The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning—Effects on Heart Function and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Isolated Heart
2.2. Experimental Protocol
- Control group preconditioned with saline (n = 12)
- Control group postconditioned with saline (n = 12)
- Group preconditioned with glutamate in dose of 100 µmol/L (n = 12)
- Group postconditioned with glutamate in dose of 100 µmol/L (n = 12)
- Group preconditioned with (RS)-(Tetrazol-5-yl)glycine in dose of 5 µmol/L (n = 12)
- Group postconditioned with (RS)-(Tetrazol-5-yl)glycine in dose of 5 µmol/L (n = 12)
- Group preconditioned with MK-801 in dose of 30 µmol/L (n = 12)
- Group postconditioned with MK-801 in dose of 30 µmol/L (n = 12)
- Group preconditioned with memantine in dose of 100 µmol/L (n = 12)
- Group postconditioned with memantine in dose of 100 µmol/L (n = 12)
- The maximum rate of pressure development in the left ventricle (dp/dt max)
- The minimum rate of pressure development in the left ventricle (dp/dt mix)
- The systolic left ventricular pressure (SLVP)
- The diastolic left ventricular pressure (DLVP)
- The heart rate (HR)
2.3. Biochemical Assays
- The index of lipid peroxidation, measured as thiobarbituric acid reactive substances (TBARS)
- The level of the superoxide anion radical (O2−)
- The level of hydrogen peroxide (H2O2)
- The level of nitrite (NO2−)
2.3.1. TBARS Determination (Index of Lipid Peroxidation)
2.3.2. Determination of the Hydrogen Peroxide Level
2.3.3. Determination of the Nitrite Level
2.3.4. Determination of the Level of the Superoxide Anion Radical
2.4. Ethical Approval
2.5. Drugs
2.6. Statistical Analysis
3. Results
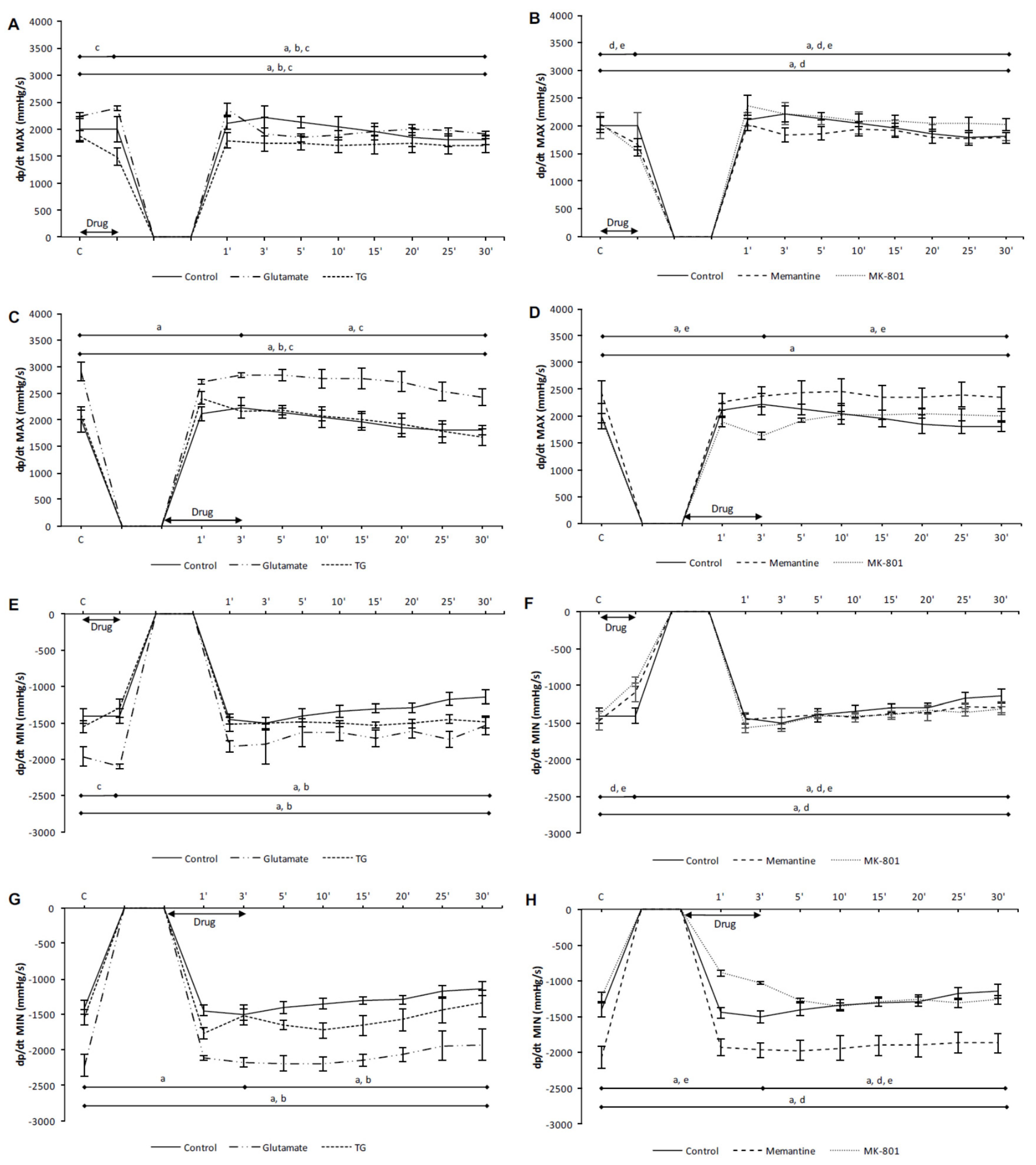
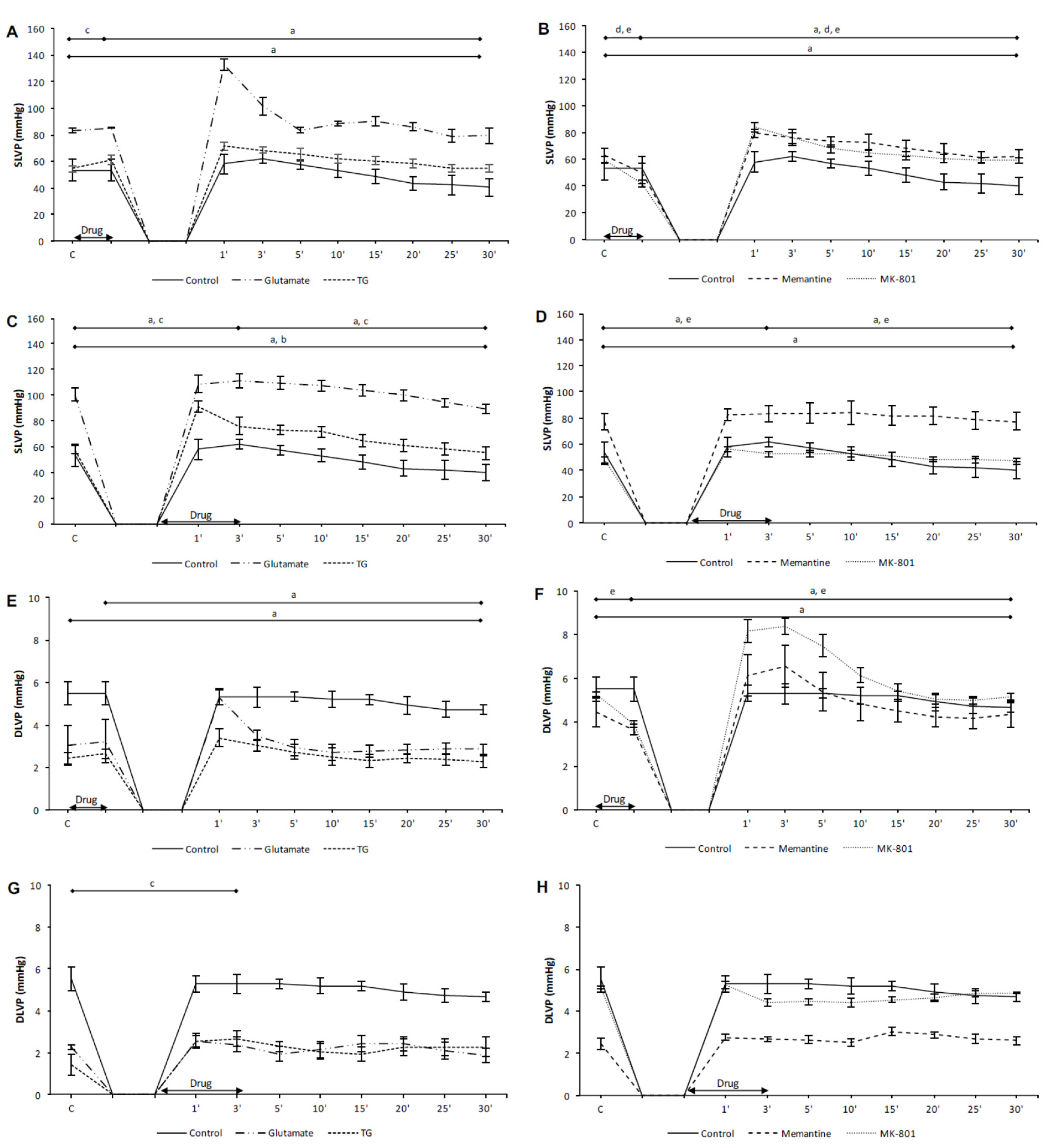
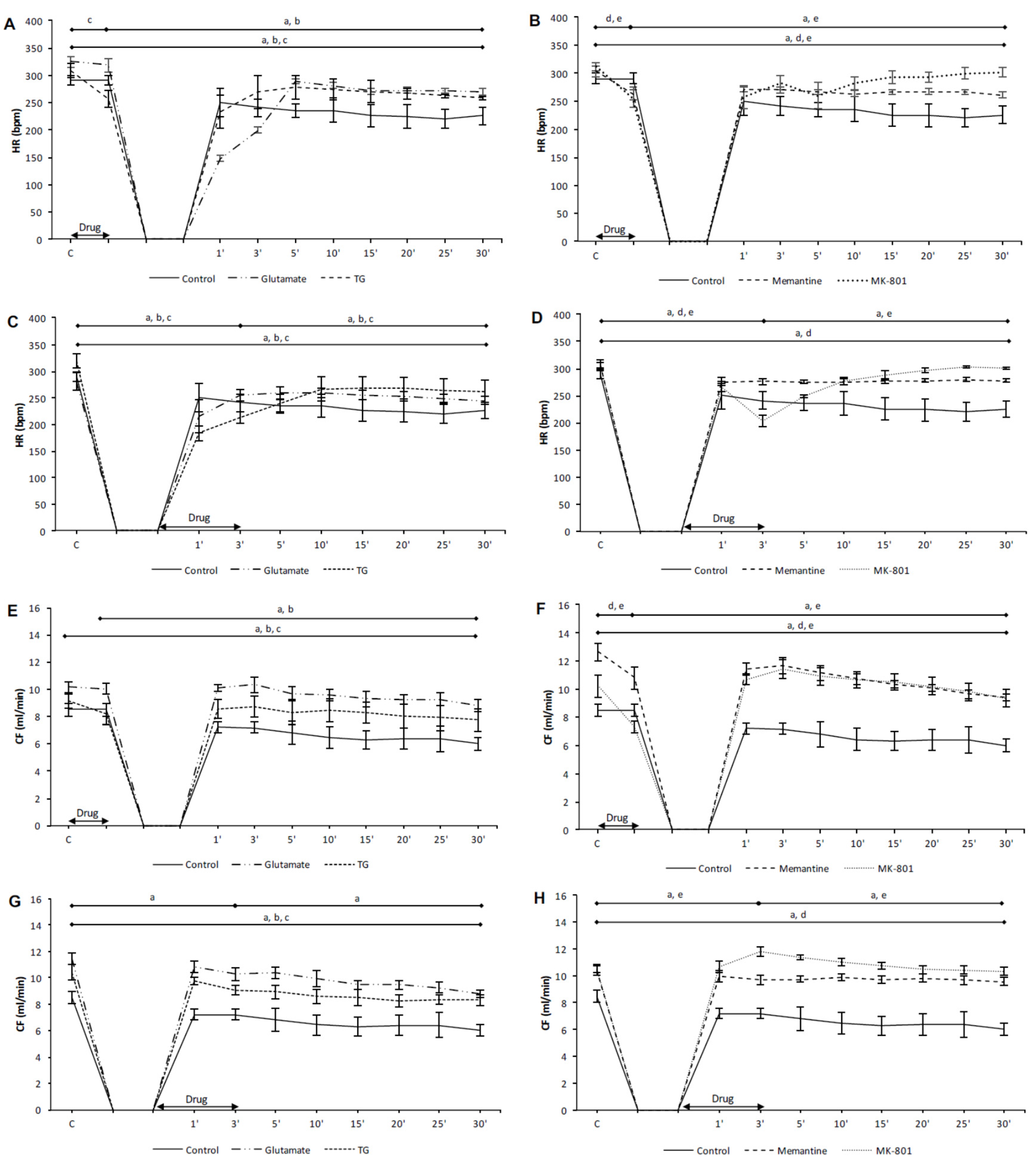
3.1. Effects of NMDA Conditioning on Cardiodynamic Parameters and Coronary Flow in Isolated Rat Heart
3.1.1. The Effects of NMDAR Conditioning with Glutamate and TG on the Cardiodynamic Parameters and Coronary Flow in Isolated Rat Heart
