The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Methamphetamine Treatment
2.3. Behavioural Testing
2.3.1. Social Interaction and Social Preference
2.3.2. Prepulse Inhibition of Acoustic Startle (PPI)
2.3.3. Locomotor Activity and Meth-Induced Locomotor Hyperactivity
2.4. Data Analysis
3. Results
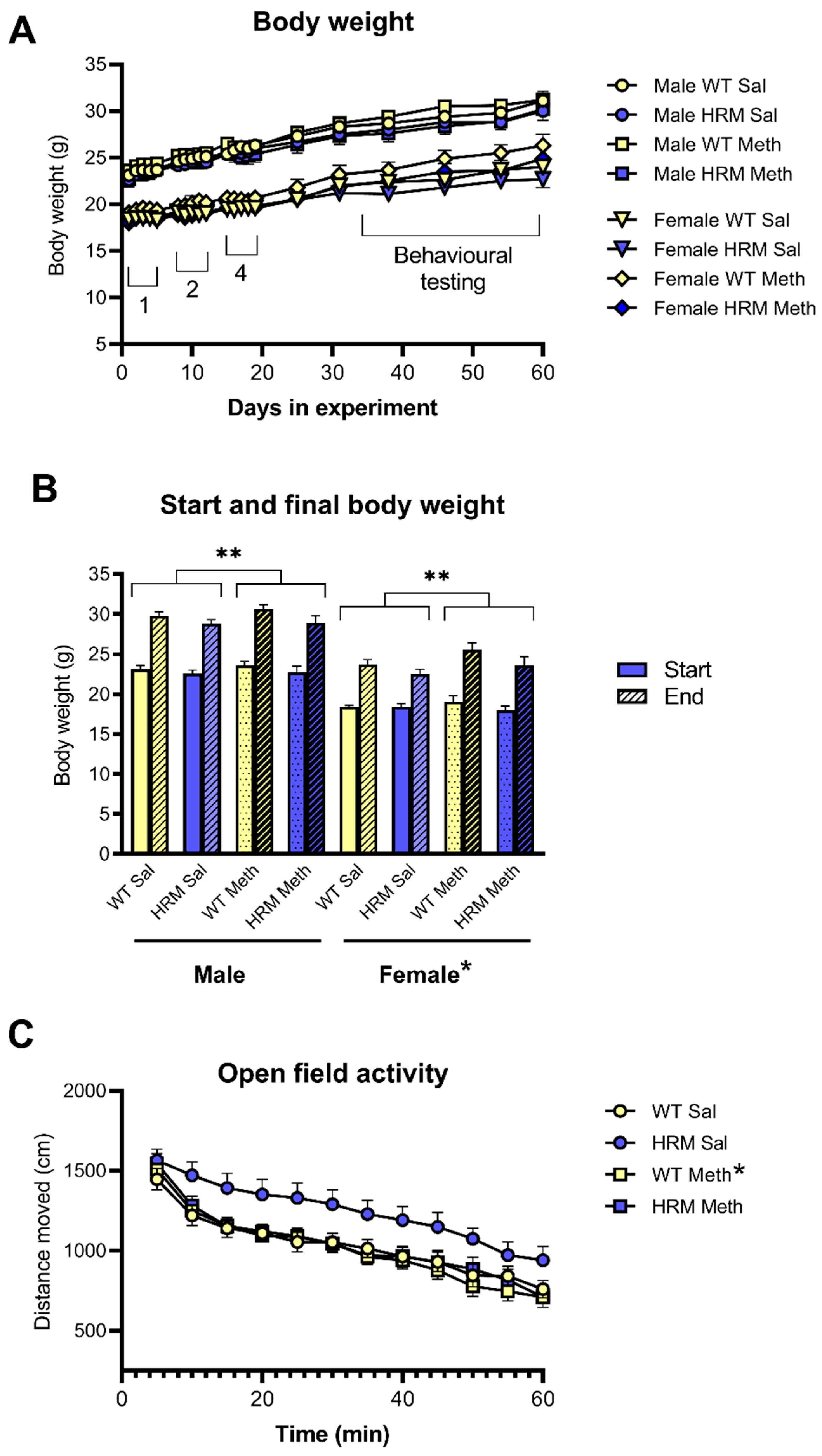
3.1. Body Weight
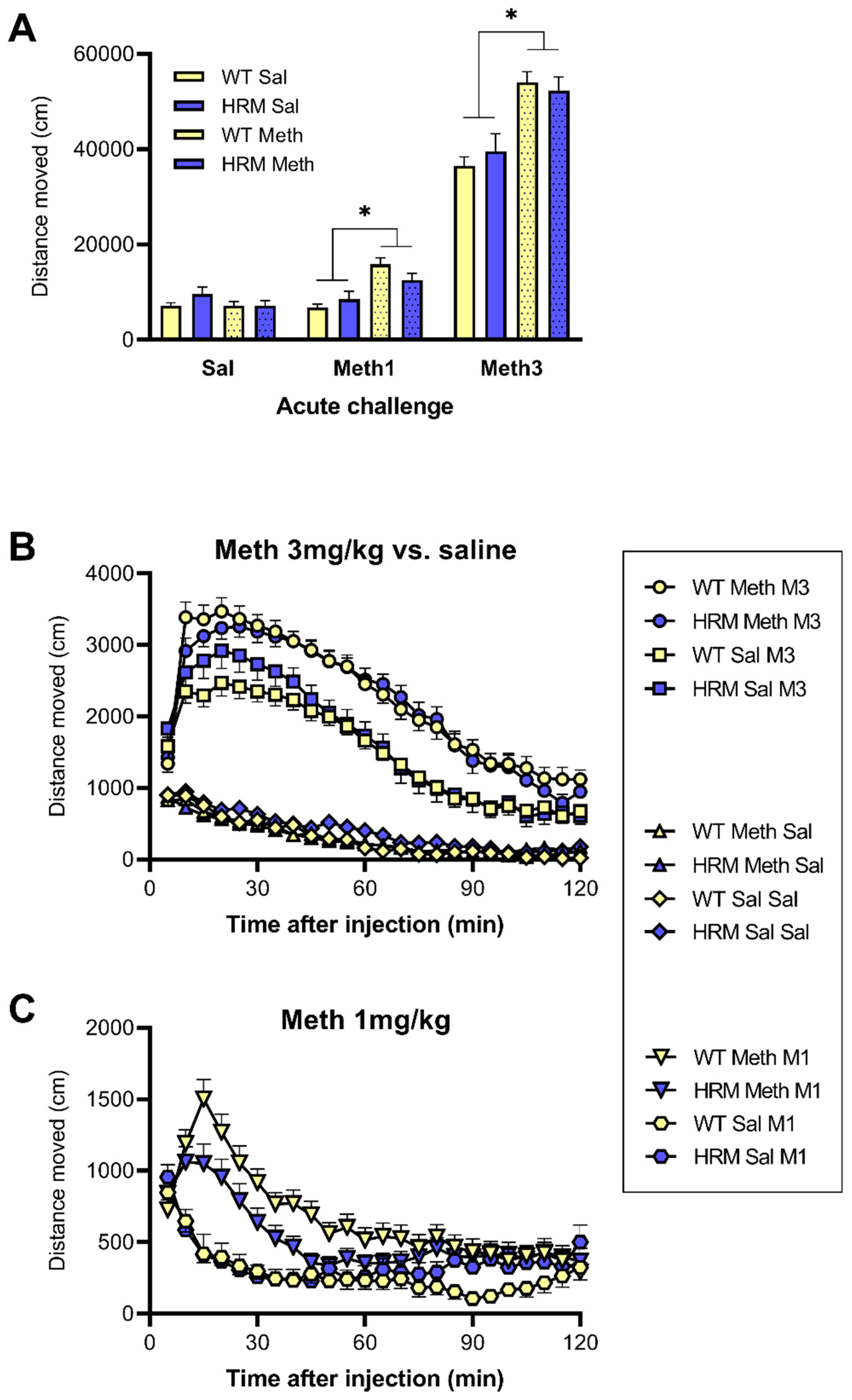
