en-20-oic acid (18) | M | N | 20 mg/kg/day:%I = 42 to | ||||
| 17-acetoxymulin-11,13-dien-20-oic acid (18) | M | N | 20 mg/kg/day:%I = 60 to | ||||
| Spermicidal/Spermatostatic | Human sperm, motile and living cells | Azorellanone (17) | A | N | 3 mM: %MC = 41% 3 mM: %LC = 57 | 0.5% ethyl acetate (vol/vol) | [68] |
| Human sperm, motile and living cells | Mulinonic acid (5) | M | N | %MC = 32% LC = 84 | No data | [31] | |
| Human sperm, motile and living cells | Azorellan-17,13-(β)olide (azorellolide) (22) | A | N | %MC = 34% LC = 82 | No data | ||
| Cytotoxic | Cancer cell line MCF-7 (ATCC, Mana-sas VA, UA), | Azorellanol (9) | A | N | IC50 = 25.64 µM | Doxorubicin IC50 = 5.52 µM | [28] |
| Mulin-11,13-dien-20-oic acid (6) | M | N | IC50 < 100 µM | ||||
| 7β-deacetylazorellanol (16) | A | N | IC50 < 100 µM | ||||
| 13β-epiazorellanol (24) | A | N | IC50 < 100 µM | ||||
| 7β-acetoxy-mulin-9,12-diene (35) | M | N | IC50 < 100 µM | ||||
| Anti-inflammatory | In vivo assay, arachidonic acid model | Azorellanol (9) | A | N | 6.3 x 10-6 mol/ear: 38.6% anti-inflammatory effect | Nimesulide 3.2 x 10-6 mol/ear: 48.8% anti-inflammatory effect. Indo-methacin 1.4 x 10-6 mol/ear: 28.0% anti-inflammatory effect | [42] |
| In vivo assay, 12-deoxy-phorbol 13 tetra-decanoate model | Azorellanol (9) | A | N | 15.0 x 10-7 mol/ear: 70.8% anti-inflammatory effect | Indomethacin 1.4 x 10-6 mol/ear: 81.8% anti-inflammatory effect | ||
| 7β-deacetylazorellanol (16) | A | N | 2.6 x 10-6 mol/ear: 79.0% anti-inflammatory effect | ||||
| anti-NF-kB | Azorellanol (9) | A | N | Inhibition at 25 mg | No data | [44] | |
| 7β-deacetylazorellanol (16) | A | N | Inhibition at 25 mg | ||||
| 13β-hydroxyazorellane (15) | A | N | Inhibition at 25 mg | ||||
| Analgesic | In vivo assay, Acetic acid model | Azorellanol (9) | A | N | 11.0 x 10-5 mol/kg: 50.7% of analgesic effect | Sodium naproxen 4.9x10-5 mol/kg: 70.0% of analgesic effect | [42] |
| 7β-deacetylazorellanol (16) | A | N | 5.7 x 10-5 mol/kg: 53.4% of analgesic effect | ||||
| Azorellanone (17) | A | N | 10.0 x 10-5 mol/kg: 59.0% of analgesic effect | ||||
| Antihyperglycemic | In vivo assay Strepto-zotocin-induced diabetic | Mulinolic acid (5) | M | N | 180 mg/mL: 48% reduction of glucose | Chlorpropamide 5 mg/mL: 50.3% reduction of glucose | [23] |
| Azorellanol (9) | A | N | 180 mg/mL: 49% reduction of glucose | ||||
| Antiulcer | In vivo assay HCl/EtOH-induced injury model | Mulin-11,13-dien-18-acetoxy-16,20-dioic acid (31) | M | N | 20 mg/kg: 73% reduction of gastric injury | Lansoprazole 20 mg/kg: 78% reduction of gastric injury | [24] |
| Azorellanol (9) | A | N | 20 mg/kg: 71% reduction of gastric injury | ||||
| 13β-hydroxyazorellane (15) | A | N | 20 mg/kg: 69% reduction of gastric injury | ||||
| 13β-hydroxymulinane (28) | M | N | 20 mg/kg: 59% reduction of gastric injury | ||||
| Mulin-11,13-dien-20-ol (29) | M | N | 20 mg/kg: 26% reduction of gastric injury | ||||
| Mulin-11,13-dien-20-oic acid (6) | M | N | 20 mg/kg: 39% reduction of gastric injury | ||||
| 13α-hydroxyazorellane (11) | A | N | 20 mg/kg: 56% reduction of gastric injury | ||||
| Mulinolic acid (5) | M | N | 20 mg/kg: 55% reduction of gastric injury | ||||
| In vivo assay HCl/EtOH-induced injury model | Mulin-11,13-dien-20-oic acid (6) | M | N | ED50 = 55 mg/kg | Lansoprazole 20 mg/kg | [36] | |
| In vivo assay HCl/EtOH-induced injury model | 16-hydroxy-mulin-11,13-dien-20-oic acid (50) | M | SS | 20 mg/kg: 59% reduction of gastric injury | Lansoprazole 20 mg/kg: 57% reduction of gastric injury | [51] | |
| 7α,16-dihydroxymulin-11,13-dien-20-oic acid (93) | M | SS | 20 mg/kg: 69% reduction of gastric injury | ||||
| 16-acetoxymulin-11,13-dien-20-oic acid (82) | M | SS | 20 mg/kg: 43% reduction of gastric injury | ||||
| 7α,16-diacetoxymulin-11,13-dien-20-oic acid (81) | M | SS | 20 mg/kg: 48% reduction of gastric injury | ||||
| 7α,16-dihydroxymulin-11,13-dien-20-oic acid methyl ester (85) | M | SS | 20 mg/kg: 36% reduction of gastric injury | ||||
| Anti-Alzheimer. | Inhibition of the enzyme acetyl-choline-esterase | Mulinolic acid (5) | M | N | IC50 = 200 µg/mL (630 µM) | Galanthamine IC50 = 1.1 µg/mL (3.0 µM) | [69] |
| Inhibition of the enzyme acetyl-choline-esterase | Mulin-11,13-dien-20-oic acid (6) | M | N | IC50 = 180 µg/mL (580 µM) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzul-Beh, A.d.J.; Uc-Cachón, A.H.; Bórquez, J.; Loyola, L.A.; Peña-Rodríguez, L.M.; Molina-Salinas, G.M. Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology. Biomolecules 2020, 10, 1333. https://doi.org/10.3390/biom10091333
Dzul-Beh AdJ, Uc-Cachón AH, Bórquez J, Loyola LA, Peña-Rodríguez LM, Molina-Salinas GM. Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology. Biomolecules. 2020; 10(9):1333. https://doi.org/10.3390/biom10091333
Chicago/Turabian StyleDzul-Beh, Angel de Jesús, Andrés Humberto Uc-Cachón, Jorge Bórquez, Luis A. Loyola, Luis Manuel Peña-Rodríguez, and Gloria María Molina-Salinas. 2020. "Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology" Biomolecules 10, no. 9: 1333. https://doi.org/10.3390/biom10091333
APA StyleDzul-Beh, A. d. J., Uc-Cachón, A. H., Bórquez, J., Loyola, L. A., Peña-Rodríguez, L. M., & Molina-Salinas, G. M. (2020). Mulinane- and Azorellane-Type Diterpenoids: A Systematic Review of Their Biosynthesis, Chemistry, and Pharmacology. Biomolecules, 10(9), 1333. https://doi.org/10.3390/biom10091333