3.1.2. The Effects of NMDAR Conditioning with Memantine and MK-801 on Cardiodynamic Parameters and Coronary Flow in Isolated Rat Heart
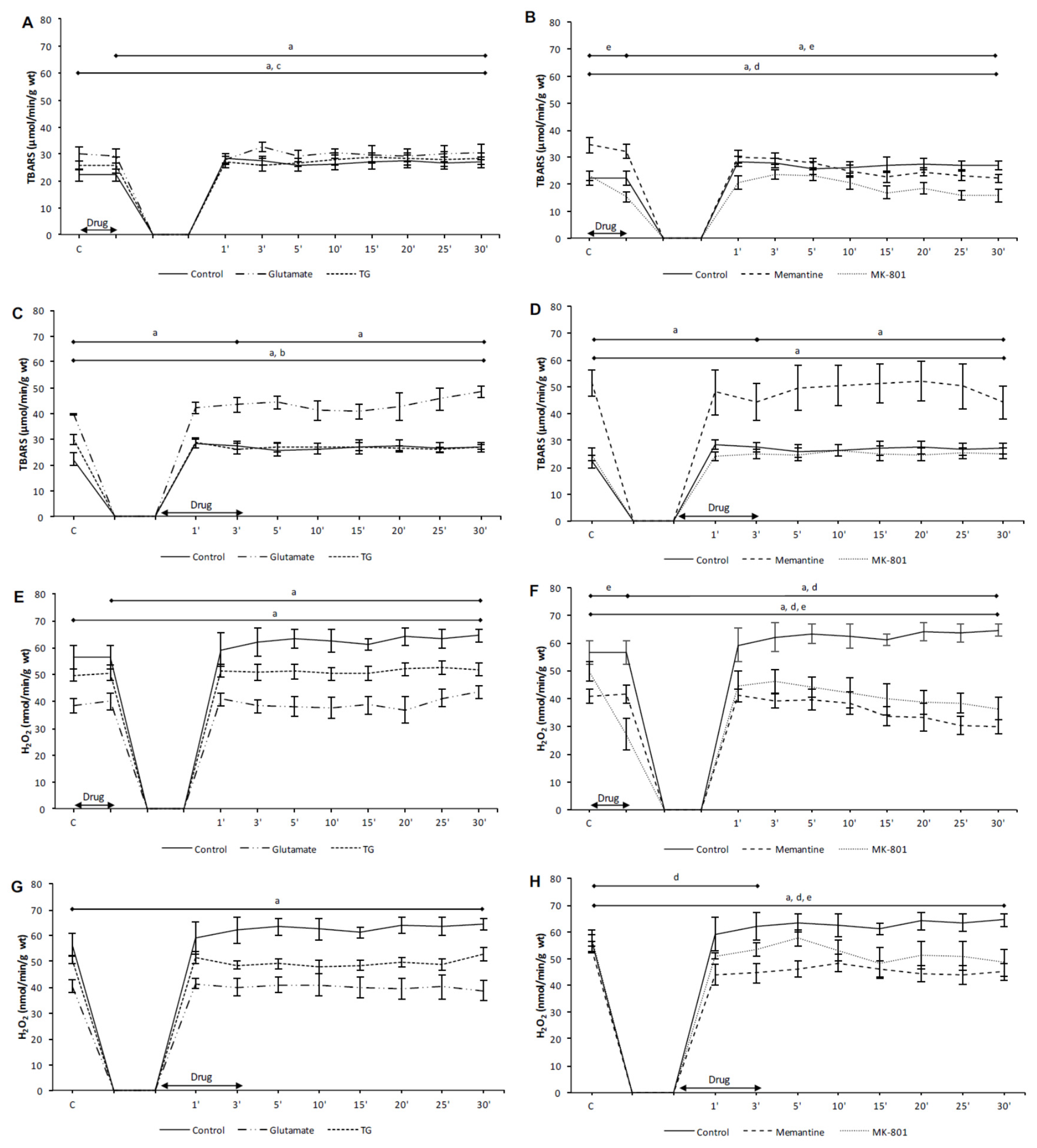
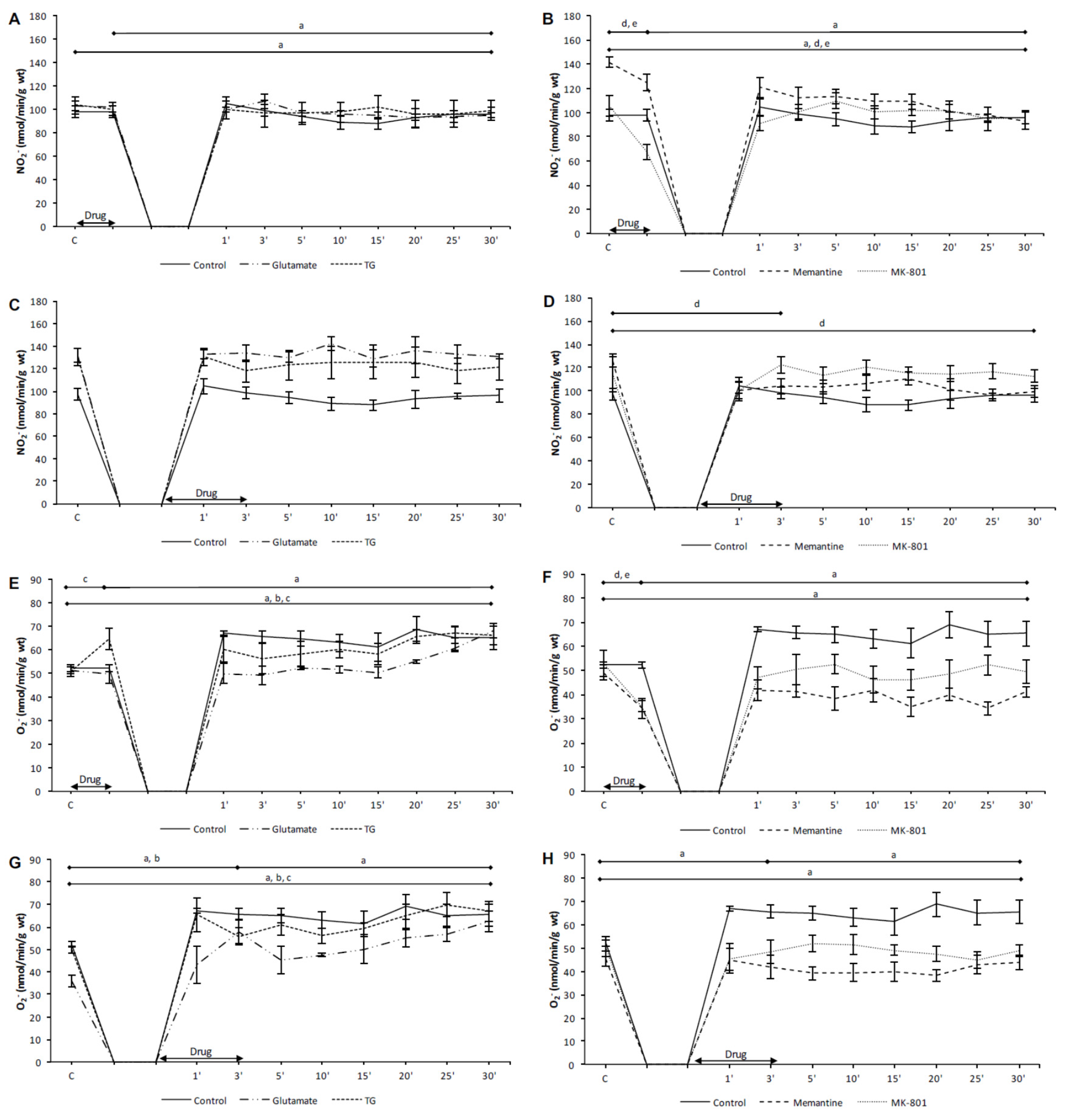
3.2. Effects of NMDA Conditioning on Biomarkers of the Oxidative Stress in Isolated Rat Heart
3.2.1. The Effects of NMDAR Conditioning with Glutamate and TG on the Biomarkers of Oxidative Stress in Isolated Rat Heart
3.2.2. The Effects of NMDAR Conditioning with Memantine and MK-801 on the Biomarkers of Oxidative Stress in Isolated Rat Heart
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef] [PubMed]
- Burnashev, N.; Zhou, Z.; Neher, E.; Sakmann, B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. 1995, 485, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Mattison, H.A.; Cerpa, W. Role of NMDA Receptor-Mediated Glutamatergic Signaling in Chronic and Acute Neuropathologies. Neural Plast. 2016, 2016, 2701526. [Google Scholar] [CrossRef]
- Kotecha, S.A.; MacDonald, J.F. Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int. Rev. Neurobiol. 2003, 54, 51–106. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Valdivielso, J.M. The potential of targeting NMDA receptors outside the CNS. Expert Opin. Ther. Targets. 2015, 19, 399–413. [Google Scholar] [CrossRef]
- Lin, Y.J.; Bovetto, S.; Carver, J.M.; Giordano, T. Cloning of the cDNA for the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery. Brain Res. Mol. Brain Res. 1996, 43, 57–64. [Google Scholar] [CrossRef]
- Leung, J.C.; Travis, B.R.; Verlander, J.W.; Sandhu, S.K.; Yang, S.G.; Zea, A.H.; Weiner, I.D.; Silverstein, D.M. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R964–R971. [Google Scholar] [CrossRef]
- McCully, K.S. Hyperhomocysteinemia, Suppressed Immunity, and Altered Oxidative Metabolism Caused by Pathogenic Microbes in Atherosclerosis and Dementia. Front. Aging Neurosci. 2017, 9, 324. [Google Scholar] [CrossRef]
