Vibrational Spectroscopy of Peritoneal Dialysis Effluent for Rapid Assessment of Patient Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Sample Collection and Preparation
2.3. FTIR Spectroscopy
2.3.1. Sample Preparation and FTIR Spectroscopy
2.3.2. Spectral Preprocessing and Analysis
2.3.3. Unsupervised and Supervised Machine Learning
2.4. Targeted Metabolomics
3. Results and Discussion
3.1. Spectral Impact on PD Fluid during Dialysis and Owing to Ala-Gln Treatment
3.2. Supervised Machine Learning of Patient-, PD-, and Infection and Inflammation-Related Parameters
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, P.K.; Chow, K.M.; van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- van de Luijtgaarden, M.W.; Jager, K.J.; Segelmark, M.; Pascual, J.; Collart, F.; Hemke, A.C.; Remon, C.; Metcalfe, W.; Miguel, A.; Kramar, R.; et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol. Dial. Transplant. 2016, 31, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Aufricht, C.; Beelen, R.; Eberl, M.; Fischbach, M.; Fraser, D.; Jorres, A.; Kratochwill, K.; LopezCabrera, M.; Rutherford, P.; Schmitt, C.P.; et al. Biomarker research to improve clinical outcomes of peritoneal dialysis: Consensus of the European training and research in peritoneal dialysis (EuTRiPD) network. Kidney Int. 2017, 92, 824–835. [Google Scholar] [CrossRef]
- Zhang, J.; Friberg, I.M.; Kift-Morgan, A.; Parekh, G.; Morgan, M.P.; Liuzzi, A.R.; Lin, C.Y.; Donovan, K.L.; Colmont, C.S.; Morgan, P.H.; et al. Machine-learning algorithms define pathogen-specific local immune fingerprints in peritoneal dialysis patients with bacterial infections. Kidney Int. 2017, 92, 179–191. [Google Scholar] [CrossRef]
- Araújo, J.E.; Jorge, S.; Magriço, R.; e Costa, T.; Ramos, A.; Reboiro-Jato, M.; Fdez-Riverola, F.; Lodeiro, C.; Capelo, J.L.; Santos, H.M. Classifying patients in peritoneal dialysis by mass spectrometry-based profiling. Talanta 2016, 152, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.E.; Santos, T.; Jorge, S.; Pereira, T.M.; Reboiro-Jato, M.; Pavón, R.; Magriço, R.; Teixeira-Costa, F.; Ramos, A.; Santos, H.M. