Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Characterization of Bacteria
2.3. Experimental Design
2.4. Determination of Phytohormones
2.5. Statistics
3. Results
3.1. Morphological Parameters
3.2. Hormones in Bacterial Culture Media
3.3. Effect of Bacteria on the Content of Hormones in the Plants
3.3.1. Auxin
3.3.2. ABA
3.3.3. Cytokinins
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Vargas, L.; Brigida, A.B.S.; Filho, J.P.M.; De Carvalho, T.G.; Rojas, C.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.G.; Vandepoele, K.; et al. Drought Tolerance Conferred to Sugarcane by Association with Gluconacetobacter diazotrophicus: A Transcriptomic View of Hormone Pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Bottini, R.; Pontin, M.; Berli, F.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilenseameliorates the response ofArabidopsis thalianato drought mainly via enhancement of ABA levels. Physiol. Plant. 2014, 153, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-arid Agroecosystem and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2017, 64, 574–587. [Google Scholar] [CrossRef]
- Rubin, R.L.; Van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Ali, S.Z.; Vardharajula, S.; Vurukonda, S.S.K.P. Transcriptomic profiling of maize (Zea mays L.) seedlings in response to Pseudomonas putida stain FBKV2 inoculation under drought stress. Ann. Microbiol. 2018, 68, 331–349. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A halotolerant growth promoting rhizobacteria triggers induced systemic resistance in plants and defends against fungal infection. Sci. Rep. 2019, 9, 4054. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Arkhipova, T.; Galimsyanova, N.; Kuzmina, L.; Vysotskaya, L.; Sidorova, L.; Gabbasova, I.; Melentiev, A.; Kudoyarova, G. Effect of seed bacterization with plant growth-promoting bacteria on wheat productivity and phosphorus mobility in the rhizosphere. Plant Soil Environ. 2019, 65, 313–319. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth ofTriticum aestivumL. