Hapten Design and Monoclonal Antibody to Fluoroacetamide, a Small and Highly Toxic Chemical
Abstract
1. Introduction
2. Materials and Methods
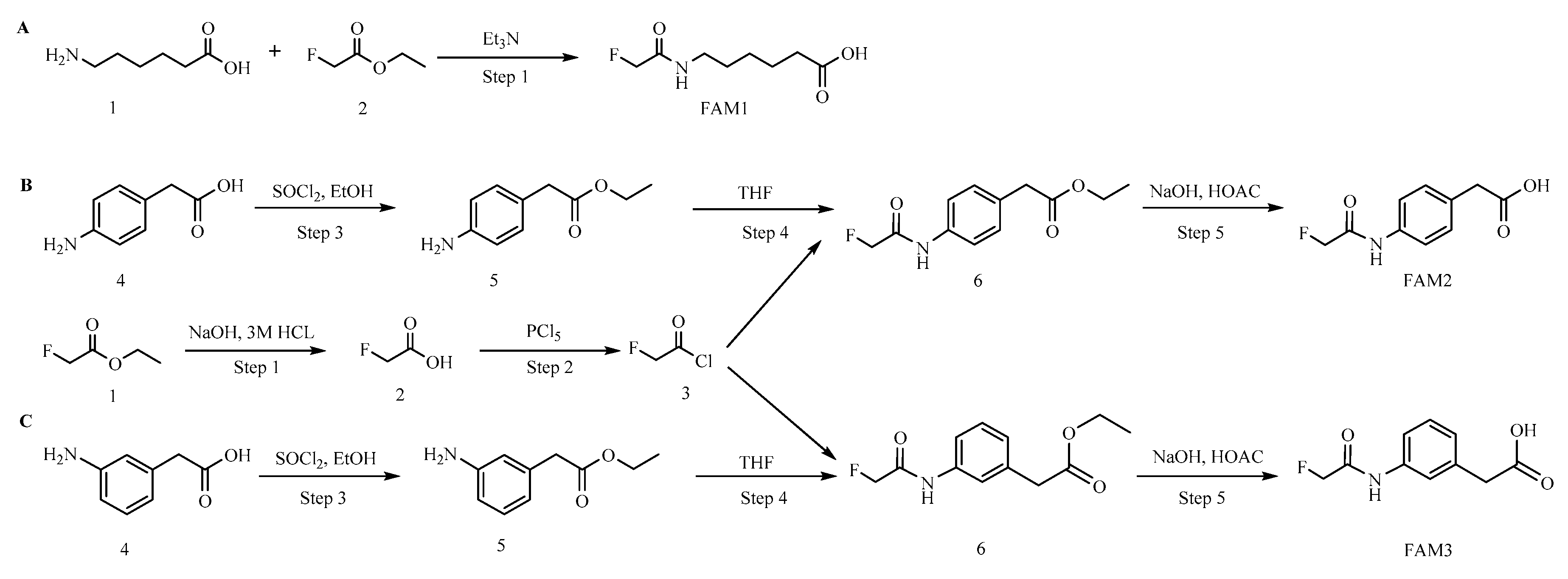
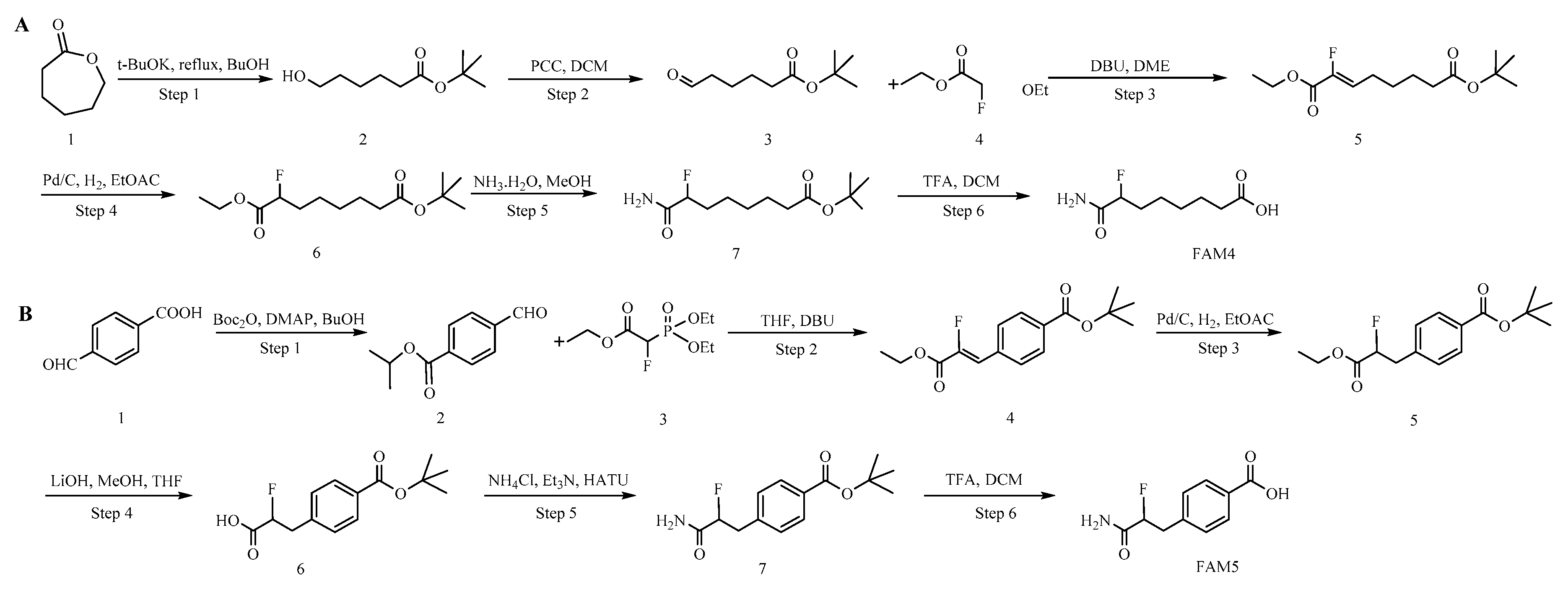
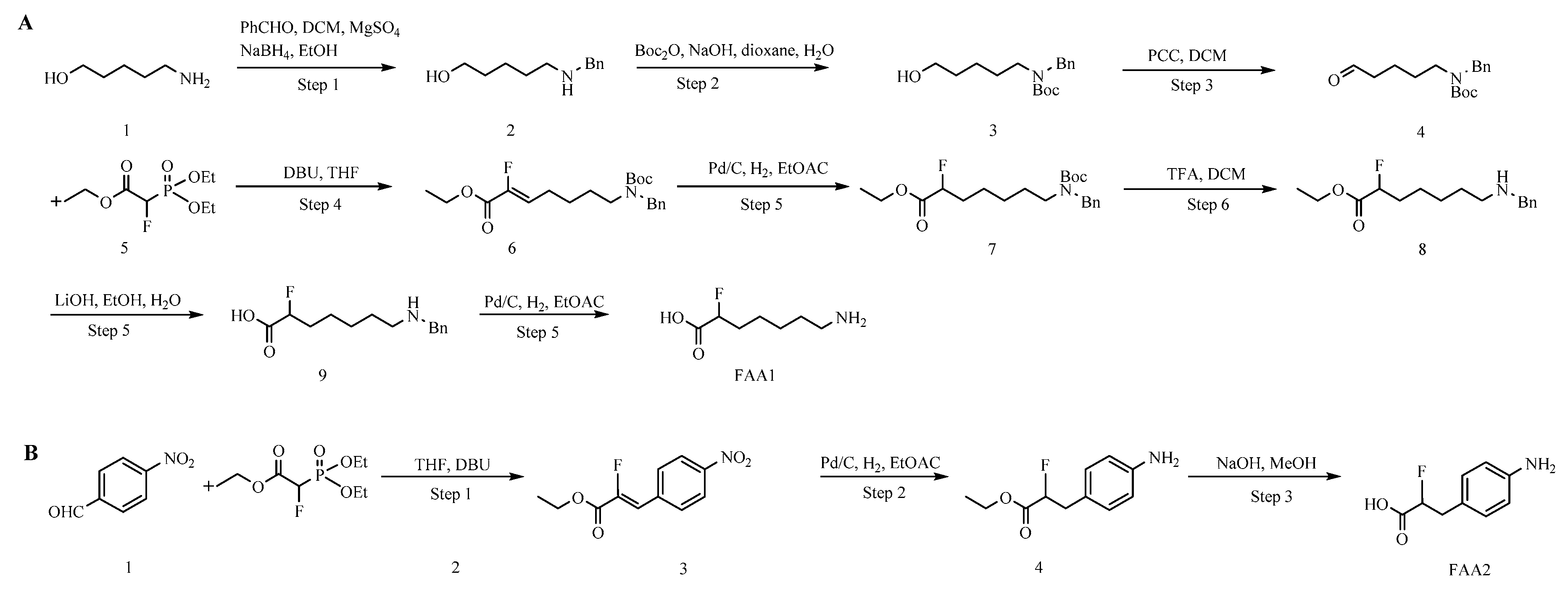
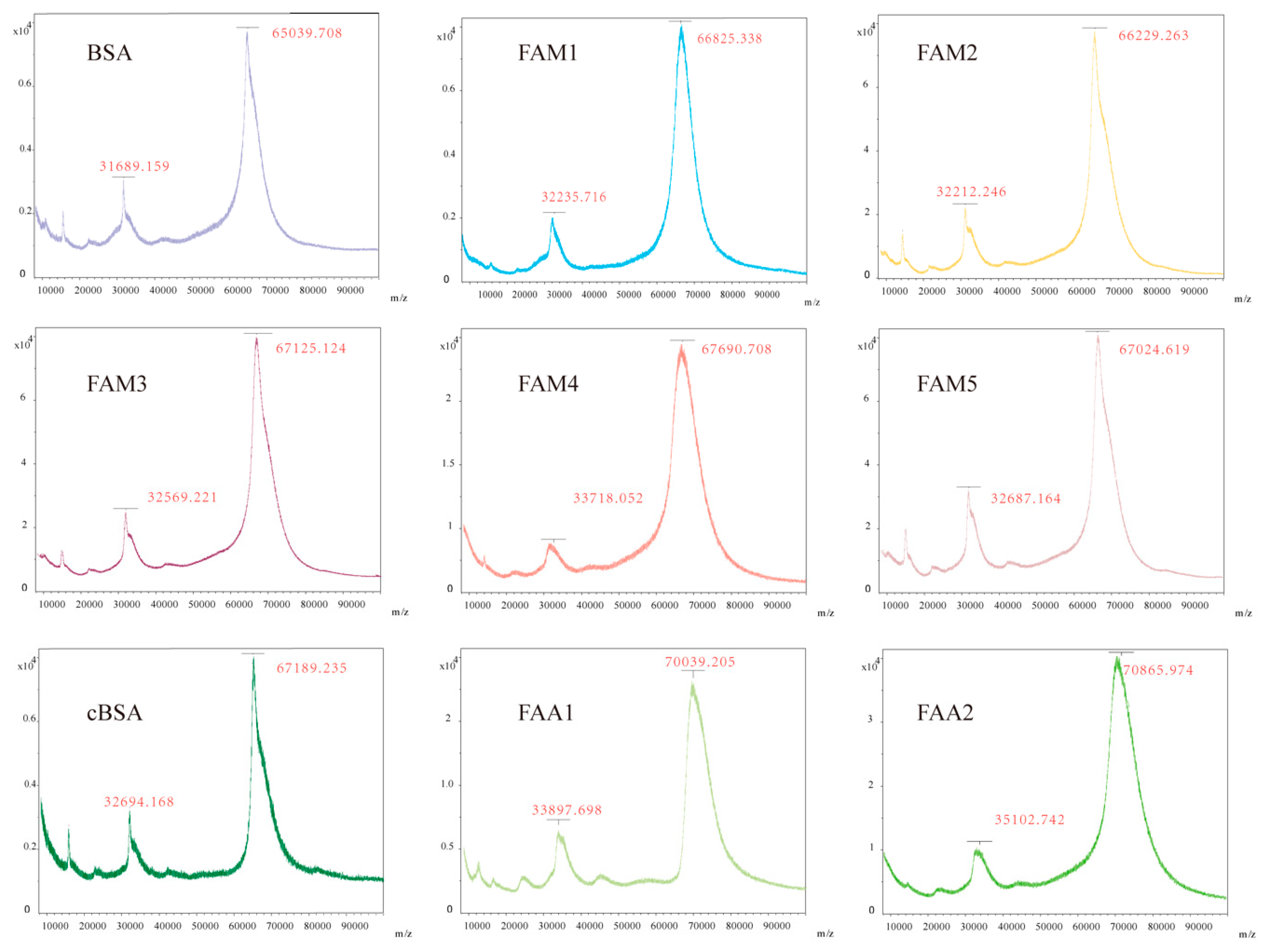
2.1. Hapten Synthesis and Conjugate Preparation
2.2. ELISA Method Development
2.3. Mice Immunization and Antisera Analysis
2.4. mAb Production
2.5. Cross-Reactivity Determination
3. Results
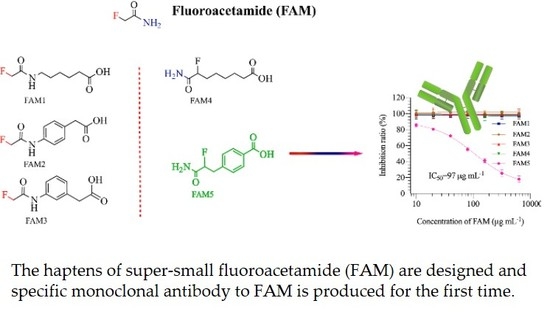
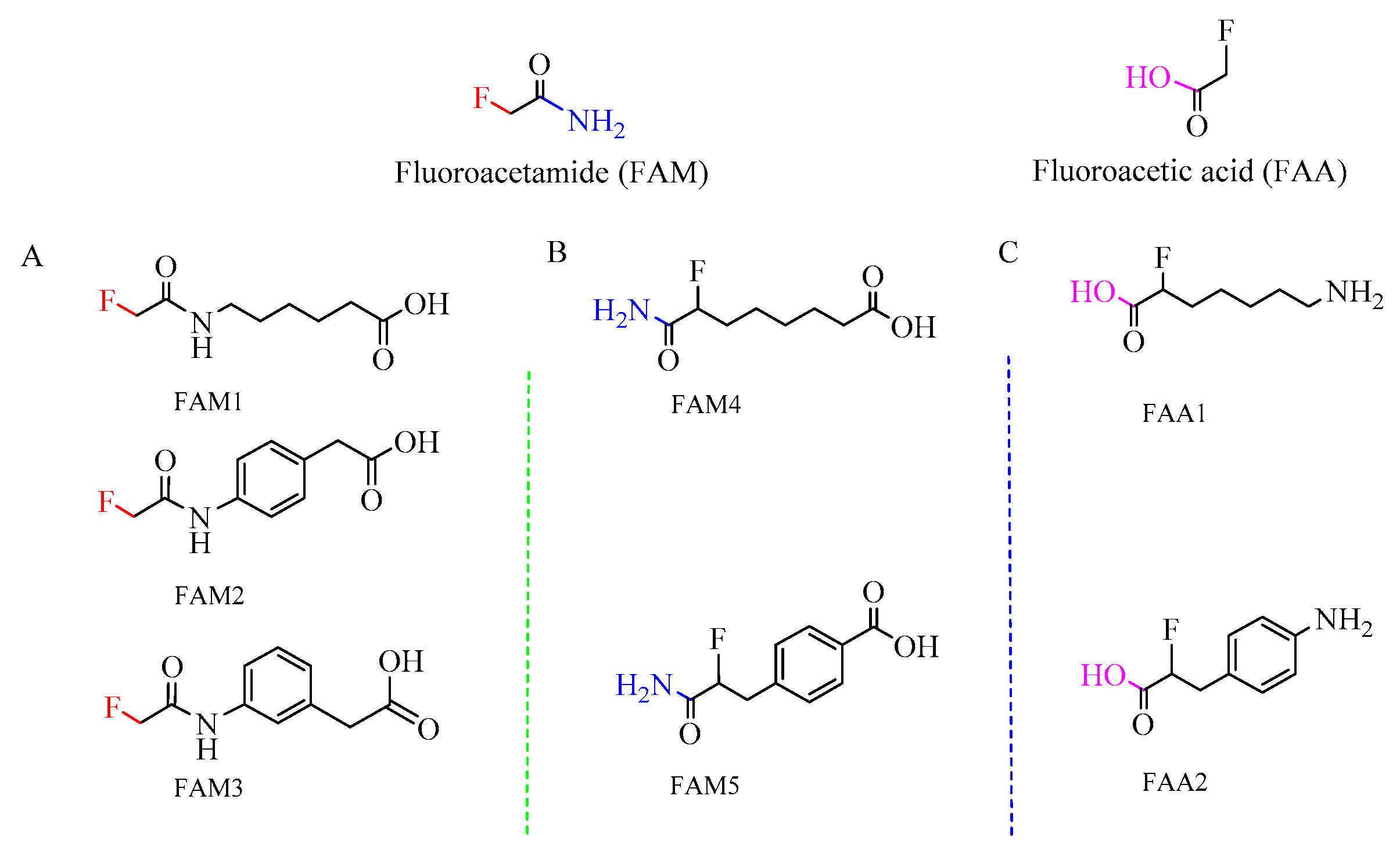
3.1. Hapten Design and Conjugate Preparation
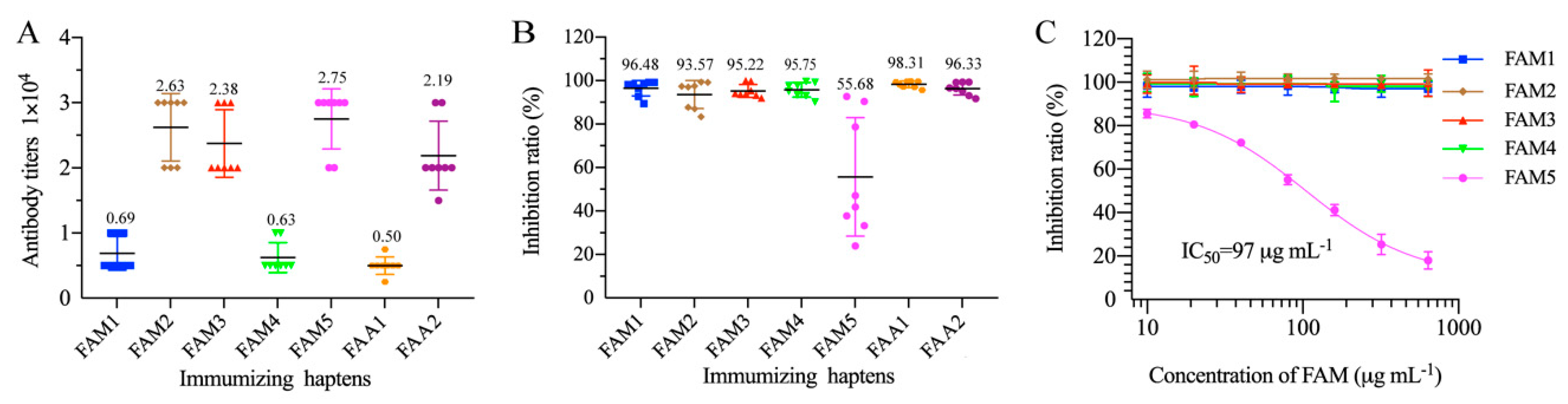
3.2. Mice Immunization and Antisera Analysis
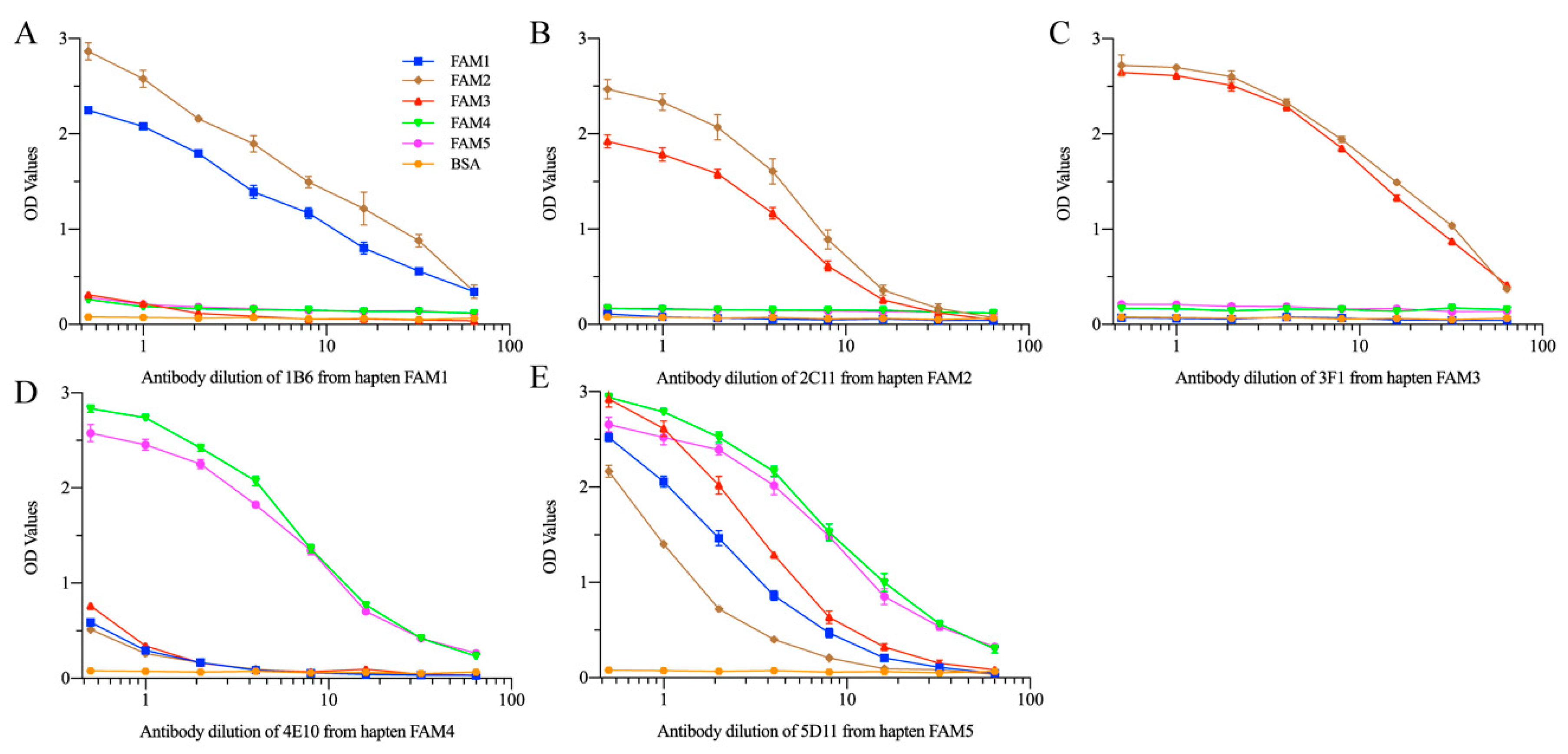
3.3. mAb Production and Characterization
3.4. mAb5D11 Characterization by ic-ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, W.A.L.; Gardiner, B.O. Persistence of fluoroacetate and fluoroacetamide in soil. Nature 1966, 209, 1367–1368. [Google Scholar] [CrossRef]
- Wang, R.S.; Zhuo, L.; Wang, Y.Y.; Ren, L.; Liu, Q.; Liu, L. Lessons learned from poisoning cases caused by 2 illegal rodenticides: Tetramine and fluoroacetamide. Medicine 2016, 95, 4–8. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.L.; Deng, H.Y.; Zhong, F.; Li, Y.J. Evaluation of charcoal hemoperfusion in dogs with acute fluoroacetamide poisoning. Chin. J. Pediatr. 2007, 45, 661–664. [Google Scholar]
