4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition
Abstract
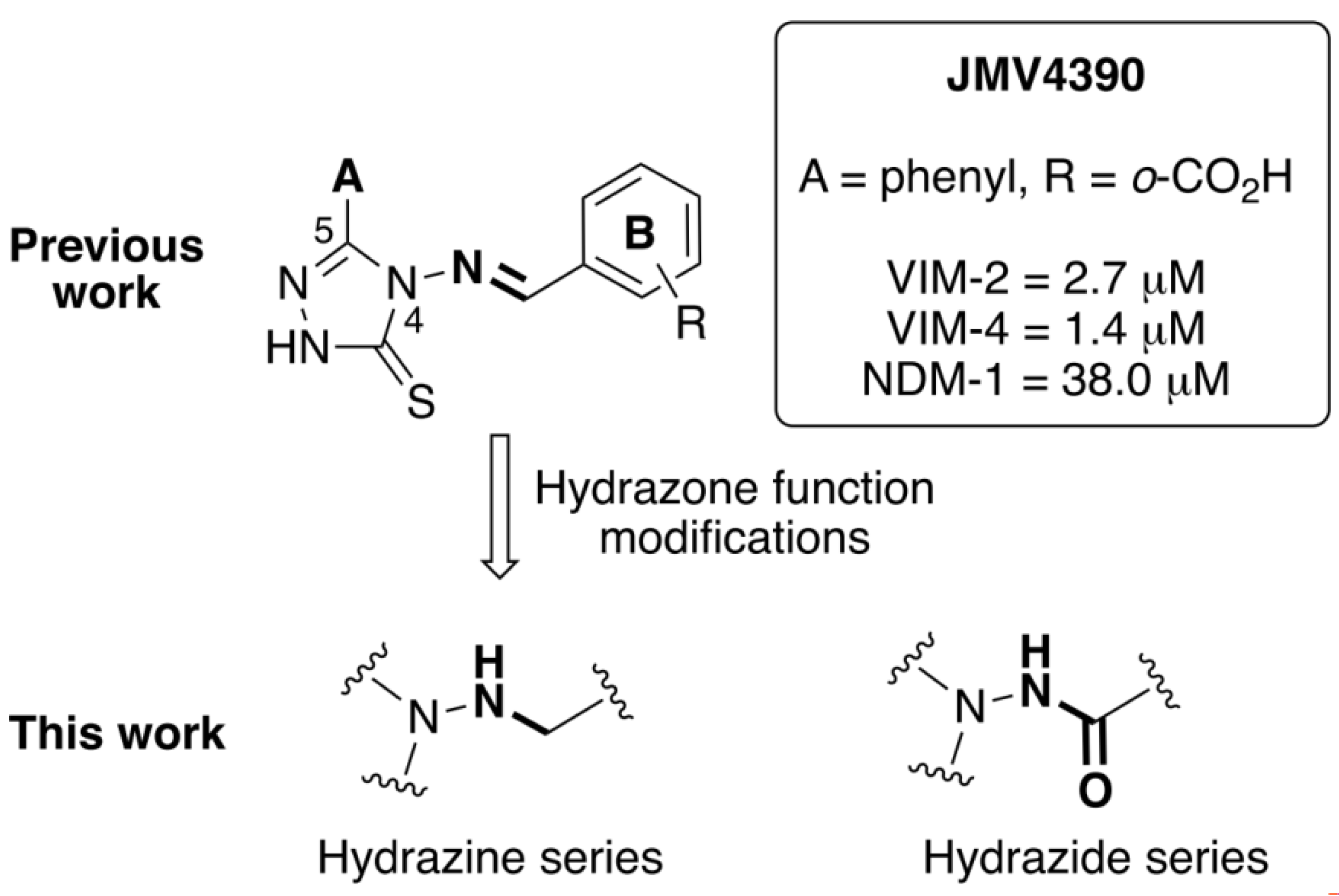
1. Introduction
2. Materials and Methods
2.1. Chemistry
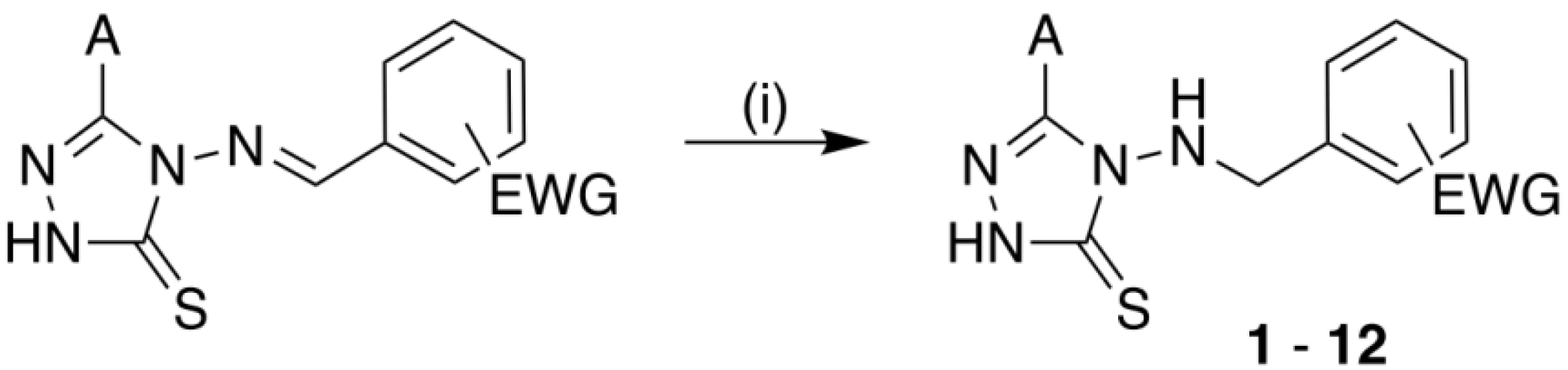
2.1.1. Typical Procedure of Hydrazone Reduction (Synthesis of Hydrazines 1–12)
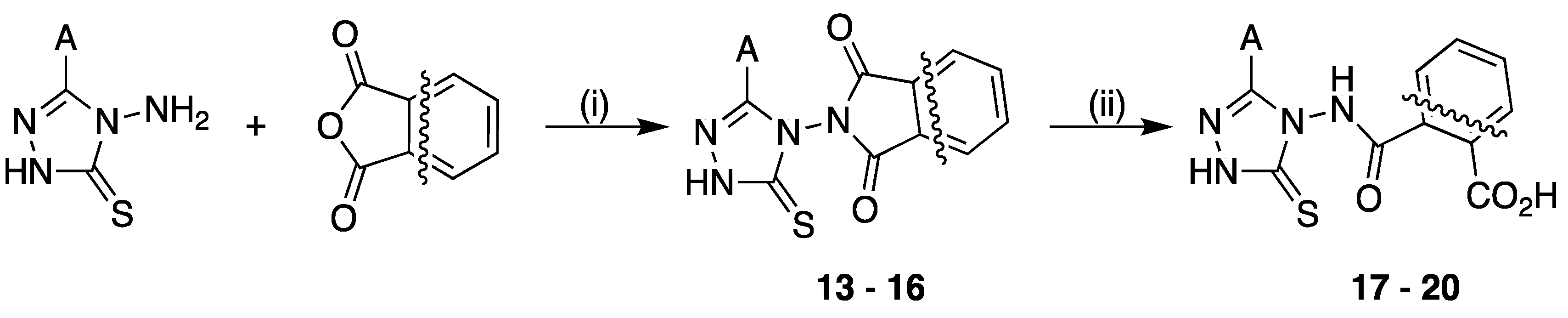
2.1.2. Typical Procedure for Succinic Anhydride Condensation (Synthesis of Succinimides 13–16)
2.1.3. Typical Procedure of Succinimide Ring-Opening Reaction (Synthesis of Hydrazides 17–20)
2.2. Metallo-β-Lactamase Inhibition Assays
2.2.1. VIM-Type Enzymes: NDM-1 and IMP-1
2.2.2. CphA and L1
2.3. Microbiological Assays
2.4. Isothermal Titration Calorimetry Analysis of Binding to VIM-2
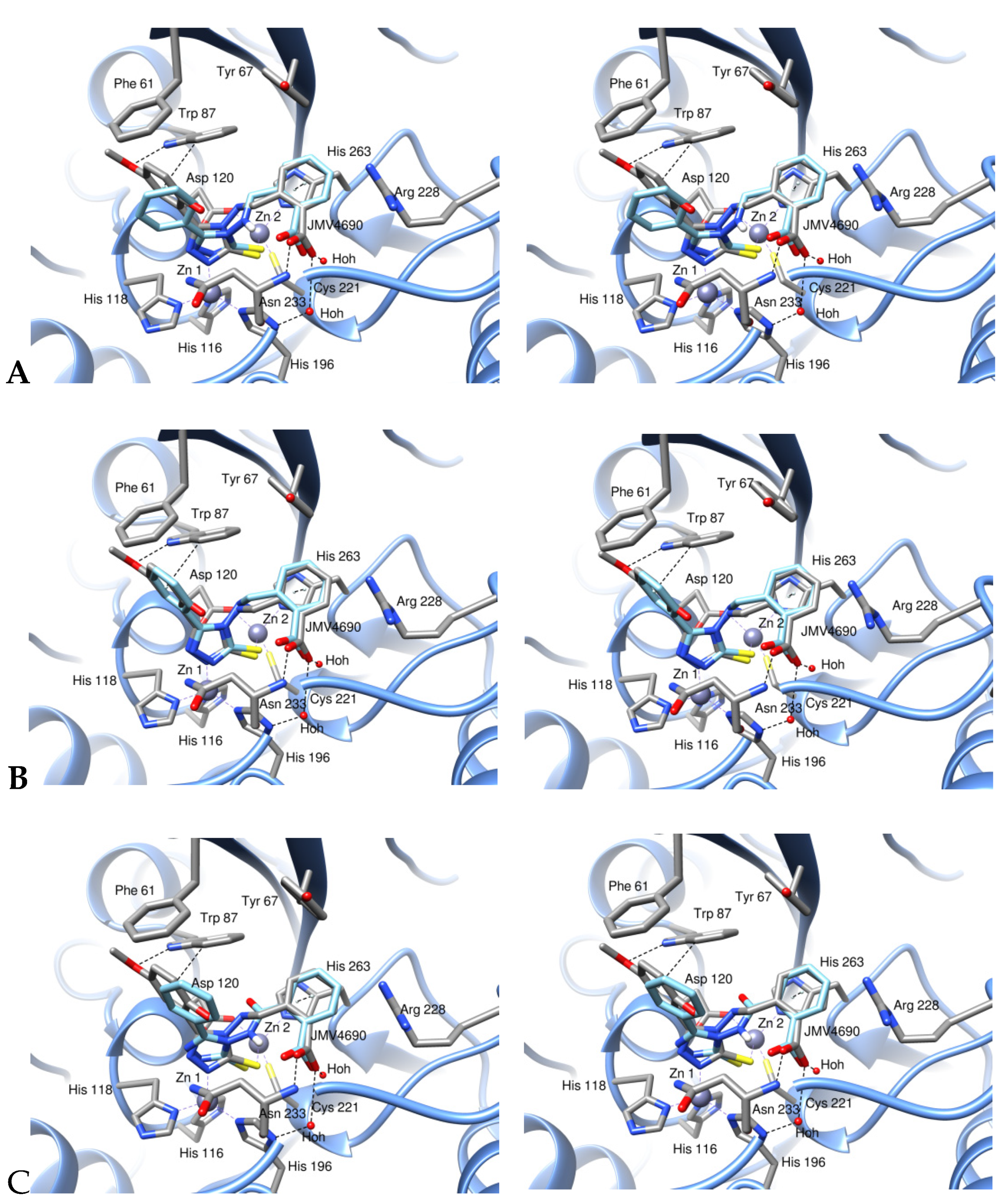
2.5. Modeling Study
3. Results and Discussion
3.1. Chemistry
3.2. MBL Inhibition
3.3. Microbiological Assays
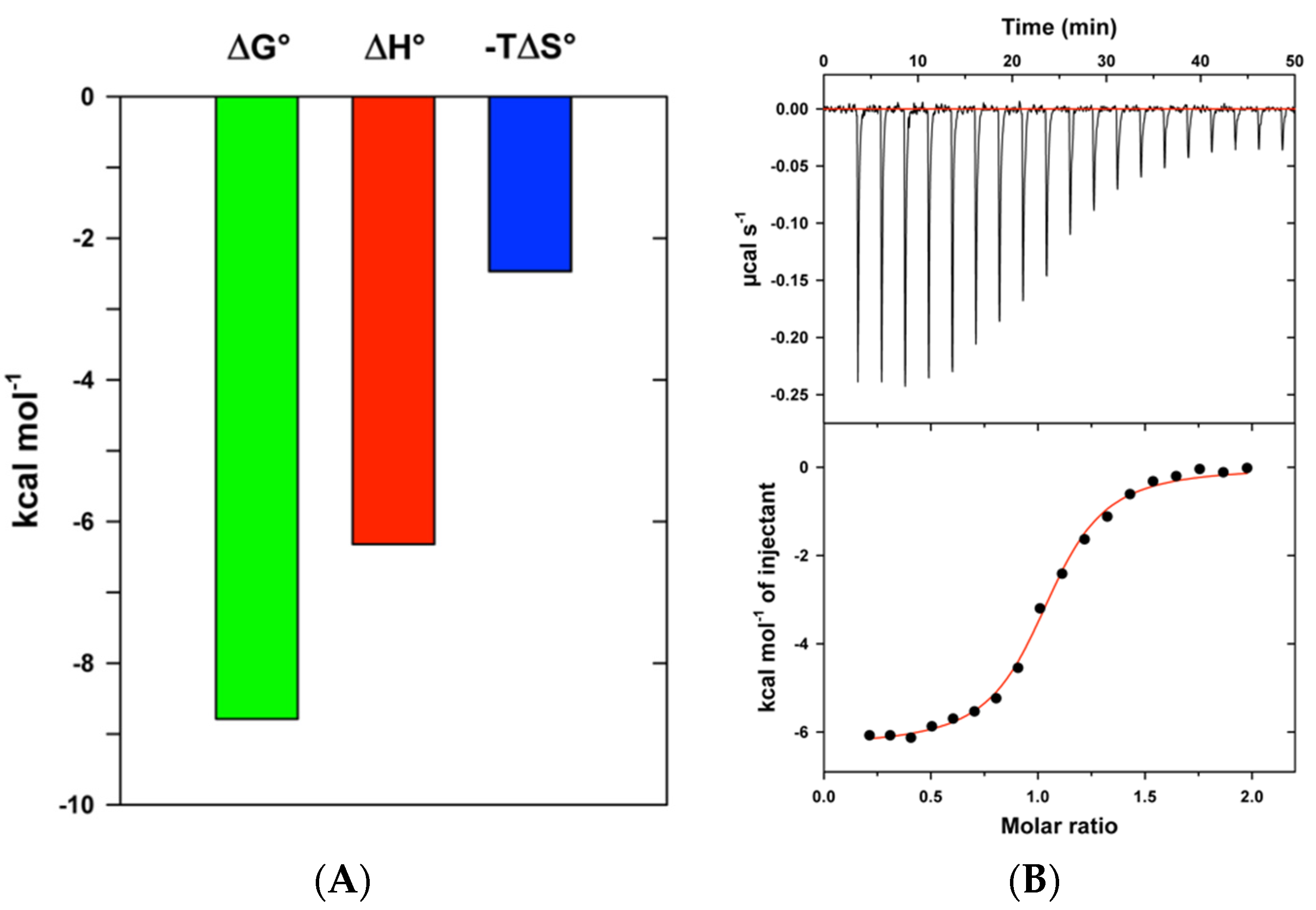
3.4. Isothermal Titration Calorimetry
3.5. Modeling Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928–2949. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. The antibiotic future. In Antibacterials; Fischer, J.F., Mobashery, S., Miller, M.J., Eds.; Springer: Cham, Germany, 2017; Volume 25, pp. 31–67. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef]
- Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally. Available online: https://amr-review.org/ (accessed on 29 November 2019).
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, 1–19. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis. Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Overcoming resistance to β-lactam antibiotics. J. Org. Chem. 2013, 78, 4207–4213. [Google Scholar] [CrossRef]