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry-based profiling as a step forward in the characterization of peritoneal dialysis effluent. Anal. Methods 2015, 7, 7467–7473. [Google Scholar] [CrossRef]
- Herzog, R.; Boehm, M.; Unterwurzacher, M.; Wagner, A.; Parapatics, K.; Majek, P.; Mueller, A.C.; Lichtenauer, A.; Bennett, K.L.; Alper, S.L.; et al. Effects of alanyl-glutamine treatment on the peritoneal dialysis effluent proteome reveal pathomechanism-associated molecular signatures. Mol. Cell Proteomics 2018, 17, 516–532. [Google Scholar] [CrossRef]
- Herzog, R.; Kuster, L.; Becker, J.; Gluexam, T.; Pils, D.; Spittler, A.; Bhasin, M.K.; Alper, S.L.; Vychytil, A.; Aufricht, C.; et al. Functional and transcriptomic characterization of peritoneal immune-modulation by addition of alanyl-glutamine to dialysis fluid. Sci. Rep. 2017, 7, 6229. [Google Scholar] [CrossRef]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C.; et al. Addition of alanyl-glutamine to dialysis fluid restores peritoneal cellular stress responses—A first-in-man trial. PLoS ONE 2016, 11, e0165045. [Google Scholar] [CrossRef]
- Wiesenhofer, F.M.; Herzog, R.; Boehm, M.; Wagner, A.; Unterwurzacher, M.; Kasper, D.C.; Alper, S.L.; Vychytil, A.; Aufricht, C.; Kratochwill, K. Targeted metabolomic profiling of peritoneal dialysis effluents shows anti-oxidative capacity of alanyl-glutamine. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- European Technology Platform Photonics21. Towards 2020—Photonics driving economic growth in Europe. In Multiannual Strategic Roadmap 2014–2020; European Technology Platform Photonics21: Brussels, Belgium, 2013. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Barlev, E.; Zelig, U.; Bar, O.; Segev, C.; Mordechai, S.; Kapelushnik, J.; Nathan, I.; Flomen, F.; Kashtan, H.; Dickman, R.; et al. A novel method for screening colorectal cancer by infrared spectroscopy of peripheral blood mononuclear cells and plasma. J. Gastroenterol. 