Untargeted Profiling of Bile Acids and Lysophospholipids Identifies the Lipid Signature Associated with Glycemic Outcome in an Obese Non-Diabetic Clinical Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Standard Solutions
2.3. Lipid Extraction
2.4. Liquid Chromatography Separation and Mass Spectrometry Detection
2.5. Data Analysis
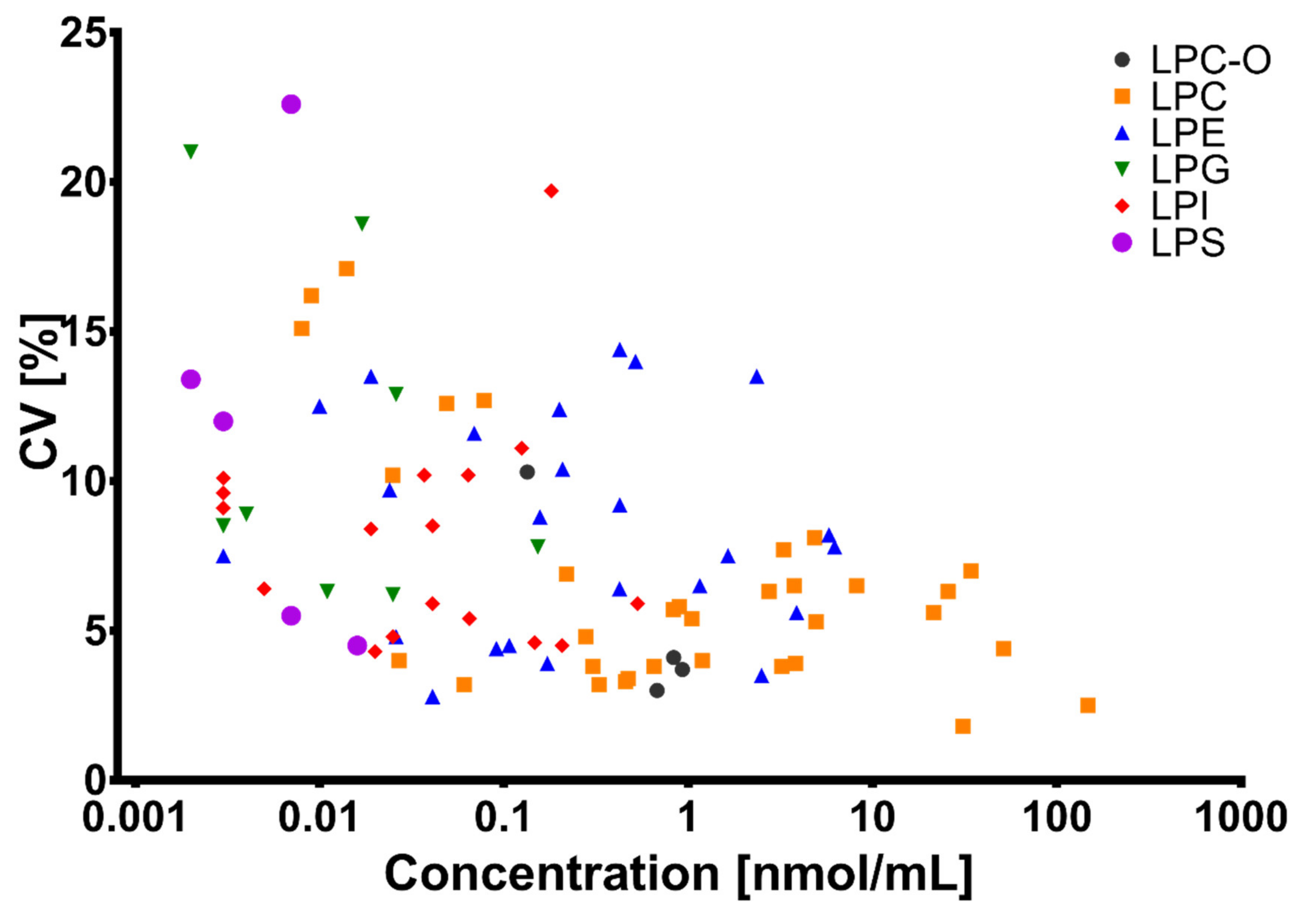
2.6. Development and Validation of the LC-MS Method
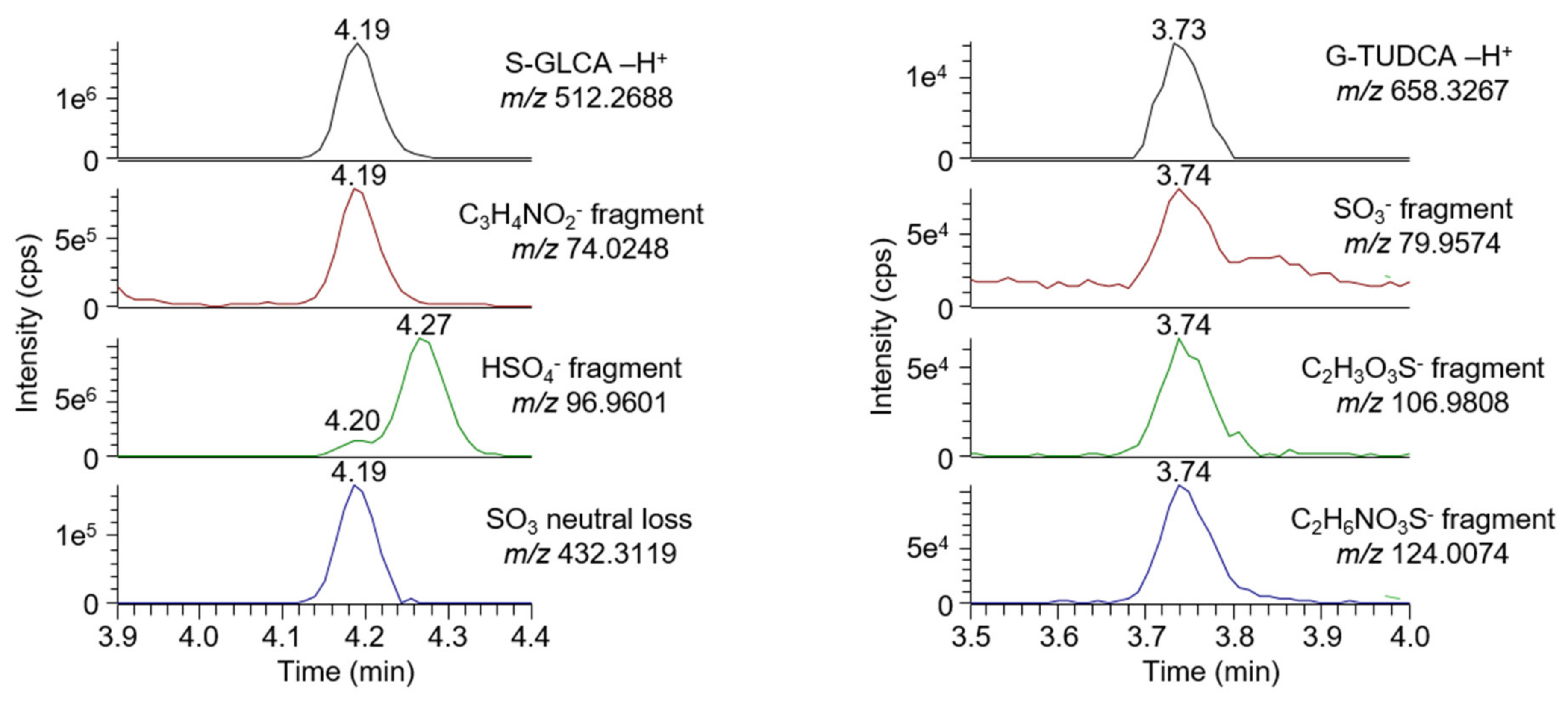
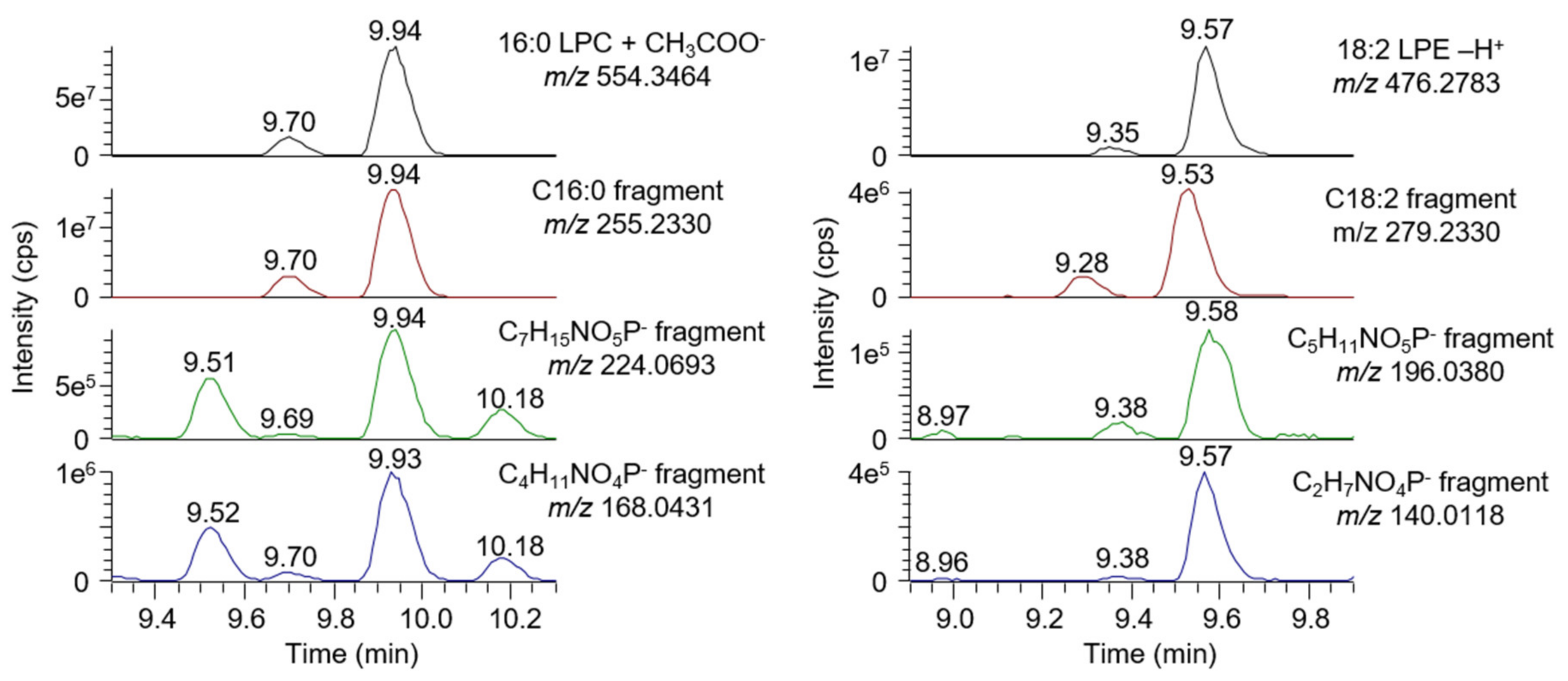
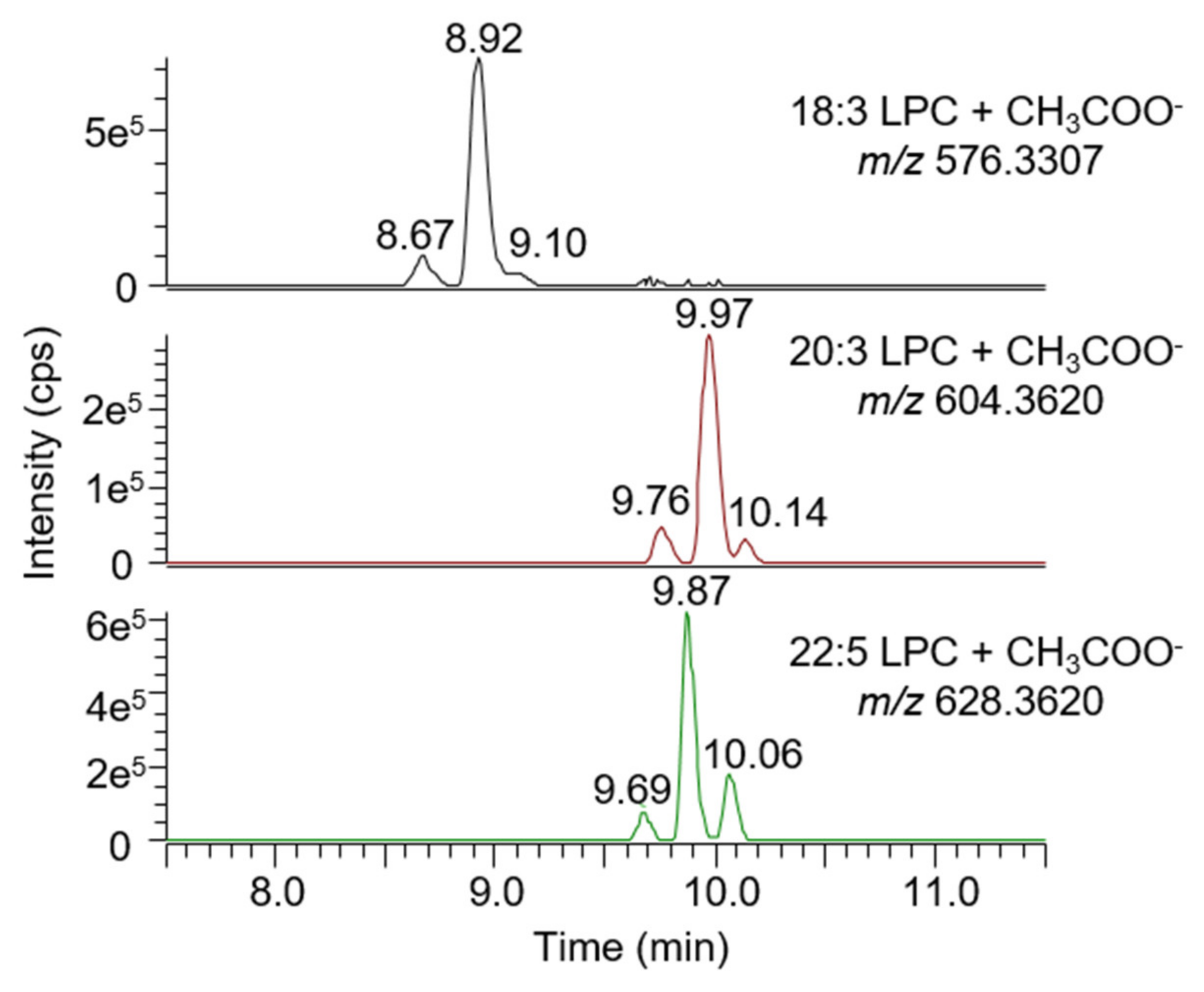
2.6.1. Selectivity
- A signal is visible on an MS1 chromatogram extracted using the analyte mass and a mass tolerance of ±5 ppm;
- A signal is visible on an MS2 chromatogram extracted using a characteristic fragment mass (fatty acid, phospholipid head group for lysophospholipids, and conjugated group for bile acids) and a mass tolerance of ±5 ppm;
- The retention time of the analyte matches the result obtained with a reference standard.
2.6.2. Recovery of Extraction
2.6.3. Dynamic Range, Limits of Detection, and Quantification
2.6.4. Matrix Effect
2.6.5. Trueness, Precision, and Repeatability
2.7. Untargeted Screening of Bile Acids and Lysophospholipids
2.8. Experimental Model and Subjects’ Details
2.9. Statistical Analysis on the Clinical Cohort
- -
- A clinical model based solely on baseline clinical parameters, including total lipid levels (triglycerides, HDL, cholesterol, and LDL), the Body Mass Index (BMI), homeostasis model assessment of insulin resistance (HOMA-IR), gender, and age;
- -
- A model consisting of only baseline bile acid and lysophoslipid levels;
- -
- A model consisting of only the log2 fold-change that occurred during weight loss in bile acids and lysophoslipids.