- Eason, C.; Miller, A.; Ogilvie, S.; Fairweather, A. An updated review of the toxicology and ecotoxicology of sodium fluoroacetate (1080) in relation to its use as a pest control tool in New Zealand. N. Zeal. J. Ecol. 2011, 35, 1–20. [Google Scholar]
- Goncharov, N.; Savelieva, E.; Zinchenko, V.; Kuznetsov, S.; Mindukshev, I.; Vinokurov, M.; Avdonin, P.; Voitenko, N.; Ukolov, A.; Orlova, T.; et al. Fluoroacetate; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 2015; pp. 193–214. [Google Scholar] [CrossRef]
- Tecle, B.; Casida, J.E. Enzymatic defluorination and metabolism of fluoroacetate, fluoroacetamide, fluoroethanol, and (-)-erythro-fluorocitrate in rats and mice examined by F-19 and C-13 NMR. Chem. Res. Toxicol. 1989, 2, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.Y. Development change of fluoroacetamide and urine fluoride of acute fluoroacetamide poisoning. J. Medical Forum 2010, 7, 44–45. [Google Scholar]
- Cai, X.L.; Zhang, D.M.; Ju, H.X.; Wu, G.P.; Liu, X.P. Fast detection of fluoroacetamide in body fluid using gas chromatography-mass spectrometry after solid-phase microextraction. J. Chromatogr. B 2004, 802, 239–245. [Google Scholar] [CrossRef]