- Bonomo, R.A. β-Lactamases: A focus on current challenges. Cold Spring Harb. Perspect. Med. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Ju, L.-C.; Cheng, Z.; Fast, W.; Bonomo, R.A.; Crowder, M.W. The continuing challenge of metallo-β-lactamase inhibition: Mechanism matters. Trends Pharmacol. Sci. 2018, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-Metallo-β-lactamases: Where do we stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Cendron, L.; Gianquinto, E.; Spyrakis, F.; Tondi, D. Ten years with New Delhi Metallo-β-Lactamase-1 (NDM-1): From structural insights to inhibitor design. ACS Infect. Dis. 2019, 5, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Makena, A.; Düzgün, A.Ö.; Brem, J.; McDonough, M.A.; Rydzik, A.M.; Abboud, M.I.; Saral, A.; Çiçek, A.Ç.; Sandalli, C.; Schofield, C.J. Comparison of Verona Integron-borne Metallo-β-lactamase (VIM) variants reveals differences in stability and inhibition profiles. Antimicrob. Agents Chemother. 2016, 60, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- McGeary, R.P.; Dan, D.T.C.; Schenk, G. Progress toward inhibitors of metallo-β-lactamases. Future Med. Chem. 2017, 9, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Liénard, B.M.; Garau, G.; Horsfall, L.; Karsisiotis, A.I.; Damblon, C.; Lassaux, P.; Papamicael, C.; Roberts, G.C.; Galleni, M.; Dideberg, O.; et al. Structural basis for the broad-spectrum inhibition of metallo-β-lactamases by thiols. Org. Biomol. Chem. 2008, 6, 2282–2294. [Google Scholar] [CrossRef]