- Moshal, K.S.; Kumar, M.; Tyagi, N.; Mishra, P.K.; Metreveli, N.; Rodriguez, W.E.; Tyagi, S.C. Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1. Am. J. Physiol. Heart Circ. Physiol 2009, 296, H887–H892. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Lisa, F.D.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, N.; Petkovic, A.; Srejovic, I.; Zivkovic, V.; Djuric, D.; Jakovljevic, V. Effects of ischemia and omeprazole preconditioning on functional recovery of isolated rat heart. Rev. Bras. Cir. Cardiovasc. 2015, 30, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Skyschally, A.; Heusch, G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017, 469, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Caricati-Neto, A.; Errante, P.R.; Menezes-Rodrigues, F.S. Recent Advances in Pharmacological and Non-Pharmacological Strategies of Cardioprotection. Int. J. Mol. Sci. 2019, 20, 4002. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Invest. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Zhou, H.; Toan, S. Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury. Biomolecules 2020, 10, 85. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef]
- Liu, Z.; Vuohelainen, V.; Tarkka, M.; Tenhunen, J.; Lappalainen, R.S.; Narkilahti, S.; Paavonen, T.; Oksala, N.; Wu, Z.; Mennander, A. Glutamate release predicts ongoing myocardial ischemia of rat hearts. Scand. J. Clin. Lab. Invest. 2010, 70, 217–224. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Hu, S.; Zhong, Q.W.; Tian, C.N.; Ma, H.M.; Yu, J.J. N-Methyl-D-Aspartate Receptor-Driven Calcium Influx Potentiates the Adverse Effects of Myocardial Ischemia-Reperfusion Injury Ex Vivo. J. Cardiovasc. Pharmacol. 2017, 70, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Srejovic, I.; Zivkovic, V.; Nikolic, T.; Jeremic, N.; Stojic, I.; Jeremic, J.; Djuric, D.; Jakovljevic, V. Modulation of N-methyl-d-aspartate receptors in isolated rat heart. Can. J. Physiol. Pharmacol. 2017, 95, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Srejovic, I.; Jakovljevic, V.; Zivkovic, V.; Barudzic, N.; Radovanovic, A.; Stanojlovic, O.; Djuric, D.M. The effects of the modulation of NMDA receptors by homocysteine thiolactone and dizocilpine on cardiodynamics and oxidative stress in isolated rat heart. Mol. Cell. Biochem. 2015, 401, 97–105. [Google Scholar] [CrossRef]