- Cooney, T.P.; Varelis, P.; Bendall, J.G. High-throughput quantification of monofluoroacetate (1080) in milk as a response to an extortion threat. J. Food Prot. 2016, 79, 273–281. [Google Scholar] [CrossRef]
- Wen, W.X.; Gao, H.X.; Kang, N.N.; Lu, A.L.; Qian, C.W.; Zhao, Y.Q. Treatment of severe fluoroacetamide poisoning in patient with combined multiple organ dysfunction syndrome by evidence-based integrated Chinese and Western medicines A case report. Medicine 2017, 96, 3–6. [Google Scholar] [CrossRef]
- Feng, S.Z.; Yu, Z.S. Analysis of fluoroacetamide in biological samples with GC/NPD, GC/FID. Fa Yi Xue Za Zhi 1999, 15, 91–92. [Google Scholar]
- Xu, X.M.; Song, G.L.; Zhu, Y.; Zhang, J.; Zhao, Y.X.; Shen, H.T.; Cai, Z.X.; Han, J.L.; Ren, Y.P. Simultaneous determination of two acute poisoning rodenticides tetramine and fluoroacetamide with a coupled column in poisoning cases. J. Chromatogr. B. 2008, 876, 103–108. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Yu, J. Simultaneous determination of five hypertoxic rodenticides in serum by gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2015, 33, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Q.; Lu, X.W.; Hu, X.L.; Zhang, Y.R.; Zeng, L.B.; Chen, L.K.; Sun, M.Q. In vitro selection of DNA aptamers binding pesticide fluoroacetamide. Biosci. Biotechnol. Biochem. 