3. Results and Discussion
3.1. Method Development and Validation
3.2. Application of the High Throughput Screening Method for Biomarker Discovery: Predicting the Response to a Controlled Weight Loss Intervention
3.3. Application of the High Throughput Screening Method for Biomarker Discovery: Predicting Insulin Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma Lysophosphatidylcholine Levels Are Reduced in Obesity and Type 2 Diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Drogan, D.; Dunn, W.B.; Lin, W.; Buijsse, B.; Schulze, M.B.; Langenberg, C.; Brown, M.; Floegel, A.; Dietrich, S.; Rolandsson, O.; et al. Untargeted Metabolic Profiling Identifies Altered Serum Metabolites of Type 2 Diabetes Mellitus in a Prospective, Nested Case Control Study. Clin. Chem. 2015, 61, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.-P.; Milburn, M.V.; Kastenmüller, G.; Adamski, J.; Tuomi, T.; et al. Early Metabolic Markers of the Development of Dysglycemia and Type 2 Diabetes and Their Physiological Significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.-J.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef]
- Klingler, C.; Zhao, X.; Adhikary, T.; Li, J.; Xu, G.; Häring, H.-U.; Schleicher, E.; Lehmann, R.; Weigert, C. Lysophosphatidylcholines activate PPARδ and protect human skeletal muscle cells from lipotoxicity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1980–1992. [Google Scholar] [CrossRef]
- Ma, H.; Patti, M.E. Bile acids, obesity, and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 573–583. [Google Scholar] [CrossRef]
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Sys. Biol. 2008, 4, 214. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Duran-Sandoval, D.; Cariou, B.; Percevault, F.; Hennuyer, N.; Grefhorst, A.; van Dijk, T.H.; Gonzalez, F.J.; Fruchart, J.-C.; Kuipers, F.; Staels, B. The Farnesoid X Receptor Modulates Hepatic Carbohydrate Metabolism during the Fasting-Refeeding Transition. J. Biol. Chem. 2005, 280, 29971–29979. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Huaman Samanez, C.; Dehondt, H.; Ploton, M.; Briand, O.; Lien, F.; Dorchies, E.; Dumont, J.; Postic, C.; Cariou, B.; et al. Farnesoid X Receptor Inhibits the Transcriptional Activity of Carbohydrate Response Element Binding Protein in Human Hepatocytes. Mol. Cell. Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rong, Z.; Xiang, D.; Zhang, C.; Liu, D. Detection technologies and metabolic profiling of bile acids: A comprehensive review. Lipids Health Dis. 2018, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Gnewuch, C.; Schmitz, G.; Liebisch, G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 3920–3925. [Google Scholar] [CrossRef]
- Perwaiz, S.; Tuchweber, B.; Mignault, D.; Gilat, T.; Yousef, I.M. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J. Lipid Res. 2001, 42, 114–119. [Google Scholar]
- Alnouti, Y.; Csanaky, I.L.; Klaassen, C.D. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC–MS/MS. J. Chromatogr. B 2008, 873, 209–217. [Google Scholar] [CrossRef]
- Bathena, S.P.R.; Mukherjee, S.; Olivera, M.; Alnouti, Y. The profile of bile acids and their sulfate metabolites in human urine and serum. J. Chromatogr. B 2013, 942, 53–62. [Google Scholar] [CrossRef]
- Amplatz, B.; Zöhrer, E.; Haas, C.; Schäffer, M.; Stojakovic, T.; Jahnel, J.; Fauler, G. Bile acid preparation and comprehensive analysis by high performance liquid chromatography–high-resolution mass spectrometry. Clin. Chim. Acta 2017, 464, 85–92. [Google Scholar] [CrossRef]
- Jäntti, S.E.; Kivilompolo, M.; Öhrnberg, L.; Pietiläinen, K.H.; Nygren, H.; Orešič, M.; Hyötyläinen, T. Quantitative profiling of bile acids in blood, adipose tissue, intestine, and gall bladder samples using ultra high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7799–7815. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.; Hong, J.; Lee, S.H.; Jung, B.H. Quantitative profiling of bile acids in rat bile using ultrahigh-performance liquid chromatography–orbitrap mass spectrometry: Alteration of the bile acid composition with aging. J. Chromatogr. B 2016, 1031, 37–49. [Google Scholar] [CrossRef]
- Sarafian, M.H.; Lewis, M.R.; Pechlivanis, A.; Ralphs, S.; McPhail, M.J.W.; Patel, V.C.; Dumas, M.-E.; Holmes, E.; Nicholson, J.K. Bile Acid Profiling and Quantification in Biofluids Using Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Chem. 2015, 87, 9662–9670. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Han, X. Comprehensive and Quantitative Analysis of Lysophospholipid Molecular Species Present in Obese Mouse Liver by Shotgun Lipidomics. Anal. Chem. 2015, 87, 4879–4887. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.E.; Bird, S.S.; Gross, V.S.; Marur, V.R.; Lazarev, A.V.; Walker, W.A.; Kristal, B.S. Method Development for Fecal Lipidomics Profiling. Anal. Chem. 2013, 85, 1114–1123. [Google Scholar] [CrossRef]
- Rampler, E.; Criscuolo, A.; Zeller, M.; El Abiead, Y.; Schoeny, H.; Hermann, G.; Sokol, E.; Cook, K.; Peake, D.A.; Delanghe, B.; et al. A Novel Lipidomics Workflow for Improved Human Plasma Identification and Quantification Using RPLC-MSn Methods and Isotope Dilution Strategies. Anal. Chem. 2018, 90, 6494–6501. [Google Scholar] [CrossRef]
- Bollinger, J.G.; Ii, H.; Sadilek, M.; Gelb, M.H. Improved method for the quantification of lysophospholipids including enol ether species by liquid chromatography-tandem mass spectrometry. J. Lipid Res. 2010, 51, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.-M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.H.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Pihlsgård, M.; et al. Diets with High or Low Protein Content and Glycemic Index for Weight-Loss Maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry, Bioanalytical Method Validation; U.S. Department of Health and Human Services Food and Drug Administration: Rockville, MD, USA, 2001.