- Stojic, I.; Srejovic, I.; Zivkovic, V.; Jeremic, N.; Djuric, M.; Stevanovic, A.; Milanovic, T.; Djuric, D.; Jakovljevic, V. The effects of verapamil and its combinations with glutamate and glycine on cardiodynamics, coronary flow and oxidative stress in isolated rat heart. J. Physiol. Biochem. 2017, 73, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Vidt, D.G.; Bredemeyer, A.; Sapirstein, E.; Sapirstein, L.A. Effect of ether anesthesia on the cardiac output, blood pressure, and distribution of blood flow in the albino rat. Circ. Res. 1959, 7, 759–764. [Google Scholar] [CrossRef]
- Zhou, X.; Hollern, D.; Liao, J.; Andrechek, E.; Wang, H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013, 4, e560. [Google Scholar] [CrossRef]
- Rae, C.; Moussa, C.-H.; Griffin, J.L.; Parekh, S.B.; Bubb, W.A.; Hunt, N.H.; Balcar, V.J. A metabolomic approach to ionotropic glutamate receptor subtype function: A nuclear magnetic resonance in vitro investigation. J. Cereb. Blood Flow Metab. 2006, 26, 1005–1017. [Google Scholar] [CrossRef]
- Gao, X.; Xu, X.; Pang, J.; Zhang, C.; Ding, J.M.; Peng, X.; Liu, Y.; Cao, J.M. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol. Res. 2007, 56, 559–569. [Google Scholar]
- Bradic, J.; Zivkovic, V.; Srejovic, I.; Jakovljevic, V.; Petkovic, A.; Turnic, T.N.; Jeremic, J.; Jeremic, N.; Mitrovic, S.; Sobot, T.; et al. Protective Effects of Galium verum L. Extract against Cardiac Ischemia/Reperfusion Injury in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2019, 2019, 4235405. [Google Scholar] [CrossRef]
- Govoruskina, N.; Srejovic, I.; Bolevich, S.; Bolevich, S.; Omarov, I.A.; Jeremic, J.; Radonjic, K.; Jakovljevic, V. The effects of N-methyl-D-aspartate receptor blockade on oxidative status in heart conditioning maneuvers. Ser. J. Exp. Clin. Res. 2019, 20, 343–350. [Google Scholar] [CrossRef]