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Boil. 2010, 3, a001438. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Shaposhnikov, A.; Azarova, T.; Makarova, N.; Davies, W.; Tikhonovich, I.A. Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann. Appl. Boil. 2015, 167, 11–25. [Google Scholar] [CrossRef]
- Blinkov, E.A.; Tsavkelova, E.A.; Selitskaya, O.V. Auxin production by the Klebsiella planticola strain TSKhA-91 and its effect on development of cucumber (Cucumis sativus L.) seeds. Microbiology 2014, 83, 531–538. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuz’Mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.Y. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 2133–2144. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Wilkinson, S.; Kudoyarova, G.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Chen, C. Humidity in Plant Tissue Culture Vessels. Biosyst. Eng. 2004, 88, 231–241. [Google Scholar] [CrossRef]
- Glaz, N.V.; Vasiliev, A.A.; Dergileva, T.T.; Mushinskiy, A.A. Middle early and mid-ripening varieties of potato: Environmental assessment of flexibility. Far East Agrar. Bull. 2019, 1, 10–19. [Google Scholar] [CrossRef]
- Assmus, B.; Hutzler, P.; Kirchhof, G.; Amann, R.; Lawrence, J.R.; Hartmann, A. In Situ Localization of Azospirillum brasilense in the Rhizosphere of Wheat with Fluorescently Labeled, rRNA-Targeted Oligonucleotide Probes and Scanning Confocal Laser Microscopy. Appl. Environ. Microbiol. 1995, 61, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Yevstigneyeva, S.S.; Sigida, E.N.; Fedonenko, Y.P.; Konnova, S.A.; Ignatov, V.V. Variability of composition and structure of extractable and membrane glycopolymers of Azospirillum brasilense bacteria under salt and temperature stress. In Proceedings of the VIII All-Russian Conference of Young Scientists “Strategy of Interaction of Microorganisms and Plants with the Environment”, Saratov, Russia, 26–30 September 2016; Book of Abstracts. p. 50. [Google Scholar]