2016, 51, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Trevisan, J.; Owens, G.; Keating, P.J.; Wood, N.J.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Fourier-transform infrared spectroscopy coupled with a classification machine for the analysis of blood plasma or serum: A novel diagnostic approach for ovarian cancer. Analyst 2013, 138, 3917–3926. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Snowden, J.S.; Saxon, J.A.; Richardson, A.M.T.; Jones, M.; Mann, D.M.A.; Allsop, D.; Martin-Hirsch, P.L.; et al. Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7929–E7938. [Google Scholar] [CrossRef]
- Jensen, P.S.; Bak, J.; Ladefoged, S.; Andersson-Engels, S. Determination of urea, glucose, and phosphate in dialysate with Fourier transform infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 899–905. [Google Scholar] [CrossRef]
- Jessen, T.E.; Hoskuldsson, A.T.; Bjerrum, P.J.; Verder, H.; Sorensen, L.; Bratholm, P.S.; Christensen, B.; Jensen, L.S.; Jensen, M.A. Simultaneous determination of glucose, triglycerides, urea, cholesterol, albumin and total protein in human plasma by Fourier transform infrared spectroscopy: Direct clinical biochemistry without reagents. Clin. Biochem. 2014, 47, 1306–1312. [Google Scholar] [CrossRef]
- Oleszko, A.; Olsztynska-Janus, S.; Walski, T.; Grzeszczuk-Kuc, K.; Bujok, J.; Galecka, K.; Czerski, A.; Witkiewicz, W.; Komorowska, M. Application of FTIR-ATR Spectroscopy to determine the extent of lipid peroxidation in plasma during haemodialysis. Biomed. Res. Int. 2015, 2015, 245607. [Google Scholar] [CrossRef]
- Naumann, D.; Helm, D.; Labischinski, H. Microbiological characterizations by FT-IR spectroscopy. Nature 1991, 351, 81–82. [Google Scholar] [CrossRef]
- Naumann, D. Infrared spectroscopy in microbiology. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Socrates, G. (Ed.) Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley and Sons: Chichester, UK, 2001; Volume 3. [Google Scholar]