- Srejovic, I.; Jakovljevic, V.; Zivkovic, V.; Jeremic, N.; Jevdjevic, M.; Stojic, I.; Djuric, D. The effects of glycine, glutamate and theic combination on cardiodynamics, coronary flow and oxidative stress in isolated rat heart. Curr. Res. Cardiol. 2015, 2, 63–68. [Google Scholar] [CrossRef]
- Moshal, K.S.; Tipparaju, S.M.; Vacek, T.P.; Kumar, M.; Singh, M.; Frank, I.E.; Patibandla, P.K.; Tyagi, N.; Rai, J.; Metreveli, N.; et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H890–H897. [Google Scholar] [CrossRef]
- Zivkovic, V.; Jakovljevic, V.; Pechanova, O.; Srejovic, I.; Joksimovic, J.; Selakovic, D.; Barudzic, N.; Djuric, D.M. Effects of DL-homocysteine thiolactone on cardiac contractility, coronary flow, and oxidative stress markers in the isolated rat heart: The role of different gasotransmitters. Biomed. Res. Int. 2013, 2013, 318471. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, V.; Jakovljevic, V.; Djordjevic, D.; Vuletic, M.; Barudzic, N.; Djuric, D. The effects of homocysteine-related compounds on cardiac contractility, coronary flow, and oxidative stress markers in isolated rat heart. Mol. Cell. Biochem. 2012, 370, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Holm, J.; Vidlund, M.; Vanky, F.; Friberg, Ö.; Yang, Y.; Svedjeholm, R. The impact of glutamate infusion on postoperative NT-proBNP in patients undergoing coronary artery bypass surgery: A randomized study. J. Transl. Med. 2020, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.H.; Reddy, W.J. Amino acid stimulation of oxygen and substrate utilization by cardiac myocytes. Am. J. Physiol. 1978, 235, E461–E466. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, S.; Magi, S.; Castaldo, P.; Preziuso, A.; Lariccia, V.; Amoroso, S. NCX and EAAT transporters in ischemia: At the crossroad between glutamate metabolism and cell survival. Cell Calcium. 2020, 86, 102160. [Google Scholar] [CrossRef] [PubMed]