- Valsesia, A.; Saris, W.H.; Astrup, A.; Hager, J.; Masoodi, M. Distinct lipid profiles predict improved glycemic control in obese, nondiabetic patients after a low-caloric diet intervention: The Diet, Obesity and Genes randomized trial. Am. J. Clin. Nutr. 2016, 104, 566–575. [Google Scholar] [CrossRef]
- Valsesia, A.; Chakrabarti, A.; Hager, J.; Langin, D.; Saris, W.H.M.; Astrup, A.; Blaak, E.E.; Viguerie, N.; Masoodi, M. Integrative Phenotyping of Glycemic Responders Upon Clinical Weight Loss Using Multi-Omics. Sci. Rep. 2020, 10, 9236. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 67. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; González, I.; Déjean, S. integrOmics: An R package to unravel relationships between two omics datasets. Bioinformatics 2009, 25, 2855–2856. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahé, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B 2012, 899, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Perreault, M.; Rudkowska, I.; Levy, C.; Dallaire-Theroux, A.; Verreault, M.; Caron, P.; Staels, B.; Vohl, M.-C.; Straka, R.J.; et al. Profiling Serum Bile Acid Glucuronides in Humans: Gender Divergences, Genetic Determinants, and Response to Fenofibrate. Clin. Pharmacol. Ther. 2013, 94, 533–543. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Turk, J. Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of glycerophospholipids: Mechanisms of fragmentation and structural characterization. J. Chromatogr. B 2009, 877, 2673–2695. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950–Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef]
- Heiskanen, L.A.; Suoniemi, M.; Ta, H.X.; Tarasov, K.; Ekroos, K. Long-Term Performance and Stability of Molecular Shotgun Lipidomic Analysis of Human Plasma Samples. Anal. Chem. 2013, 85, 8757–8763. [Google Scholar] [CrossRef]
- Surma, M.A.; Herzog, R.; Vasilj, A.; Klose, C.; Christinat, N.; Morin-Rivron, D.; Simons, K.; Masoodi, M.; Sampaio, J.L. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1540–1549. [Google Scholar] [CrossRef]