- Trevisan, J.; Angelov, P.P.; Carmichael, P.L.; Scott, A.D.; Martin, F.L. Extracting biological information with computational analysis of Fourier-transform infrared (FTIR) biospectroscopy datasets: Current practices to future perspectives. Analyst 2012, 137, 3202–3215. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Monahan, A.; Lassnig, C.; Vogl, C.; Müller, M.; Ehling-Schulz, M. Deciphering host genotype-specific impacts on the metabolic fingerprint of Listeria monocytogenes by FTIR spectroscopy. PLoS ONE 2014, 9, e115959. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Stephan, R.; Ehling-Schulz, M.; Johler, S. Fourier transform infrared spectroscopy enables rapid differentiation of fresh and frozen/thawed chicken. Food Control 2016, 60, 361–364. [Google Scholar] [CrossRef]
- Mester, P.; Jehle, A.K.; Leeb, C.; Kalb, R.; Grunert, T.; Rossmanith, P. FTIR metabolomic fingerprint reveals different modes of action exerted by active pharmaceutical ingredient based ionic liquids (API-ILs) on Salmonella typhimurium. RSC Adv. 2016, 6, 32220–32227. [Google Scholar] [CrossRef]
- Helm, D.; Labischinski, H.; Schallehn, G.; Naumann, D. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J. Gen. Microbiol. 1991, 137, 69–79. [Google Scholar] [CrossRef]
- Allwood, J.W.; Chandra, S.; Xu, Y.; Dunn, W.B.; Correa, E.; Hopkins, L.; Goodacre, R.; Tobin, A.K.; Bowsher, C.G. Profiling of spatial metabolite distributions in wheat leaves under normal and nitrate limiting conditions. Phytochemistry 2015, 115, 99–111. [Google Scholar] [CrossRef]
- Duarte, I.F.; Barros, A.; Almeida, C.; Spraul, M.; Gil, A.M. Multivariate analysis of NMR and FTIR data as a potential tool for the quality control of beer. J. Agric. Food Chem. 2004, 52, 1031–1038. [Google Scholar] [CrossRef]
- Lambie, M.; Chess, J.; Donovan, K.L.; Kim, Y.L.; Do, J.Y.; Lee, H.B.; Noh, H.; Williams, P.F.; Williams, A.J.; Davison, S.; et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J. Am. Soc. Nephrol. 2013, 24, 2071–2080. [Google Scholar] [CrossRef]