- Burygin, G.L.; Popova, I.; Kargapolova, K.; Tkachenko, O.; Matora, L.Y.; Shchyogolev, S. A bacterial isolate from the rhizosphere of potato (solanum tuberosum l.) identified as Ochrobactrum lupini IPA7.2. sel’skokhozyaistvennaya Boil. 2017, 52, 105–115. [Google Scholar] [CrossRef][Green Version]
- Burygin, G.L.; Kargapolova, K.Y.; Kryuchkova, Y.V.; Avdeeva, E.S.; Gogoleva, N.E.; Ponomaryova, T.S.; Tkachenko, O.V. Ochrobactrum cytisi IPA7.2 promotes growth of potato microplants and is resistant to abiotic stress. World J. Microbiol. Biotechnol. 2019, 35, 55. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.V.; Evseeva, N.V.; Boikova, N.V.; Matora, L.Y.; Burygin, G.L.; Lobachev, Y.V.; Shchyogolev, S.Y. Improved potato microclonal reproduction with the plant growth-promoting rhizobacteria Azospirillum. Agron. Sustain. Dev. 2015, 35, 1167–1174. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Evseeva, N.; Tkachenko, O.V.; Denisova, A.Y.; Burygin, G.L.; Veselov, D.S.; Matora, L.Y.; Shchyogolev, S.Y. Functioning of plant-bacterial associations under osmotic stress in vitro. World J. Microbiol. Biotechnol. 2019, 35, 195. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G. Effect of Competition and Treatment with Inhibitor of Ethylene Perception on Growth and Hormone Content of Lettuce Plants. J. Plant Growth Regul. 2017, 36, 450–459. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of free cytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Anokhina, N.L. Effects of wheat plant inoculation with cytokinin-producing microorganisms on plant growth at increasing level of mineral nutrition. Russ. J. Plant Physiol. 2009, 56, 814–819. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.Y.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Effect of cytokinin producing microorganisms on plant growth. Biotechnol. Russ. 2006, 4, 66–73. [Google Scholar]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.C.; Mulbry, W.W.; Cohen, J.D. The gene for indole-3-acetyl-L-aspartic acid hydrolase from Enterobacter agglomerans : Molecular cloning, nucleotide sequence, and expression in Escherichia coli. Mol. Genet. Genom. 