- Jannesar, K.; Abbaszadeh, S.; Malekinejad, H.; Soraya, H. Cardioprotective effects of memantine in myocardial ischemia: Ex vivo and in vivo studies. Eur. J. Pharmacol. 2020, 882, 173277. [Google Scholar] [CrossRef] [PubMed]
- Słomka, M.; Kuszczyk, M.; Łazarewicz, J.W.; Makarewicz, D. NMDA receptor antagonists MK-801 and memantine induce tolerance to oxygen and glucose deprivation in primary cultures of rat cerebellar granule cells. Acta Neurobiol. Exp. 2014, 74, 396–404. [Google Scholar]
- Tremblay, R.; Chakravarthy, B.; Hewitt, K.; Tauskela, J.; Morley, P.; Atkinson, T.; Durkin, J.P. Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of death signals. J. Neurosci. 2000, 20, 7183–7192. [Google Scholar] [CrossRef]
- Kang, M.Y.; Zhang, Y.; Matkovich, S.J.; Diwan, A.; Chishti, A.H.; Dorn, G.W., 2nd. Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ. Res. 2010, 107, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Matkovich, S.J.; Duan, X.; Diwan, A.; Kang, M.Y.; Dorn, G.W., 2nd. Receptor-independent protein kinase C alpha (PKCalpha) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J. Biol. Chem. 2011, 286, 26943–26951. [Google Scholar] [CrossRef]
- Lai, C.C.; Fang, C.; Kuo, C.Y.; Wu, Y.W.; Lin, H.H. Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats. Biomolecules 2020, 10, 149. [Google Scholar] [CrossRef]
- McGee, M.A.; Abdel-Rahman, A.A. Enhanced vascular PI3K/Akt-NOX signaling underlies the peripheral NMDAR-mediated pressor response in conscious rats. J. Cardiovasc. Pharmacol. 2014, 63, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Zhong, Q.W.; Tian, C.N.; Ma, H.M.; Yu, J.J.; Hu, S. NMDA receptor-driven Ca2+ influx promotes ischemic human cardiomyocyte apoptosis through a p38 MAPK-mediated mechanism. J. Cell. Biochem. 2019, 120, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, T.; Li, Y.; Qin, M.; Tang, Y.; Shen, J.Y.; Liang, J.; Yang, B.; Huang, C. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin. Electrophysiol. 2014, 37, 1367–1377. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, J.; Wang, D.; Xu, J.; Su, H.; An, C.; Zhu, H.; Yan, J. Increasing glutamate promotes ischemia-reperfusion-induced ventricular arrhythmias in rats in vivo. Pharmacology 2014, 93, 4–9. [Google Scholar] [CrossRef]