- Lassaux, P.; Hamel, M.; Gulea, M.; Delbrück, H.; Mercuri, P.S.; Horsfall, L.; Dehareng, D.; Kupper, M.; Frère, J.-M.; Hoffmann, K.; et al. Mercaptophosphonate compounds as broad-spectrum inhibitors of the metallo-β-lactamases. J. Med. Chem. 2010, 53, 4862–4876. [Google Scholar] [CrossRef]
- Toney, J.H.; Hammond, G.G.; Fitzgerald, P.M.; Sharma, N.; Balkovec, J.M.; Rouen, G.P.; Olson, S.H.; Hammond, M.L.; Greenlee, M.L.; Gao, Y.D. Succinic acids as potent inhibitors of plasmid-borne IMP-1 metallo-β-lactamase. J. Biol. Chem. 2001, 276, 31913–31918. [Google Scholar] [CrossRef]
- Chen, A.Y.; Thomas, P.W.; Stewart, A.C.; Bergstrom, A.; Cheng, Z.; Miller, C.; Bethel, C.R.; Marshall, S.H.; Credille, C.V.; Riley, C.L.; et al. Dipicolinic acid derivatives as inhibitors of New Delhi Metallo-β-lactamase-1. J. Med. Chem. 2017, 60, 7267–7283. [Google Scholar] [CrossRef]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Matsuura, A.; Okumura, H.; Asakura, R.; Ashizawa, N.; Takahashi, M.; Kobayashi, F.; Ashikawa, N.; Arai, K. Pharmacological profiles of aspergillomarasmines as endothelin converting enzyme inhibitors. JPN J. Pharmacol. 1993, 63, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Daigle, D.; Liu, B.; McGarry, D.; Pevear, D.C.; Trout, R.E. β-Lactamase Inhibitors. WO Patent WO2014/089365 A1, 12 June 2014. [Google Scholar]
- Hamrick, J.C.; Docquier, J.