2016, 80, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Liu, X.; Zhang, D.; Ju, H. Study on chip determining of F-of the metabolizability of fluoroacetamide. Chin. J. Forensic Med. 2010, 25, 175–177. [Google Scholar]
- Hauptman, P.J.; Blume, S.W.; Lewis, E.F.; Ward, S. Digoxin toxicity and use of digoxin immune fab insights from a national hospital database. JACC-Heart Fail. 2016, 4, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Takaishi, S.; Yasumoto, K.; Watabe, S. Novel Polyclonal Antibody Raised against Tetrodotoxin Using Its Haptenic Antigen Prepared from 4,9-anhydrotetrodotoxin Reacted with 1,2-Ethaneditiol and Further Reacted with Keyhole Limpet Hemocyanin. Toxins 2019, 11, 551. [Google Scholar] [CrossRef]
- Gao, A.Z.; Chen, Q.L.; Cheng, Y.; Lei, J.; Zeng, L.W. Preparation of monoclonal antibodies against a derivative of semicarbazide as a metabolic target of nitrofurazone. Anal. Chim. Acta 2007, 592, 58–63. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Wang, D.; Zhao, M.P. Antigen synthetic strategy and immunoassay development for detection of acrylamide in foods. Analyst 2008, 133, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wei, X.Q.; Jia, B.Z.; Yang, J.Y.; Shen, Y.D.; Hammock, B.; Dong, J.X.; Wang, H.; Lei, H.T.; Xu, Z.L. Modulating linker composition of haptens resulted in improved immunoassay for histamine. Biomolecules 2019, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Liu, M.X.; Shi, W.M.; Li, C.L.; Zhang, S.X.; Shen, J.Z. New haptens and antibodies for ractopamine. Food Chem. 2015, 183, 111–114. [Google Scholar] [CrossRef]
- Jalah, R.; Torres, O.B.; Mayorov, A.V.; Li, F.Y.; Antoline, J.F.G.; Jacobson, A.E.; Rice, K.C.; Deschamps, J.R.; Beck, Z.; Alving, C.R.; et al. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on crm197 carriers. Bioconjug. Chem. 2015, 26, 1041–1053. [Google Scholar] [CrossRef]
- Carroll, F.I.; Blough, B.E.; Pidaparthi, R.R.; Abraham, P.; Gong, P.K.; Deng, L.; Huang, X.D.; Gunnell, M.; Lay, J.O.; Peterson, E.C.; et al. Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J. Med. Chem. 2011, 54, 5221–5228. [Google Scholar] [CrossRef]
- Pieper, K.; Tan, J.; Piccoli, L.; Foglierini, M.; Barbieri, S.; Chen, Y.; Silacci-Fregni, C.; Wolf, T.; Jarrossay, D.; Anderle, M.; et al. Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature 2017, 548, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Iverson, B.L. The influence of hapten size and hydrophobicity on the catalytic activity of elicited polyclonal antibodies. J. Am. Chem. Soc. 1996, 118, 251–252. [Google Scholar] [CrossRef]