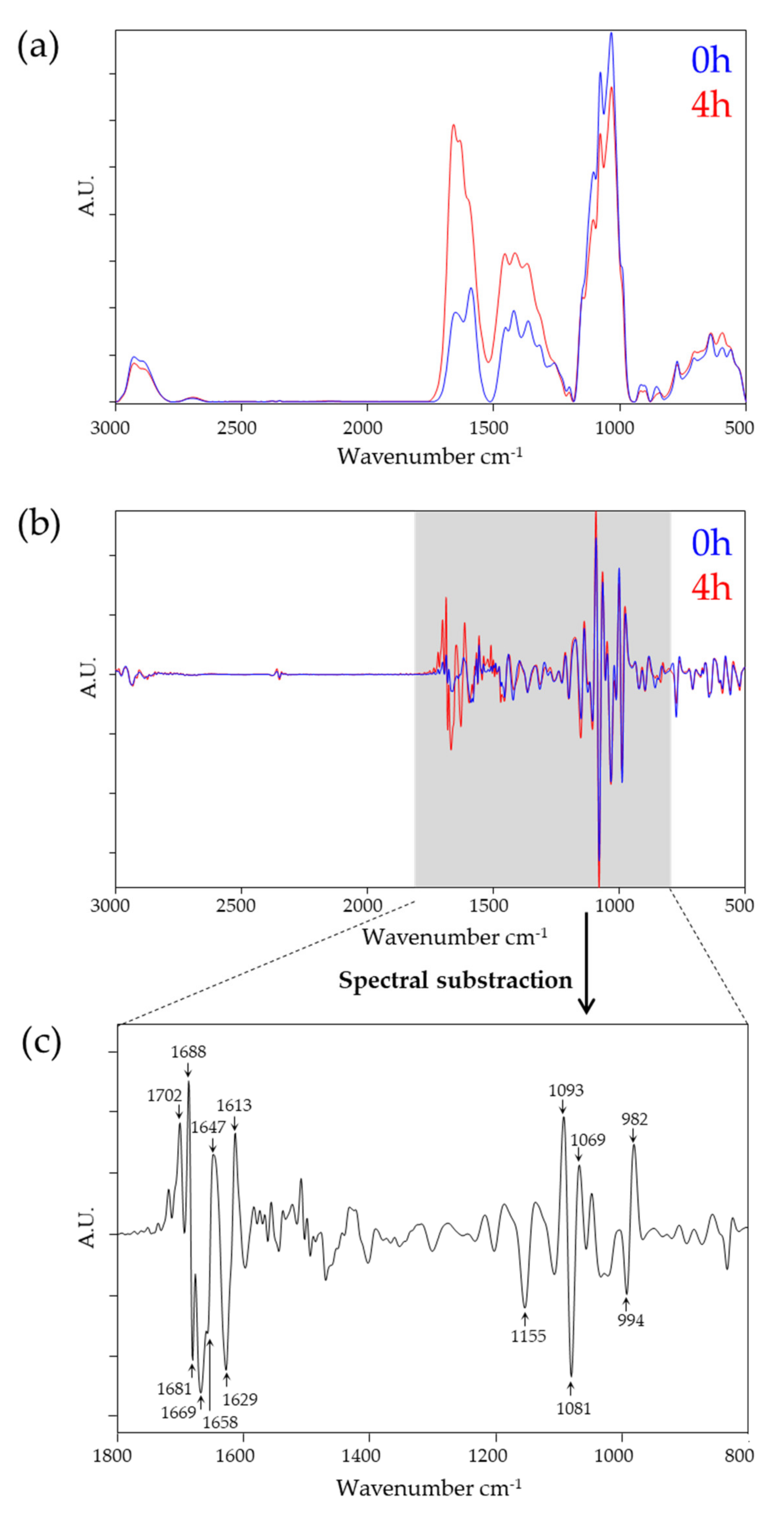
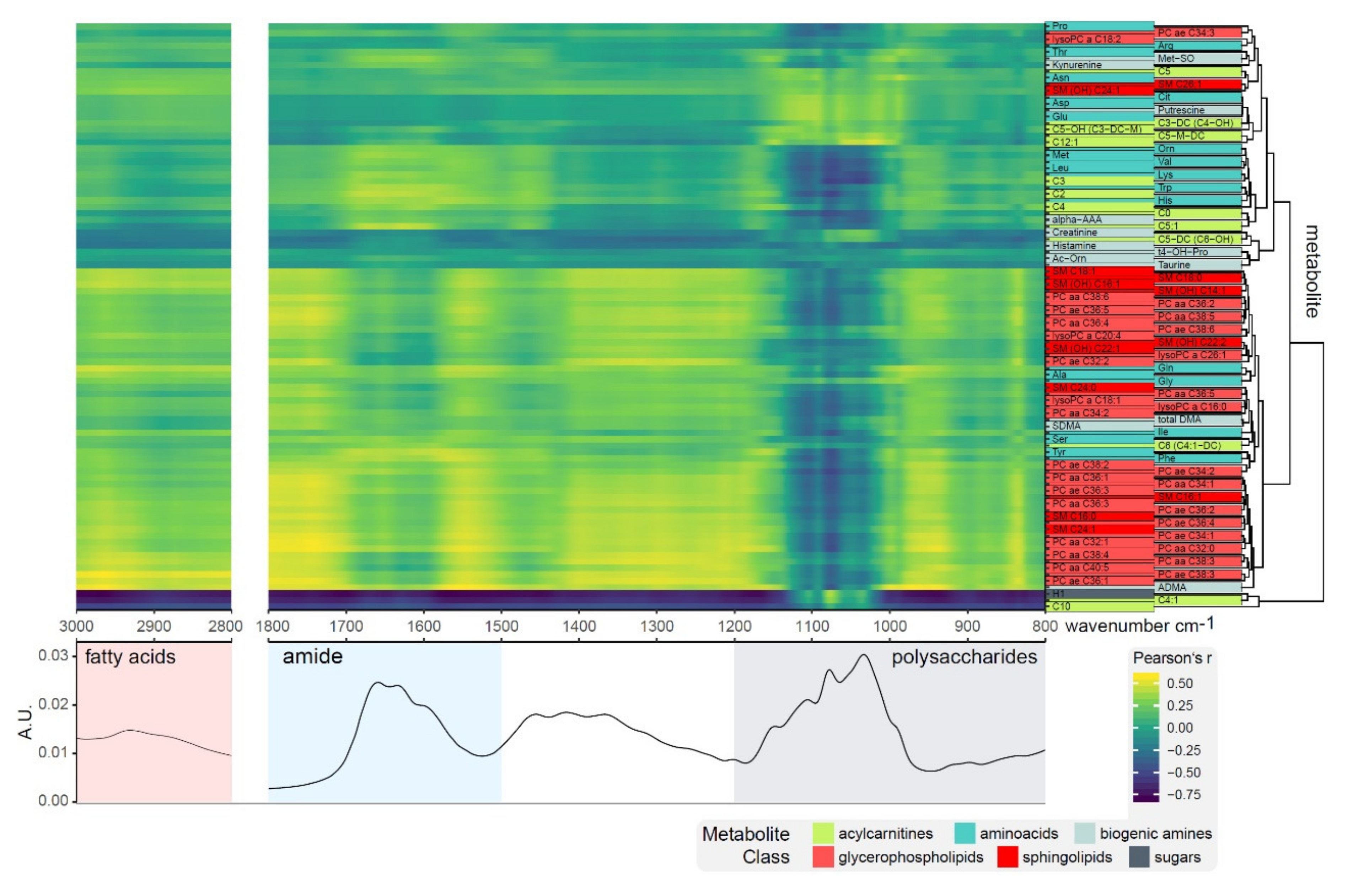
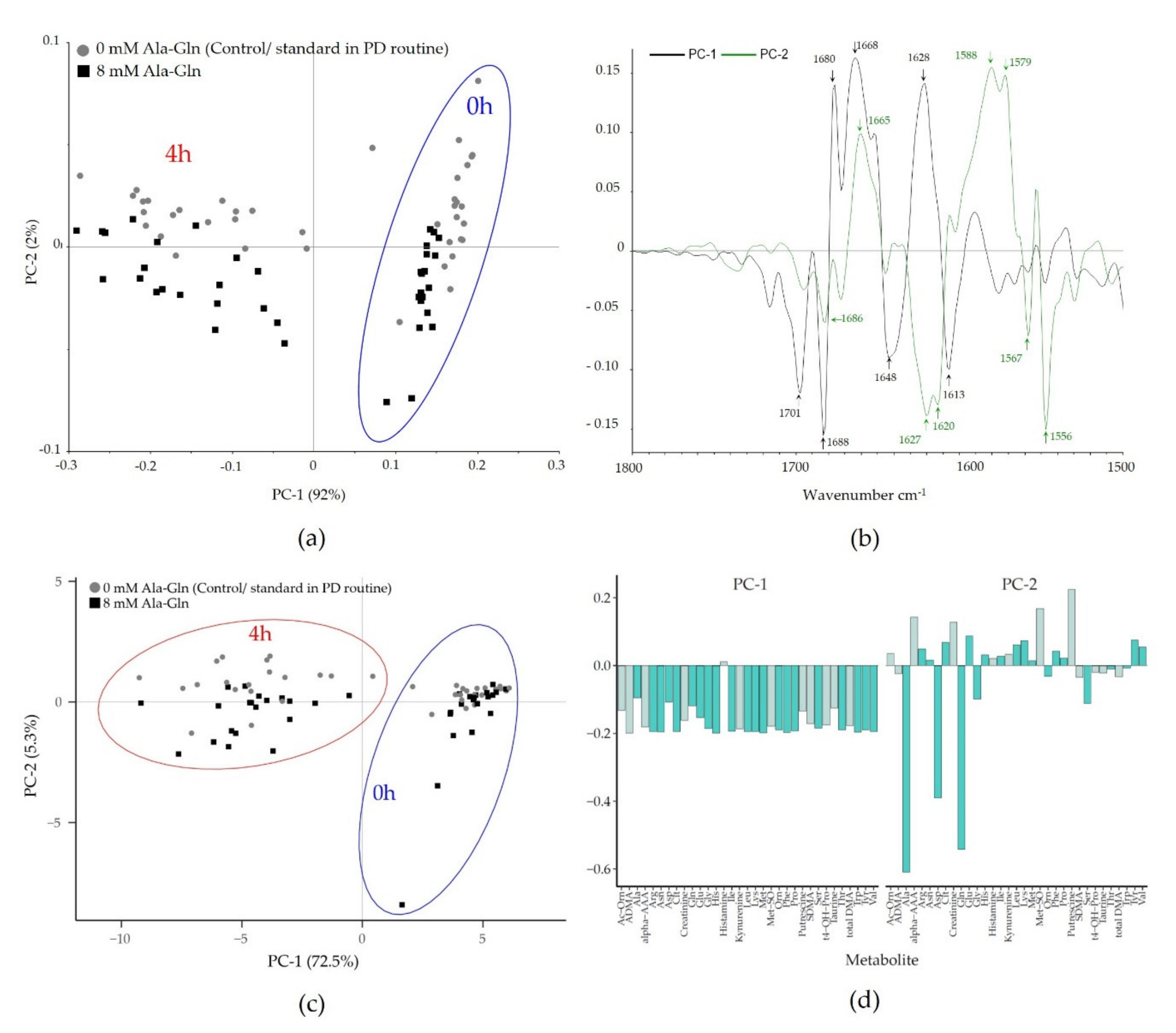
| Frequency (cm−1) | Spectral Region | Assignment |
|---|---|---|
| 3000–2800 | Fatty acids | dominated by fatty acid chains (e.g., phospholipids) |
| 1800–1500 | Proteins | dominated by conformation-sensitive amide I and amide II bands of proteins and peptides |
| 1500–1200 | Proteins and fatty acids (’mixed region’) | a region with complex absorption profiles from proteins as well as fatty acids |
| 1230/1085 | Phosphorus-containing biomolecules | detects vibrations of, for example, phospholipids or other phosphorus-containing biomolecules |
| 1200–800 | Polysaccharides | dominated by vibrations of various oligo- and poly-saccharides and their specific type of glycosidic linkages |
| Protein Region | Polysaccharide Region | |||
|---|---|---|---|---|
| (1800–1500 cm−1) | (1200–800 cm−1) | |||
| (A) Patient-related data | LDA | MDA | LDA | MDA |
| Age (<60/≥60 years) | ||||
| control, 4 h (n = 20) | 80.0 | 100.0 | 95.0 | 100.0 |
| all, 4 h (n = 40) | 70.0 | 67.5 | 72.5 | 60.0 |
| Sex (female/male) | ||||
| control, 4 h (n = 20) | 78.0 | 64.3 | 78.0 | 71.4 |
| all, 4 h (n = 40) | 45.8 | 66.7 | 45.8 | 49.0 |
| (B) PD-related data | LDA | MDA | LDA | MDA |
| Modality (CAPD/APD-Cycler) | ||||
| control, 4 h (n = 20) | 80.0 | 90.0 | 95.0 | 95.0 |
| all, 4 h (n = 40) | 87.5 | 77.5 | 77.5 | 82.5 |
| Glucose/Icodextrin | ||||
| control, 4 h (n = 20) | n.d. | n.d. | n.d. | n.d. |
| all, 4 h (n = 40) | 64.1 | 50.0 | 81.3 | 50.0 |
| Time on PD (<1/≥1 year) | ||||
| control, 4 h (n = 20) | n.d. | n.d. | n.d. | n.d. |
| all, 4 h (n = 40) | 68.3 | 35.0 | 66.7 | 46.7 |
| UrinVolOut (</≥1000 mL) | ||||
| control, 4 h (n = 20) | 93.8 | 100.0 | 75.0 | 93.8 |
| all, 4 h (n = 40) | 69.8 | 70.8 | 62.5 | 66.7 |
| UF (</≥550 mL) | ||||
| control, 4 h (n = 20) | 75.0 | 95.0 | 90.0 | 100.0 |
| all, 4 h (n = 40) | 63.6 | 62.4 | 65.2 | 56.8 |
| ResidualCl (</≥3 mL/min/1.73 m2) | ||||