-D.; Uehara, T.; Myers, C.L.; Six, D.A.; Chatwin, C.L.; John, K.J.; Vernacchio, S.F.; Cusick, S.M.; Trout, R.E.L.; et al. VNRX-5133 (Taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamase, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Trout, R.E.L.; Chu, G.H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of Taniborbactam (VNRX-5133): A broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.; Brem, J.; Hinchliffe, P.; Calvopiña, K.; Panduwawala, T.D.; Lang, P.A.; Kamps, J.J.A.; Tyrrell, J.M.; Widlake, E.; Saward, B.G.; et al. Bicyclic boronate VNRX-5133 inhibits metallo- and serine β-lactamases. J. Med. Chem. 2019, 62, 8544–8556. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.; Sprynski, N.; Coelho, A.; Castandet, J.; Bayet, M.; Bougnon, J.; Lozano, C.; Davies, D.T.; Leiris, S.; Zalacain, M.; et al. Discovery of a novel metallo-β-lactamase inhibitor that potentiates meropenem activity against carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, 7–18. [Google Scholar] [CrossRef]
- Leiris, S.; Coelho, A.; Castandet, J.; Bayet, M.; Lozano, C.; Bougnon, J.; Bousquet, J.; Everett, M.; Lemonnier, M.; Sprynski, N.; et al. SAR studies leading to the identification of a novel series of metallo-β-lactamase inhibitors for the treatment of carbapenem-resistant Enterobacteriaceae infections that display efficacy in an animal infection model. ACS Infect. Dis. 2019, 5, 131–140. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of cyclic boronic acid QPX7728, an ultra-broad-spectrum inhibitor of serine and metallo-β-lactamases. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Docquier, J.-D.; Mangani, S. An update on β-lactamase inhibitor discovery and development. Drug Resist. Update 2018, 36, 13–29. [Google Scholar] [CrossRef]