- Moreno, A.Y.; Mayorov, A.V.; Janda, K.D. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [Google Scholar] [CrossRef]
- Ceballos-Alcantarilla, E.; Abad-Fuentes, A.; Aloisio, V.; Agullo, C.; Abad-Somovilla, A.; Mercader, J.V. Haptens, bioconjugates, and antibodies for penthiopyrad immunosensing. Analyst 2014, 139, 5358–5361. [Google Scholar] [CrossRef]
- Reindl, M.; Hoffmann-Roder, A. Antibody recognition of fluorinated haptens and antigens. Curr. Top. Med. Chem. 2014, 14, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Han, J.L.; Wang, J.D.; Shibata, N.; Sodeoka, M.; Soloshonok, V.A.; Coelho, J.A.S.; Toste, F.D. Modern approaches for asymmetric construction of carbon-fluorine quaternary stereogenic centers: Synthetic challenges and pharmaceutical needs. Chem. Rev. 2018, 118, 3887–3964. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Q.; Tsuchikama, K.; Janda, K.D. Modulating cocaine vaccine potency through hapten fluorination. J. Am. Chem. Soc. 2013, 135, 2971–2974. [Google Scholar] [CrossRef]
- Pryde, D.C.; Jones, L.H.; Gervais, D.P.; Stead, D.R.; Blakemore, D.C.; Selby, M.D.; Brown, A.D.; Coe, J.W.; Badland, M.; Beal, D.M.; et al. Selection of a novel anti-nicotine vaccine: Influence of antigen design on antibody function in mice. PLoS ONE 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Huang, J.X.; Yao, C.Y.; Yang, J.Y.; Li, Z.F.; He, F.; Tian, Y.X.; Wang, H.; Xu, Z.L.; Shen, Y.D. Design of Novel Haptens and Development of Monoclonal Antibody-Based Immunoassays for the Simultaneous Detection of Tylosin and Tilmicosin in Milk and Water Samples. Biomolecules 2019, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, Y.D.; Lei, H.T.; Sun, Y.M.; Yang, J.Y.; Xiao, Z.L.; Wang, H.; Xu, Z.L. Hapten synthesis and development of a competitive indirect enzyme-linked immunosorbent assay for acrylamide in food samples. J. Agric. Food. Chem. 2014, 62, 7078–7084. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage–associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
| Compounds | S/F 1 | IC50 (μg/mL) | CR | Structure |
|---|---|---|---|---|
| FAM | S&F | 100 | 100% | 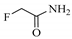 |
| Bromoacetic acid | F | _ | _ |  |
| Chloroacetamide | F | _ | _ | 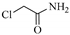 |
| Chloroacetic acid | F | _ | _ | 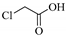 |
| Difluoroacetic Acid | F | _ | _ |  |