- Meyer, A.; Montastier, E.; Hager, J.; Saris, W.H.M.; Astrup, A.; Viguerie, N.; Valsesia, A. Plasma metabolites and lipids predict insulin sensitivity improvement in obese, nondiabetic individuals after a 2-phase dietary intervention. Am. J. Clin. Nutr. 2018, 108, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Heimerl, S.; Fischer, M.; Baessler, A.; Liebisch, G.; Sigruener, A.; Wallner, S.; Schmitz, G. Alterations of Plasma Lysophosphatidylcholine Species in Obesity and Weight Loss. PLoS ONE 2014, 9, e111348. [Google Scholar] [CrossRef]
- Eisinger, K.; Liebisch, G.; Schmitz, G.; Aslanidis, C.; Krautbauer, S.; Buechler, C. Lipidomic Analysis of Serum from High Fat Diet Induced Obese Mice. Int. J. Mol. Sci. 2014, 15, 2991–3002. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; del Bas, J.M.; Caimari, A.; Arola, L.; Zulet, M.A.; Martinez, J.A. Changes in lysophospholipids and liver status after weight loss: The RESMENA study. Nutr. Metab. 2018, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Ginos, B.N.R.; Navarro, S.L.; Schwarz, Y.; Gu, H.; Wang, D.; Randolph, T.W.; Shojaie, A.; Hullar, M.A.J.; Lampe, P.D.; Kratz, M.; et al. Circulating bile acids in healthy adults respond differently to a dietary pattern characterized by whole grains, legumes and fruits and vegetables compared to a diet high in refined grains and added sugars: A randomized, controlled, crossover feeding study. Metab. Clin. Exp. 2018, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Yang, Y.; McCullough, A.J.; Marczewski, S.; Bennett, C.; Kalhan, S.C. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 382–388. [Google Scholar] [CrossRef]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.R.; Sidney Barritt, A. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar]