- González-Bello, C.; Rodríguez, D.; Pernas, M.; Rodríguez, Á.; Colchón, E. β-Lactamase inhibitors to restore the efficacy of antibiotics against superbugs. J. Med. Chem. 2020, 63, 1859–1881. [Google Scholar] [CrossRef]
- Olsen, L.; Jost, S.; Adolph, H.W.; Pettersson, I.; Hemmingsen, L.; Jørgensen, F.S. New leads of metallo-β-lactamase inhibitors from structure-based pharmacophore design. Bioorg. Med. Chem. 2006, 14, 2627–2635. [Google Scholar] [CrossRef]
- Nauton, L.; Kahn, R.; Garau, G.; Hernandez, J.-F.; Dideberg, O. Structural insights into the design of inhibitors for the L1 metallo-β-lactamase from Stenotrophomonas maltophilia. J. Mol. Biol. 2008, 375, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Sevaille, L.; Gavara, L.; Bebrone, C.; De Luca, F.; Nauton, L.; Achard, M.; Mercuri, P.; Tanfoni, S.; Borgianni, L.; Guyon, C.; et al. 1,2,4-Triazole-3-thione compounds as inhibitors of dizinc metallo-β-lactamases. Chem. Med. Chem. 2017, 12, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Kwapien, K.; Damergi, M.; Nader, S.; El Khoury, L.; Hobaika, Z.; Maroun, R.G.; Piquemal, J.-P.; Gavara, L.; Berthomieu, D.; Hernandez, J.-F.; et al. Calibration of 1,2,4-triazole-3-thione, an original Zn-binding group of metallo-β-lactamase inhibitors. Validation of a polarizable MM/MD potential by quantum chemistry. J. Phys. Chem. B 2017, 121, 6295–6312. [Google Scholar] [CrossRef]
- Gavara, L.; Sevaille, L.; De Luca, F.; Mercuri, P.; Bebrone, C.; Feller, G.; Legru, A.; Cerboni, G.; Tanfoni, S.; Baud, D.; et al. 4-Amino-1,2,4-triazole-3-thione-derived Schiff bases as metallo-β-lactamase inhibitors. Eur. J. Med. Chem. under review.