| FAA | S&F | _ | _ | 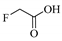 |
| Thiosemicarbazide | F | _ | _ |  |
| 1-Chloro-3-fluoroisopropanol | F | _ | _ |  |
| 1,3-Difluoro-2-propanol | F | _ | _ | 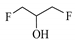 |
| 2,2,2-Trifluoroacetamide | F | _ | _ |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, X.; Shen, D.; Yu, X.; Li, Y.; Wen, K.; Shen, J.; Wang, Z. Hapten Design and Monoclonal Antibody to Fluoroacetamide, a Small and Highly Toxic Chemical. Biomolecules 2020, 10, 986. https://doi.org/10.3390/biom10070986
Yang L, Zhang X, Shen D, Yu X, Li Y, Wen K, Shen J, Wang Z. Hapten Design and Monoclonal Antibody to Fluoroacetamide, a Small and Highly Toxic Chemical. Biomolecules. 2020; 10(7):986. https://doi.org/10.3390/biom10070986
Chicago/Turabian StyleYang, Ling, Xiya Zhang, Dongshuai Shen, Xuezhi Yu, Yuan Li, Kai Wen, Jianzhong Shen, and Zhanhui Wang. 2020. "Hapten Design and Monoclonal Antibody to Fluoroacetamide, a Small and Highly Toxic Chemical" Biomolecules 10, no. 7: 986. https://doi.org/10.3390/biom10070986
APA StyleYang, L., Zhang, X., Shen, D., Yu, X., Li, Y., Wen, K., Shen, J., & Wang, Z. (2020). Hapten Design and Monoclonal Antibody to Fluoroacetamide, a Small and Highly Toxic Chemical. Biomolecules, 10(7), 986. https://doi.org/10.3390/biom10070986