1998, 259, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Egorshina, A.A.; Khairullin, R.M.; Sakhabutdinova, A.R.; Luk’Yantsev, M.A. Involvement of phytohormones in the development of interaction between wheat seedlings and endophytic Bacillus subtilis strain 11BM. Russ. J. Plant Physiol. 2011, 59, 134–140. [Google Scholar] [CrossRef]
- Procko, C.; Burko, Y.; Jaillais, Y.; Ljung, K.; Long, J.A.; Chory, J. The epidermis coordinates auxin-induced stem growth in response to shade. Genes Dev. 2016, 30, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Prasad, V.; Prasad, M. A Functional Genomic Perspective on Drought Signalling and its Crosstalk with Phytohormone-mediated Signalling Pathways in Plants. Curr. Genom. 2017, 18, 469–482. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Kim, J.I.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Cha, J.Y.; Kim, W.Y.; Kim, M.C.; Chung, W.S.; et al. Overexpression of Arabidopsis YUCCA6 in Potato Results in High-Auxin Developmental Phenotypes and Enhanced Resistance to Water Deficit. Mol. Plant 2013, 6, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Schopfer, P. Effect of auxin and abscisic acid on cell wall extensibility in maize coleoptiles. Planta 1986, 167, 527–535. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Veselov, D.S.; Kudoyarova, G.R.; Kudryakova, N.V.; Kusnetsov, V.V. Role of cytokinins in stress resistance of plants. Russ. J. Plant Physiol. 2017, 64, 15–27. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the Crossroads of Abiotic Stress Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [PubMed]
- Dobránszki, J.; Mendler-Drienyovszki, N. Cytokinin-induced changes in the chlorophyll content and fluorescence of in vitro apple leaves. J. Plant Physiol. 2014, 171, 1472–1478. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Korobova, A.V.; Vysotskaya, L.B.; Vasinskaya, A.N.; Kuluev, B.R.; Veselov, S.Y.; Kudoyarova, G. Dependence of root biomass accumulation on the content and metabolism of cytokinins in ethylene-insensitive plants. Russ. J. Plant Physiol. 2016, 63, 597–603. [Google Scholar] [CrossRef]