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Laraki, N.; Franceschini, N.; Rossolini, G.M.; Santucci, P.; Meunier, C.; de Pauw, E.; Amicosante, G.; Frère, J.-M.; Galleni, M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 1999, 43, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Docquier, J.-D.; Pantanella, F.; Giuliani, F.; Thaller, M.C.; Amicosante, G.; Galleni, M.; Frère, J.-M.; Bush, K.; Rossolini, G.M. CAU-1, a subclass B3 metallo-β-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 2002, 46, 1823–1830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Docquier, J.-D.; Lamotte-Brasseur, J.; Galleni, M.; Amicosante, G.; Frère, J.-M.; Rossolini, G.M. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 2003, 51, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Villadares, M.; Galleni, M.; Frère, J.-M.; Felici, A.; Perilli, M.; Franceschini, N.; Rossolini, G.M.; Oratore, A.; Amicosante, G. Overproduction and purification of the Aeromonas hydrophila CphA metallo-β-lactamse expressed in Escherichia coli. Microb. Drug Resist. 1996, 2, 253–256. [Google Scholar] [CrossRef]
- Bebrone, C.; Anne, C.; De Vriendt, K.; Devreese, B.; Rossolini, G.M.; Van Beeumen, J.; Frère, J.-M.; Galleni, M. Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-β-lactamase by site-directed mutagenesis. J. Biol. Chem. 2005, 280, 28195–28202. [Google Scholar] [CrossRef]
- Wikler, M.A.; Cockerill, F.R.; Craig, W.A.; Dudley, M.N.; Eliopoulos, G.M.; Hecht, D.W.; Hindler, J.F.; Ferraro, M.J.; Swenson, J.M.; Low, D.E.; et al. M02-A12: Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 20th ed.; CLSI: Wayne, PA, USA, 2015; Volume M0–A12. [Google Scholar]
- Borgianni, L.; Vandenameele, J.; Matagne, A.; Bini, L.; Bonomo, R.; Frère, J.-M.; Rossolini, G.M.; Docquier, J.-D. Mutational analysis of VIM-2 reveals an essential determinant for metallo-β-lactamase stability and folding. Antimicrob. Agents Chemother. 2010, 54, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Clayton, S.R.; Zechiedrich, E.L. Relative contributions of the AcrAB, MdfA and NorE efflux pumps to quinolone resistance in Escherichia coli. J. Antimicrob. Chemother. 2003, 51, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Mod. 1999, 17, 57–61. [Google Scholar]
- Brindisi, M.; Brogi, S.; Giovani, S.; Gemma, S.; Lamponi, S.; Luca, F.D.; Novellino, E.; Campiani, G.; Docquier, J.-D.; Butini, S. Targeting clinically-relevant metallo-β-lactamases: From high-throughput docking to broad-spectrum inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lassaux, P.; Traoré, D.A.K.; Loisel, E.; Favier, A.; Docquier, J.-D.; Sohier, J.-S.; Laurent, C.; Bebrone, C.; Frère, J.-M.; Ferrer, J.-L.; et al. Biochemical and structural characterization of the subclass B1 metallo-β-lactamase VIM-4. Antimicrob. Agents Chemother. 2011, 55, 1248–1255. [Google Scholar] [CrossRef]
- Moali, C.; Anne, C.; Lamotte-Brasseur, J.; Groslambert, S.; Devreese, B.; Van Beeumen, J.; Galleni, M.; Frère, J.-M. Analysis of the importance of the metallo-β-lactamase active site loop in substrate binding and catalysis. Chem. Biol. 2003, 10, 319–329. [Google Scholar] [CrossRef]
- Freire, E. Do enthalpy and entropy distinguish first class from best in class? Drug Discov. Today 2008, 13, 869–874. [Google Scholar] [CrossRef]