- D’Amico, M.; Di Filippo, C.; Rossi, F.; Rossi, F. Arrhythmias induced by myocardial ischaemia-reperfusion are sensitive to ionotropic excitatory amino acid receptor antagonists. Eur. J. Pharmacol. 1999, 366, 167–174. [Google Scholar] [CrossRef]
- Bennett, A.J.; DePetrillo, P.B. Differential effects of MK801 and lorazepam on heart rate variability in adolescent rhesus monkeys (macaca mulatta). J. Cardiovasc. Pharmacol. 2005, 45, 383–388. [Google Scholar] [CrossRef]
- Shi, S.; Liu, T.; Wang, D.; Zhang, Y.; Liang, J.; Yang, B.; Hu, D. Activation of N-methyl-d-aspartate receptors reduces heart rate variability and facilitates atrial fibrillation in rats. Europace. 2017, 19, 1237–1243. [Google Scholar] [CrossRef]
- Dean, J.M.; Gunn, A.J.; Wassink, G.; Bennet, L. Transient NMDA receptor-mediated hypoperfusion following umbilical cord occlusion in preterm fetal sheep. Exp. Physiol. 2006, 91, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Northington, F.J.; Carhuapoma, J.R.; Falck, J.R.; Harder, D.R.; Traystman, R.J.; Koehler, R.C. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1616–H1624. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Ke, T.; Li, R.; Chen, W. Inhibition of the NMDA receptor protects the rat sciatic nerve against ischemia/reperfusion injury. Exp. Ther. Med. 2016, 11, 1563–1572. [Google Scholar] [CrossRef][Green Version]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef]
- McGee, M.A.; Abdel-Rahman, A.A. Ethanol attenuates peripheral NMDAR-mediated vascular oxidative stress and pressor response. Alcohol 2015, 49, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J. Alcohol related changes in regulation of NMDA receptor functions. Curr. Neuropharmacol. 