- Cariou, B.; Chetiveaux, M.; Zaïr, Y.; Pouteau, E.; Disse, E.; Guyomarc’h-Delasalle, B.; Laville, M.; Krempf, M. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr. Metab. 2011, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Wewalka, M.; Patti, M.-E.; Barbato, C.; Houten, S.M.; Goldfine, A.B. Fasting Serum Taurine-Conjugated Bile Acids Are Elevated in Type 2 Diabetes and Do Not Change with Intensification of Insulin. J. Clin. Endocrinol. Metab. 2014, 99, 1442–1451. [Google Scholar] [CrossRef]
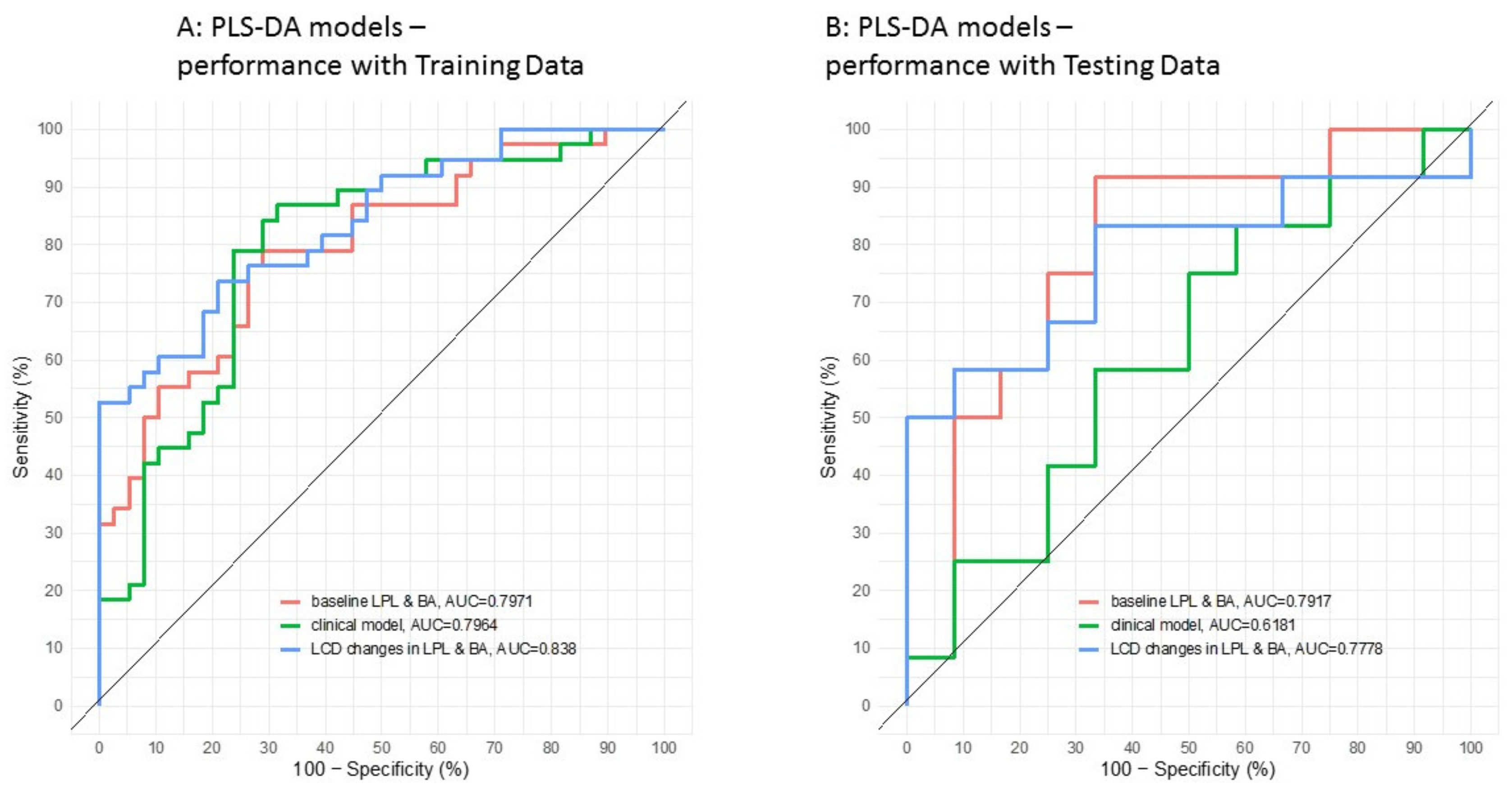
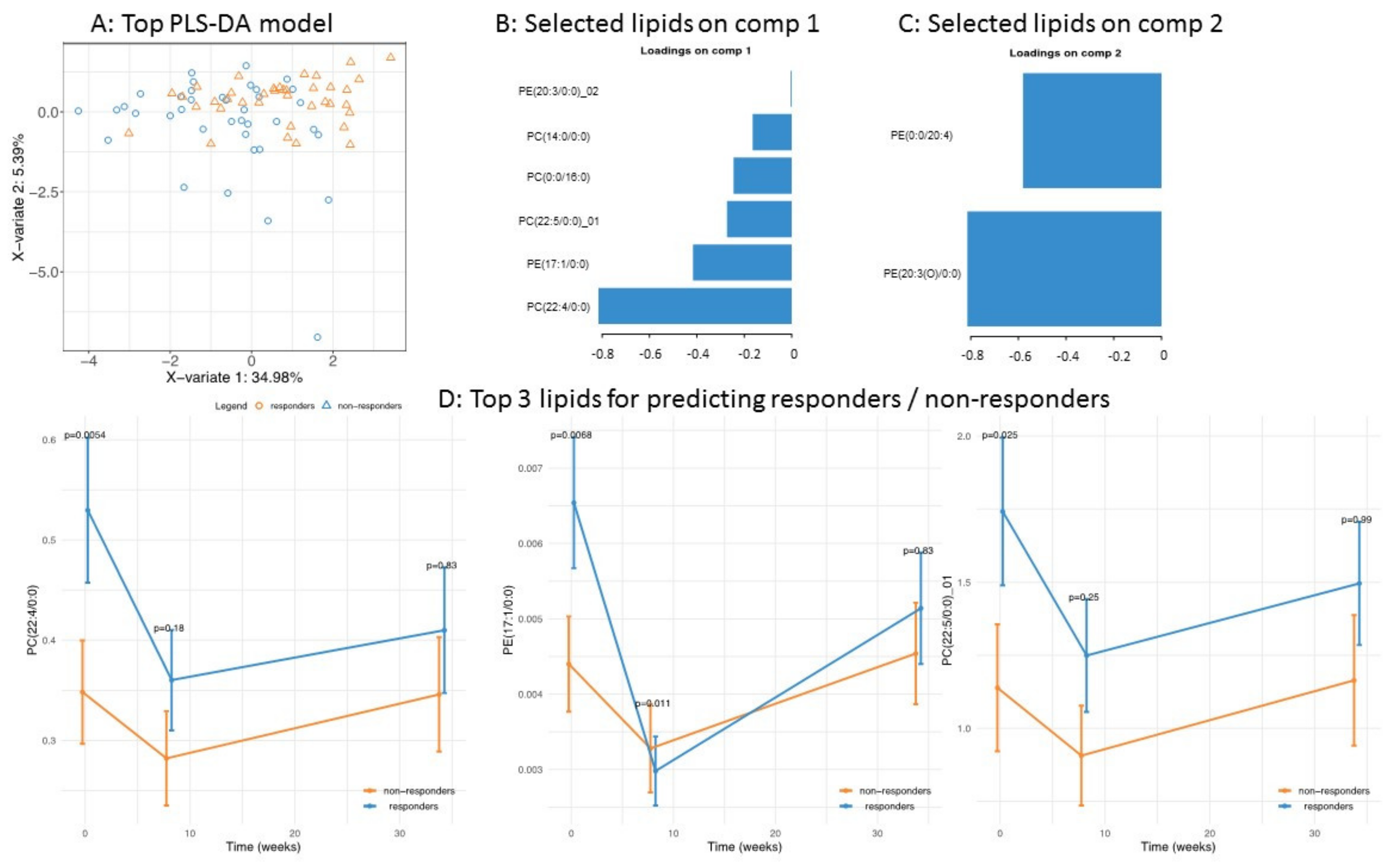
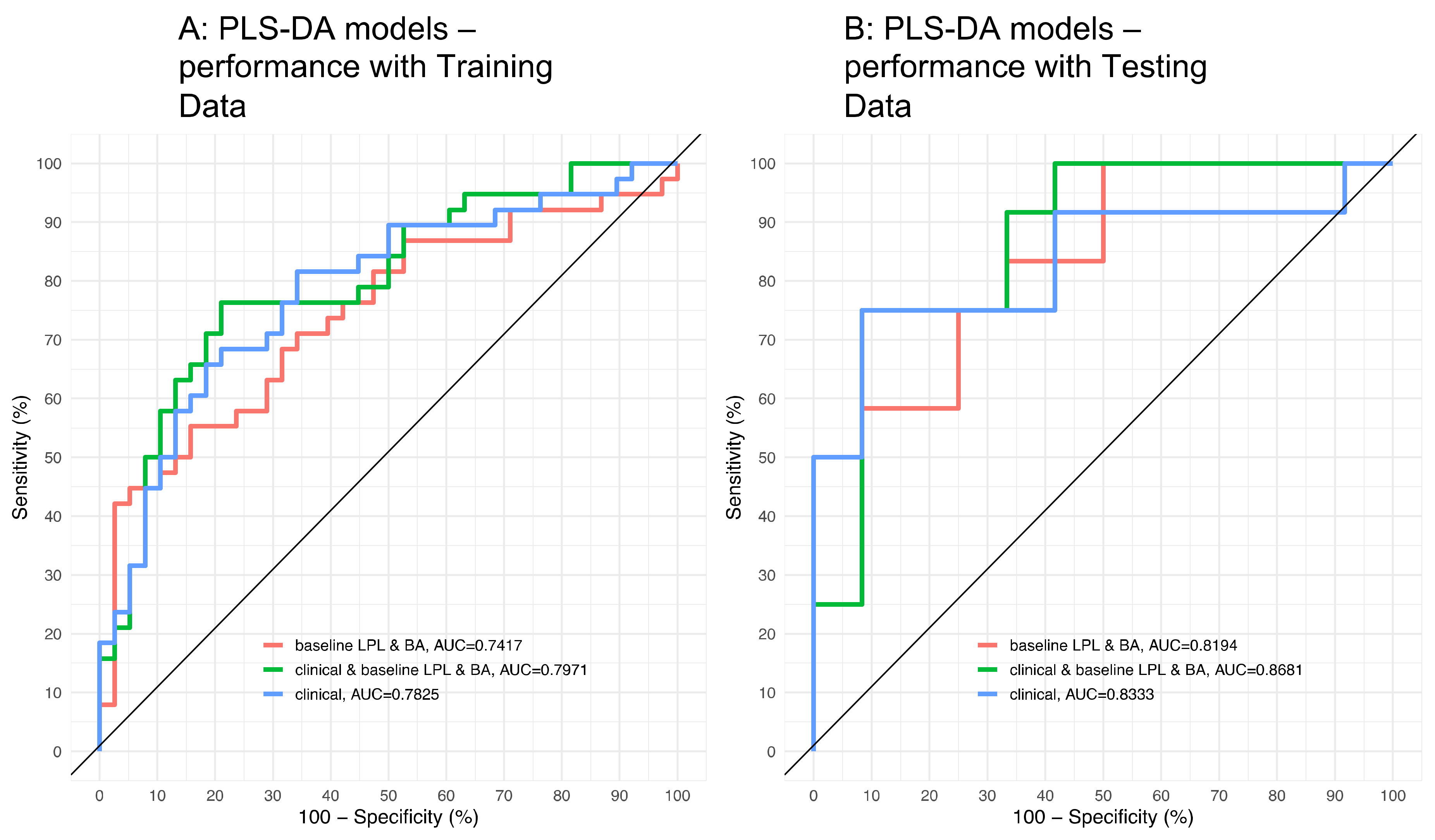
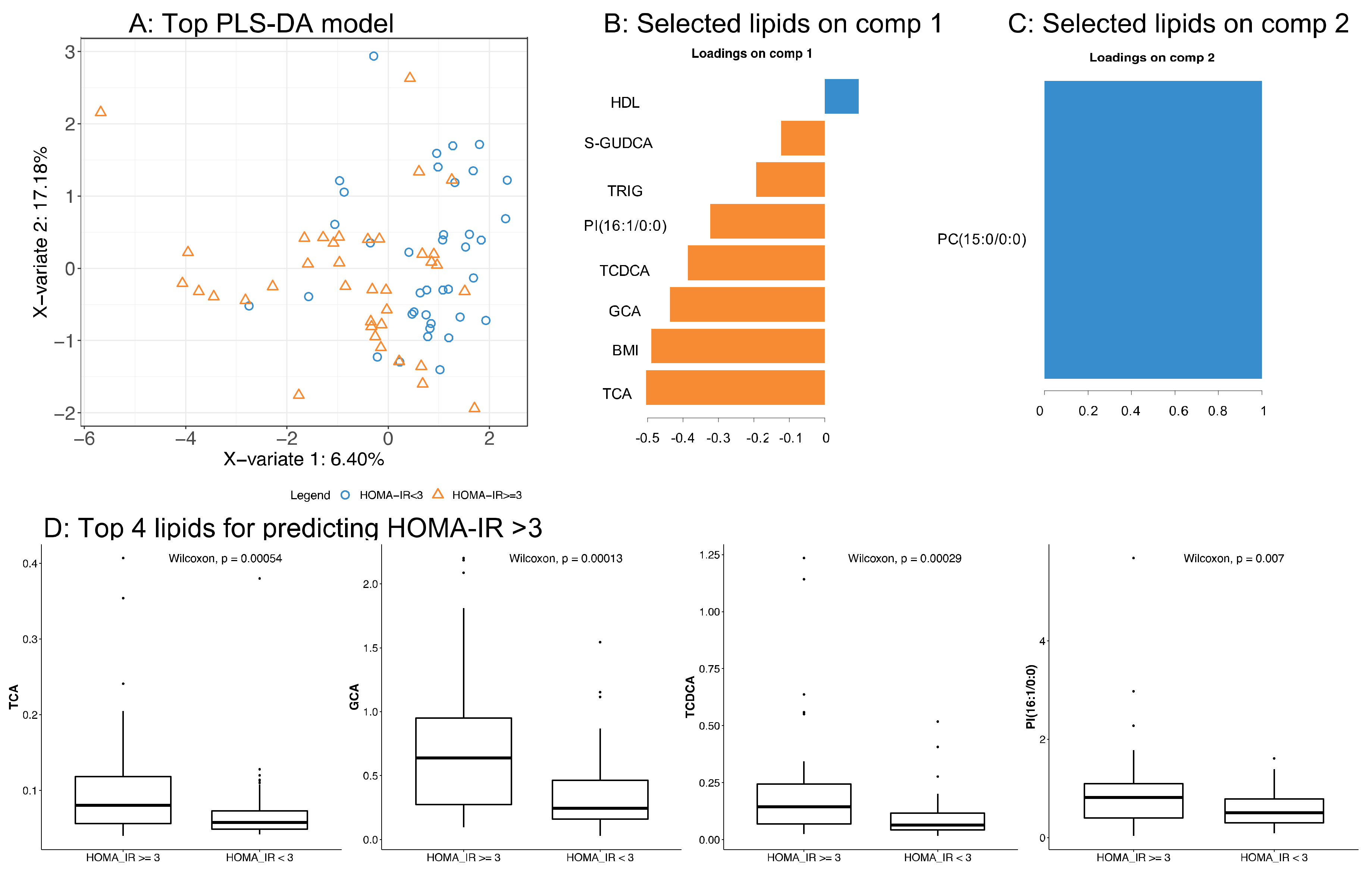
| Parameter | All Subjects (N = 100) | Non Responders (N = 50) | Responders (N = 50) | p-Value |
|---|---|---|---|---|
| age, y | 42+/−6 | 41+/−6 | 43+/−6 | 0.0423 |
| BMI, kg/2 | 35.2703+/−5.1284 | 36.0502+/−5.3902 | 34.4904+/−4.7794 | 0.129 |
| fasting cholesterol levels, mmol/L | 4.6770+/−0.9588 | 4.5218+/−0.8869 | 4.8323+/−1.0107 | 0.106 |
| HOMA-IR | 3.4239+/−1.8902 | 3.2890+/−2.1510 | 3.5588+/−1.5983 | 0.478 |
| fasting LDL, mmol/L | 2.8845+/−0.8245 | 2.7662+/−0.7372 | 3.0053+/−0.8964 | 0.151 |
| gender | M = 44, F = 56 | M = 14, F = 36 | M = 30, F = 20 | 0.002333 |
| fasting HDL, mmol/L | 1.1104+/−0.3039 | 1.2044+/−0.3291 | 1.0164+/−0.2453 | 0.00168 |
| fasting triglycerides, mmol/L | 1.4660+/−0.6817 | 1.2244+/−0.5851 | 1.7124+/−0.6904 | 0.000265 |
| Symbol | Bile Acid | Concentration (µM) |
|---|---|---|
| CA | Cholic acid | 0.132 (0.069–0.976) |
| CDCA | Chenodeoxycholic acid | 0.265 (0.058–2.027) |
| DCA | Deoxycholic acid | 1.221 (0.208–3.697) |
| LCA | Lithocholic acid | 0.125 (0.038–0.318) |
| UDCA | Ursodeoxycholic acid | 0.728 (0.210–3.343) |
| HCA | Hyocholic acid | 0.016 (0.008–0.042) |
| HDCA | Hyodeoxycholic acid | 0.075 (0.025–0.185) |
| GCA | Glycocholic acid | 0.282 (0.095–0.955) |
| GCDCA | Glycochenodeoxycholic acid | 1.445 (0.406–4.155) |
| GDCA | Glycodeoxycholic acid | 0.252 (0.060–1.017) |
| GLCA | Glycolithocholic acid | ND |