- Ladbury, J.E. Calorimetry as a tool for understanding biomolecular interactions and an aid to drug design. Biochem. Soc. Trans. 2010, 38, 888–893. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Vella, P.; Hussein, W.M.; Leung, E.W.; Clayton, D.; Ollis, D.L.; Mitić, N.; Schenk, G.; McGeary, R.P. The identification of new metallo-β-lactamase inhibitor leads from fragment-based screening. Bioorg. Med. Chem. Lett. 2011, 21, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, T.; Carlsen, T.J.; Helland, R.; Leiros, H.K. Discovery of novel inhibitor scaffolds against the metallo-β-lactamase VIM-2 by surface plasmon resonance (SPR) based fragment screening. J. Med. Chem. 2015, 58, 8671–8682. [Google Scholar] [CrossRef] [PubMed]
- Spyrakis, F.; Celenza, G.; Marcoccia, F.; Santucci, M.; Cross, S.; Bellio, P.; Cendron, L.; Perilli, M.; Tondi, D. Structure-based virtual screening for the discovery of novel inhibitors of New Delhi Metallo-β-lactamase-1. ACS Med. Chem. Lett. 2018, 9, 45–50. [Google Scholar] [CrossRef]
- Faridoon; Hussein, W.M.; Vella, P.; Ul Islam, N.; Ollis, D.L.; Schenk, G.; McGeary, R.P. 3-Mercapto-1,2,4-triazoles and N-acylated thiosemicarbazides as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 383–386. [Google Scholar] [CrossRef]
- Feng, L.; Yang, K.-W.; Zhou, L.-S.; Xiao, J.-M.; Yang, X.; Zhai, L.; Zhang, Y.-L.; Crowder, M.W. N-Heterocyclic dicarboxylic acids: Broad-spectrum inhibitors of metallo-β-lactamases with co-antibacterial effect against antibiotic-resistant bacteria. Bioorg. Med. Chem. Lett. 2012, 22, 5185–5189. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Gianquinto, E.; Montanari, M.; Maso, L.; Bellio, P.; Cebrian-Sastre, E.; Celenza, G.; Blazquez, J.; Cendron, L.; Spyrakis, F.; et al. 4-Amino-1,2,4-triazole-3-thione as a promising scaffold for the inhibition of serine and metallo-β-lactamases. Pharmaceuticals 2020, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Yang, K.-W.; Zhou, Y.-J.; LaCuran, A.E.; Oelschlaeger, P.; Crowder, M.W. Diaryl-substituted azolylthioacetamides: Inhibitor discovery of New Delhi Metallo-β-lactamase-1 (NDM-1). Chem. Med. Chem. 2014, 9, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, T.; Yang, K.-W.; Yang, S.-K.; Leiros, H.K. The structure of the metallo-β-lactamase VIM-2 in complex with a triazolylthioacetamide inhibitor. Acta Cryst. 2016, 72, 813–819. [Google Scholar] [CrossRef]
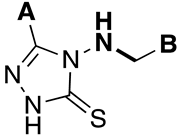 | Ki Value (μM) | ||||
|---|---|---|---|---|---|
| Compound | A | B | IMP-1 | VIM-2 | NDM-1 |
| 1 | 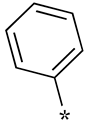 |  | -2 (-) | 1.40 ± 0.02 (2.7) | - (38) |
| 2 | 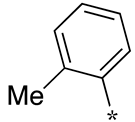 | 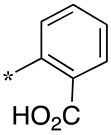 | - (58) | 8.0 ± 0.7 (3.0) | - (-) |
| 3 |  |  | 76 ± 13 (18) | 0.24 ± 0.02 (0.20) | 8.2 ± 0.7 (4.2) |
| 4 | 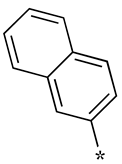 | 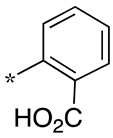 | - (6.9) | 4.2 ± 0.9 (0.7) | - (2.3) |
| 5 | 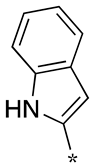 | 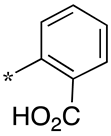 | - (55) | - (-) | - (9.4) |
| 6 | 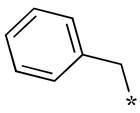 | 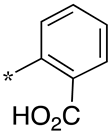 | - (28) | 8.5 ± 2.3 (3.2) | - (42) |