2008, 6, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Gubandru, M.; Margina, D.; Tsitsimpikou, C.; Goutzourelas, A.; Tsarouhas, K.; Ilie, M.; Tsatsakis, A.M.; Kouretas, D. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem. Toxicol. 2013, 61, 209–214. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.; Deng, Y.; Xu, B.; Wei, Y.; Yang, T. Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex. Neurotox. Res. 2013, 24, 320–337. [Google Scholar] [CrossRef]
- Lionetti, V.; Barile, L. Perioperative cardioprotection: Back to bedside. Minerva. Anestesiol. 2020, 86, 445–454. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govoruskina, N.; Jakovljevic, V.; Zivkovic, V.; Milosavljevic, I.; Jeremic, J.; Bradic, J.; Bolevich, S.; Omarov, I.A.; Djuric, D.; Radonjic, K.; et al. The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning—Effects on Heart Function and Oxidative Stress. Biomolecules 2020, 10, 1065. https://doi.org/10.3390/biom10071065
Govoruskina N, Jakovljevic V, Zivkovic V, Milosavljevic I, Jeremic J, Bradic J, Bolevich S, Omarov IA, Djuric D, Radonjic K, et al. The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning—Effects on Heart Function and Oxidative Stress. Biomolecules. 2020; 10(7):1065. https://doi.org/10.3390/biom10071065
Chicago/Turabian StyleGovoruskina, Natalia, Vladimir Jakovljevic, Vladimir Zivkovic, Isidora Milosavljevic, Jovana Jeremic, Jovana Bradic, Sergey Bolevich, Israpil Alisultanovich Omarov, Dragan Djuric, Katarina Radonjic, and et al. 2020. "The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning—Effects on Heart Function and Oxidative Stress" Biomolecules 10, no. 7: 1065. https://doi.org/10.3390/biom10071065
APA StyleGovoruskina, N., Jakovljevic, V., Zivkovic, V., Milosavljevic, I., Jeremic, J., Bradic, J., Bolevich, S., Omarov, I. A., Djuric, D., Radonjic, K., Andjic, M., Draginic, N., Stojanovic, A., & Srejovic, I. (2020). The Role of Cardiac N-Methyl-D-Aspartate Receptors in Heart Conditioning—Effects on Heart Function and Oxidative Stress. Biomolecules, 10(7), 1065. https://doi.org/10.3390/biom10071065