| GUDCA | Glycoursodeoxycholic acid | 0.113 (0.060–0.387) |
| GHDCA | Glycohyodeoxycholic acid | ND |
| TCA | Taurocholic acid | 0.058 (0.044–0.129) |
| TCDCA | Taurochenodeoxycholic acid | 0.081 (0.026–0.270) |
| TDCA | Taurodeoxycholic acid | 0.033 (0.014–0.109) |
| TLCA | Taurolithocholic acid | 0.022 (0.009–0.042) |
| TUDCA | Tauroursodeoxycholic acid | 0.025 (0.016–0.246) |
| THDCA | Taurohyodeoxycholic acid | ND |
| α-MCA | α-muricholic acid | 0.015 (0.008–0.031) |
| β-MCA | β-muricholic acid | ND |
| ω-MCA | ω-muricholic acid | 0.027 (0.012–0.074) |
| 7S-CA | Cholic acid 7-sulfate | ND |
| 3S-TLCA | Taurolitocholic acid 3-sulfate | 0.055 (0.016–0.174) |
| 3S-TCA | Taurocholic acid 3-sulfate | ND |
| MDCA | Murideoxycholic acid | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christinat, N.; Valsesia, A.; Masoodi, M. Untargeted Profiling of Bile Acids and Lysophospholipids Identifies the Lipid Signature Associated with Glycemic Outcome in an Obese Non-Diabetic Clinical Cohort. Biomolecules 2020, 10, 1049. https://doi.org/10.3390/biom10071049
Christinat N, Valsesia A, Masoodi M. Untargeted Profiling of Bile Acids and Lysophospholipids Identifies the Lipid Signature Associated with Glycemic Outcome in an Obese Non-Diabetic Clinical Cohort. Biomolecules. 2020; 10(7):1049. https://doi.org/10.3390/biom10071049
Chicago/Turabian StyleChristinat, Nicolas, Armand Valsesia, and Mojgan Masoodi. 2020. "Untargeted Profiling of Bile Acids and Lysophospholipids Identifies the Lipid Signature Associated with Glycemic Outcome in an Obese Non-Diabetic Clinical Cohort" Biomolecules 10, no. 7: 1049. https://doi.org/10.3390/biom10071049
APA StyleChristinat, N., Valsesia, A., & Masoodi, M. (2020). Untargeted Profiling of Bile Acids and Lysophospholipids Identifies the Lipid Signature Associated with Glycemic Outcome in an Obese Non-Diabetic Clinical Cohort. Biomolecules, 10(7), 1049. https://doi.org/10.3390/biom10071049