| 7 | 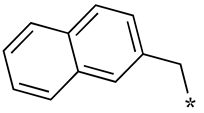 | 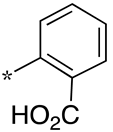 | - (34) | 1.8 ± 0.2 (2.4) | - (1.6) |
| 8 | 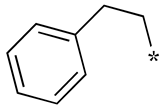 | 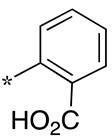 | - (-) | - (7.0) | - (-) |
| 9 | 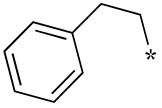 | 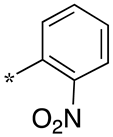 | - (-) | - (-) | - (-) |
| 10 | 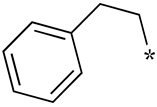 |  | - (11) | - (-) | - (-) |
| 11 |  | 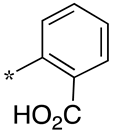 | - (-) | - (25) | 16.4 ± 2.6 (2.7) |
| 12 | 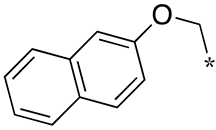 |  | - (-) | - (2.0) | - (2.4) |
 | Percentage of Inhibition at 100 μM Inhibitor 1 | ||||
|---|---|---|---|---|---|
| Compound | A | B | IMP-1 | VIM-2 | NDM-1 |
| 17 | 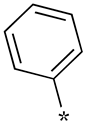 |  | -2 | - | - |
| 18 | 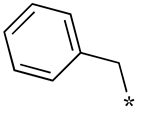 | 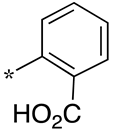 | - | - | - |
| 19 | 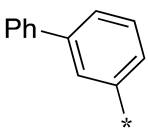 |  | 40 | 36 | 42 |
| 20 | 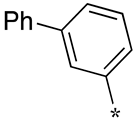 | 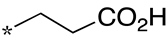 | - | - | - |
| Compounds | Ki Values (μM) or Percentage of Inhibition at 100 μM Inhibitor | |||
|---|---|---|---|---|
| VIM-1 | VIM-4 | CphA | L1 | |
| 1 | -1 | - | 60% | - |
| 2 | - | 10 ± 1 | 100 ± 10 | 110 ± 10 |
| 3 | 3.6 ± 0.2 | 0.57 ± 0.03 | nd | nd |
| 4 | 32% | 6.3 ± 0.7 | nd | - |
| 6 | - | - | nd | - |
| 7 | 19.5 ± 1.3 | 4.7 ± 0.3 | nd | nd |
| 19 | - | 41% | nd | nd |
| Compound Added to Cefoxitin Disk 2 | Ki (μM) on VIM-2 | Inhibition Zone Diameter (mm) 3 |
|---|---|---|
| none | - | 10 |
| EDTA (220 μg) | - | 28 |
| DMSO (100%) | - | 10 |
| 1 (16 μg) | 1.4 | 13 |
| 1 (32 μg) | 1.4 | 16 |
| 1 (48 μg) | 1.4 | 20 |
| 2 (40 μg) | 8.0 | 11 |
| 3 (120 μg) | 0.24 | 11 |
| 4 (120 μg) | 4.2 | 10 |
| 6 (40 μg) | 8.5 | 10 |
| 7 (120 μg) | 1.8 | 11 |
| Compound | n 1 | Ka (μM−1) | Kd (nM) | ∆G°b (kcal.mol−1) | ∆H°b (kcal.mol−1) | T∆S°b (kcal.mol−1) |
|---|---|---|---|---|---|---|
| 1 | 1.01 ± 0.01 | 2.8 ± 0.4 | 358 | −8.8 | −6.3 ± 0.1 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavara, L.; Verdirosa, F.; Legru, A.; Mercuri, P.S.; Nauton, L.; Sevaille, L.; Feller, G.; Berthomieu, D.; Sannio, F.; Marcoccia, F.; et al. 4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition. Biomolecules 2020, 10, 1094. https://doi.org/10.3390/biom10081094
Gavara L, Verdirosa F, Legru A, Mercuri PS, Nauton L, Sevaille L, Feller G, Berthomieu D, Sannio F, Marcoccia F, et al. 4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition. Biomolecules. 2020; 10(8):1094. https://doi.org/10.3390/biom10081094
Chicago/Turabian StyleGavara, Laurent, Federica Verdirosa, Alice Legru, Paola Sandra Mercuri, Lionel Nauton, Laurent Sevaille, Georges Feller, Dorothée Berthomieu, Filomena Sannio, Francesca Marcoccia, and et al. 2020. "4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition" Biomolecules 10, no. 8: 1094. https://doi.org/10.3390/biom10081094
APA StyleGavara, L., Verdirosa, F., Legru, A., Mercuri, P. S., Nauton, L., Sevaille, L., Feller, G., Berthomieu, D., Sannio, F., Marcoccia, F., Tanfoni, S., De Luca, F., Gresh, N., Galleni, M., Docquier, J.-D., & Hernandez, J.-F. (2020). 4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition. Biomolecules, 10(8), 1094. https://doi.org/10.3390/biom10081094