| control, 4 h (n = 20) | 89.0 | 85.7 | 78.0 | 57.1 |
| all, 4 h (n = 40) | 70.7 | 71.3 | 50.7 | 65.3 |
| DP Crea (</≥ 0.8 (-)) | ||||
| control, 4 h (n = 20) | 85.4 | 95.5 | 94.4 | 100.0 |
| all, 4 h (n = 40) | 76.7 | 65.3 | 71.3 | 78.0 |
| (C) Immunesystem-related data | LDA | MDA | LDA | MDA |
| IL-8 (</≥3 pg/mL) | ||||
| control, 4 h (n = 20) | 66.2 | 83.3 | 66.2 | 83.3 |
| all, 4 h (n = 40) | 45.3 | 50.3 | 54.7 | 44.4 |
| IL-6 (</≥200 pg/mL) | ||||
| control, 4 h (n = 20) | 85.7 | 50.0 | 78.0 | 50.0 |
| all, 4 h (n = 40) | 70.8 | 57.7 | 67.9 | 57.7 |
| Hsp-72 (</≥0.5 AU) | ||||
| control, 4 h (n = 20) | 100.0 | 100.0 | 79.8 | 100.0 |
| all, 4 h (n = 40) | 63.4 | 44.5 | 50.3 | 59.8 |
| Previous peritonitis (yes/no) | ||||
| control, 4 h (n = 20) | n.d. | n.d. | n.d. | n.d. |
| all, 4 h (n = 40) | 53.3 | 48.3 | 66.7 | 51.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunert, T.; Herzog, R.; Wiesenhofer, F.M.; Vychytil, A.; Ehling-Schulz, M.; Kratochwill, K. Vibrational Spectroscopy of Peritoneal Dialysis Effluent for Rapid Assessment of Patient Characteristics. Biomolecules 2020, 10, 965. https://doi.org/10.3390/biom10060965
Grunert T, Herzog R, Wiesenhofer FM, Vychytil A, Ehling-Schulz M, Kratochwill K. Vibrational Spectroscopy of Peritoneal Dialysis Effluent for Rapid Assessment of Patient Characteristics. Biomolecules. 2020; 10(6):965. https://doi.org/10.3390/biom10060965
Chicago/Turabian StyleGrunert, Tom, Rebecca Herzog, Florian M. Wiesenhofer, Andreas Vychytil, Monika Ehling-Schulz, and Klaus Kratochwill. 2020. "Vibrational Spectroscopy of Peritoneal Dialysis Effluent for Rapid Assessment of Patient Characteristics" Biomolecules 10, no. 6: 965. https://doi.org/10.3390/biom10060965
APA StyleGrunert, T., Herzog, R., Wiesenhofer, F. M., Vychytil, A., Ehling-Schulz, M., & Kratochwill, K. (2020). Vibrational Spectroscopy of Peritoneal Dialysis Effluent for Rapid Assessment of Patient Characteristics. Biomolecules, 10(6), 965. https://doi.org/10.3390/biom10060965