- Ko, D.; Kang, J.; Kiba, T.; Park, J.; Kojima, M.; Do, J.; Kim, K.Y.; Kwon, M.; Endler, A.; Song, W.Y.; et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA 2014, 111, 7150–7155. [Google Scholar] [CrossRef]
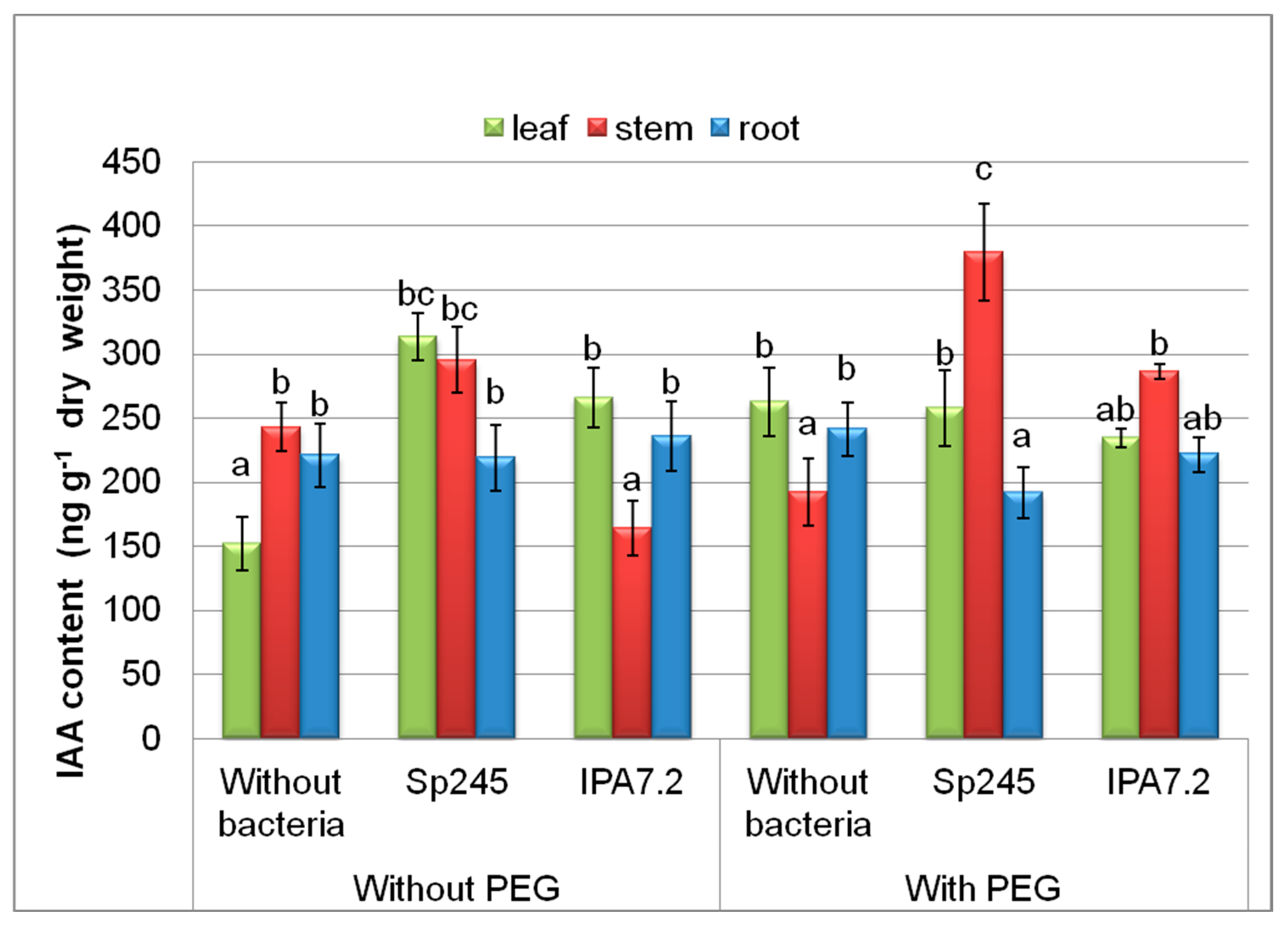

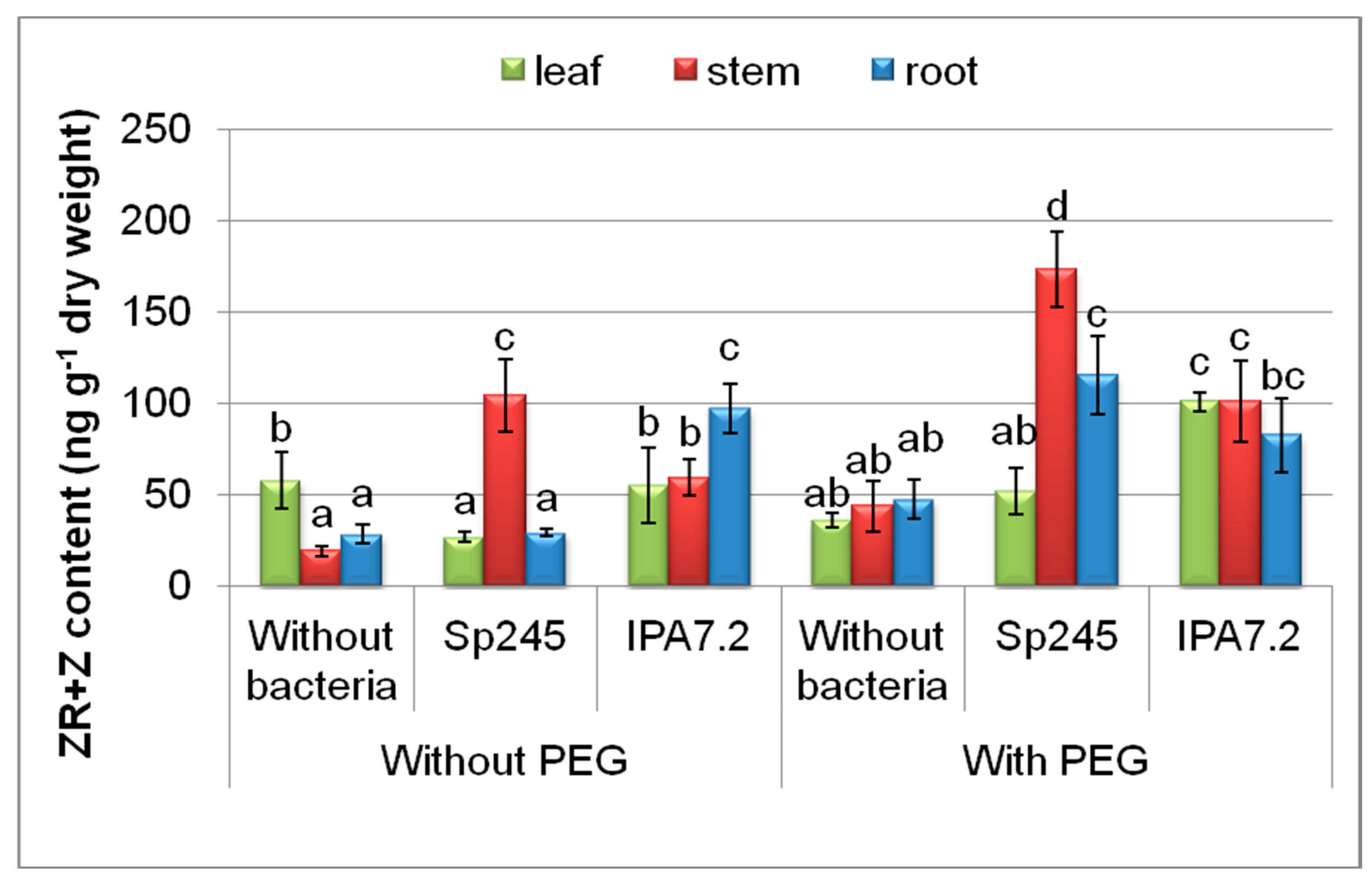
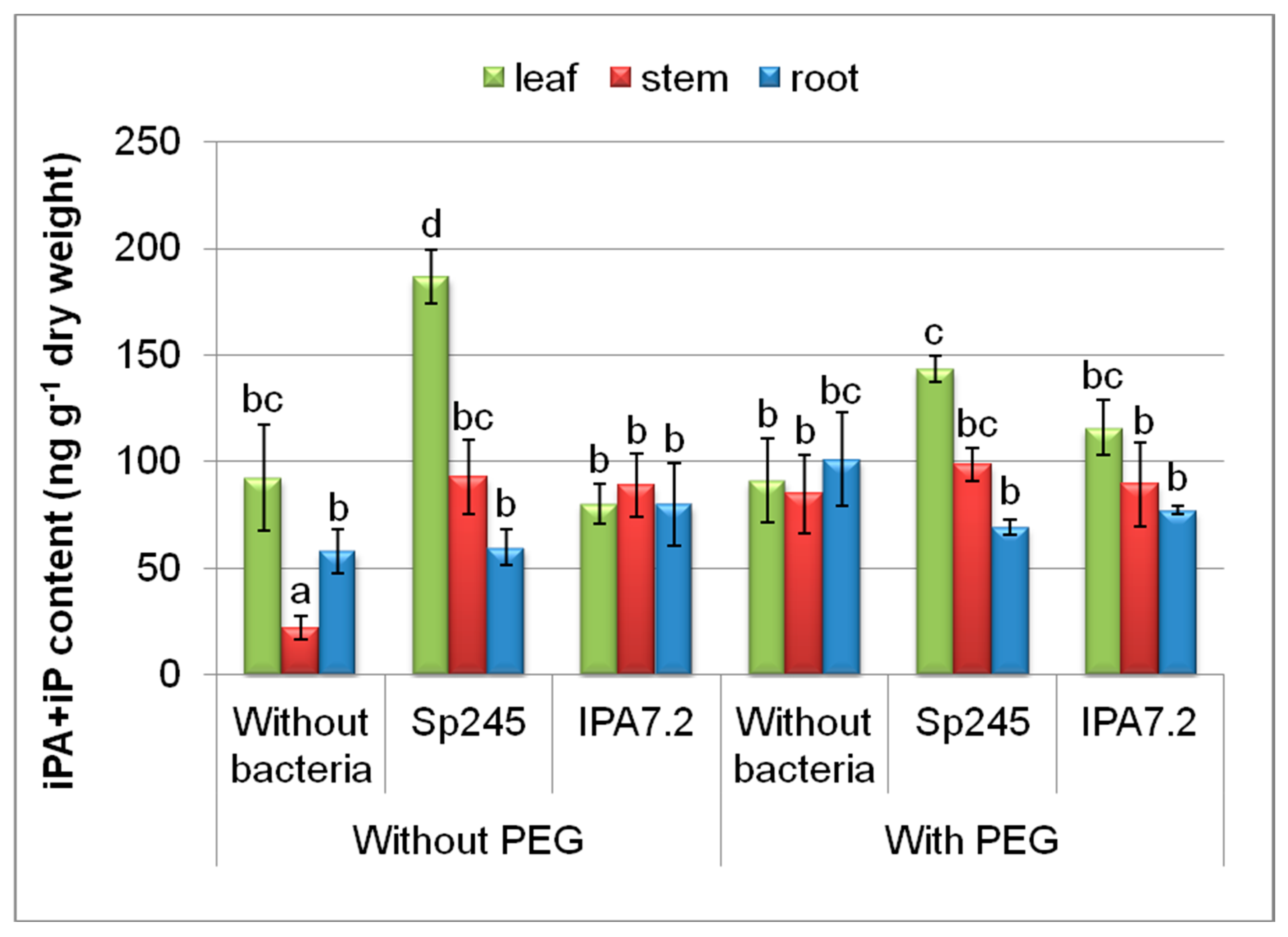
| Variants | Stem Length mm | Fresh Mass, mg | |||
|---|---|---|---|---|---|
| Stem | Leaves | Roots | |||
| Without PEG | Without bacteria | 59.7 bc* | 119.0 b | 115.0 c | 97.0 cd |
| +Sp245 | 65.4 c | 153.0 c | 152.0 d | 112.0 d | |
| +IPA7.2 | 57.9 b | 121.0 b | 141.0 cd | 77.0 bc | |
| With PEG | Without bacteria | 46.1 a | 62.1 a | 43.7 a | 37.7 a |
| +Sp245 | 59.6 bc | 56.6 a | 85.3 b | 33.0 a | |
| +IPA7.2 | 64.0 bc | 56.9 a | 119.0 c | 87.0 bc | |
| Bacterial Strains | Duration of Cultivation | |
|---|---|---|
| 36 h | 102 h | |
| O. cytisi IPA7.2 | 6.3 a* | 12.1 b |
| A. brasilense Sp245 | 9.7 a | 192.4 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress. Biomolecules 2020, 10, 1231. https://doi.org/10.3390/biom10091231
Arkhipova TN, Evseeva NV, Tkachenko OV, Burygin GL, Vysotskaya LB, Akhtyamova ZA, Kudoyarova GR. Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress. Biomolecules. 2020; 10(9):1231. https://doi.org/10.3390/biom10091231
Chicago/Turabian StyleArkhipova, Tatiana N., Nina V. Evseeva, Oksana V. Tkachenko, Gennady L. Burygin, Lidiya B. Vysotskaya, Zarina A. Akhtyamova, and Guzel R. Kudoyarova. 2020. "Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress" Biomolecules 10, no. 9: 1231. https://doi.org/10.3390/biom10091231
APA StyleArkhipova, T. N., Evseeva, N. V., Tkachenko, O. V., Burygin, G. L., Vysotskaya, L. B., Akhtyamova, Z. A., & Kudoyarova, G. R. (2020). Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress. Biomolecules, 10(9), 1231. https://doi.org/10.3390/biom10091231