3.2. Spontaneous Locomotor Activity in WT and HRM
3.3. Locomotor Hyperactivity Induced by Acute Meth Treatment in WT and HRM
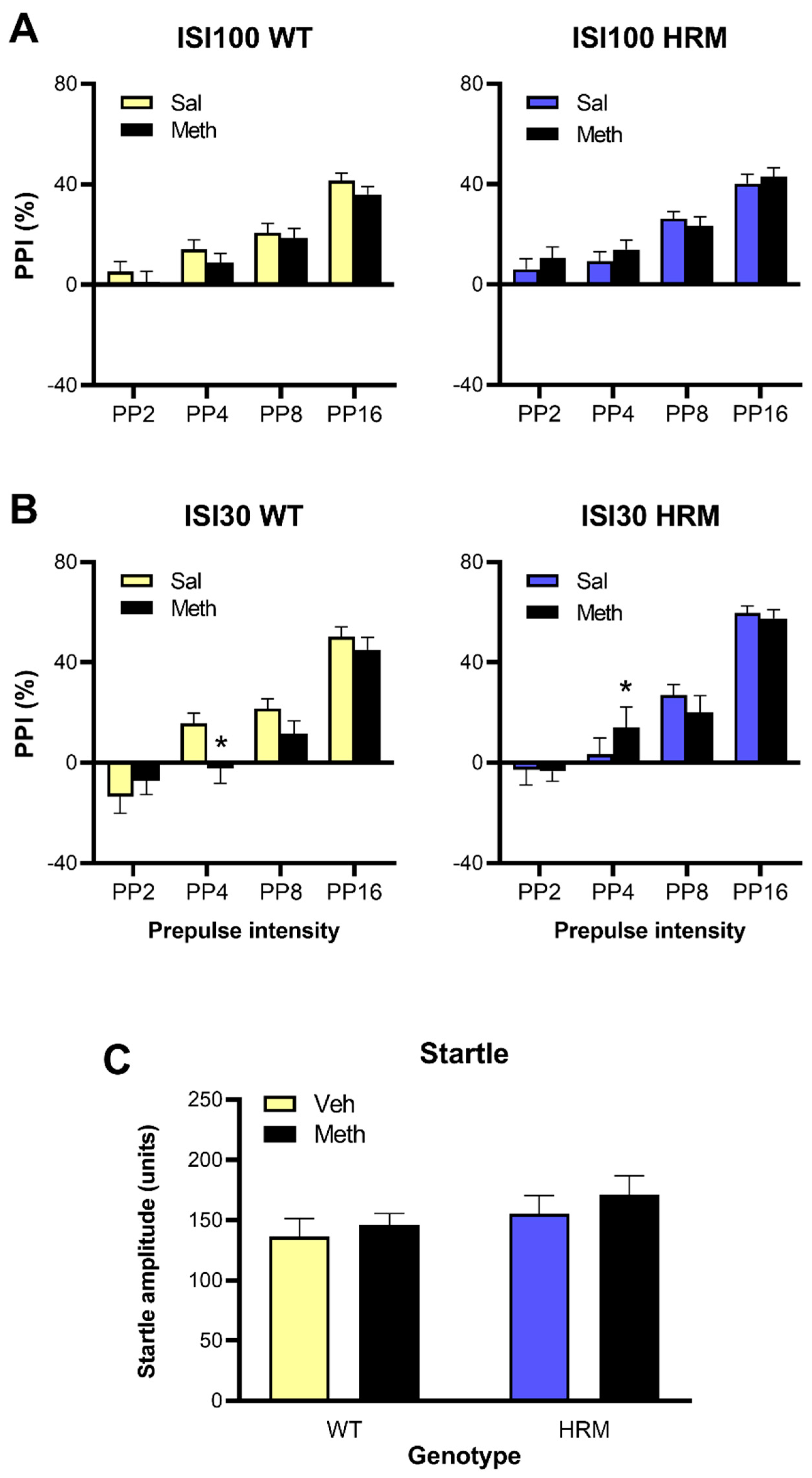
3.4. Effect of Meth Pretreatment on PPI in WT and HRM
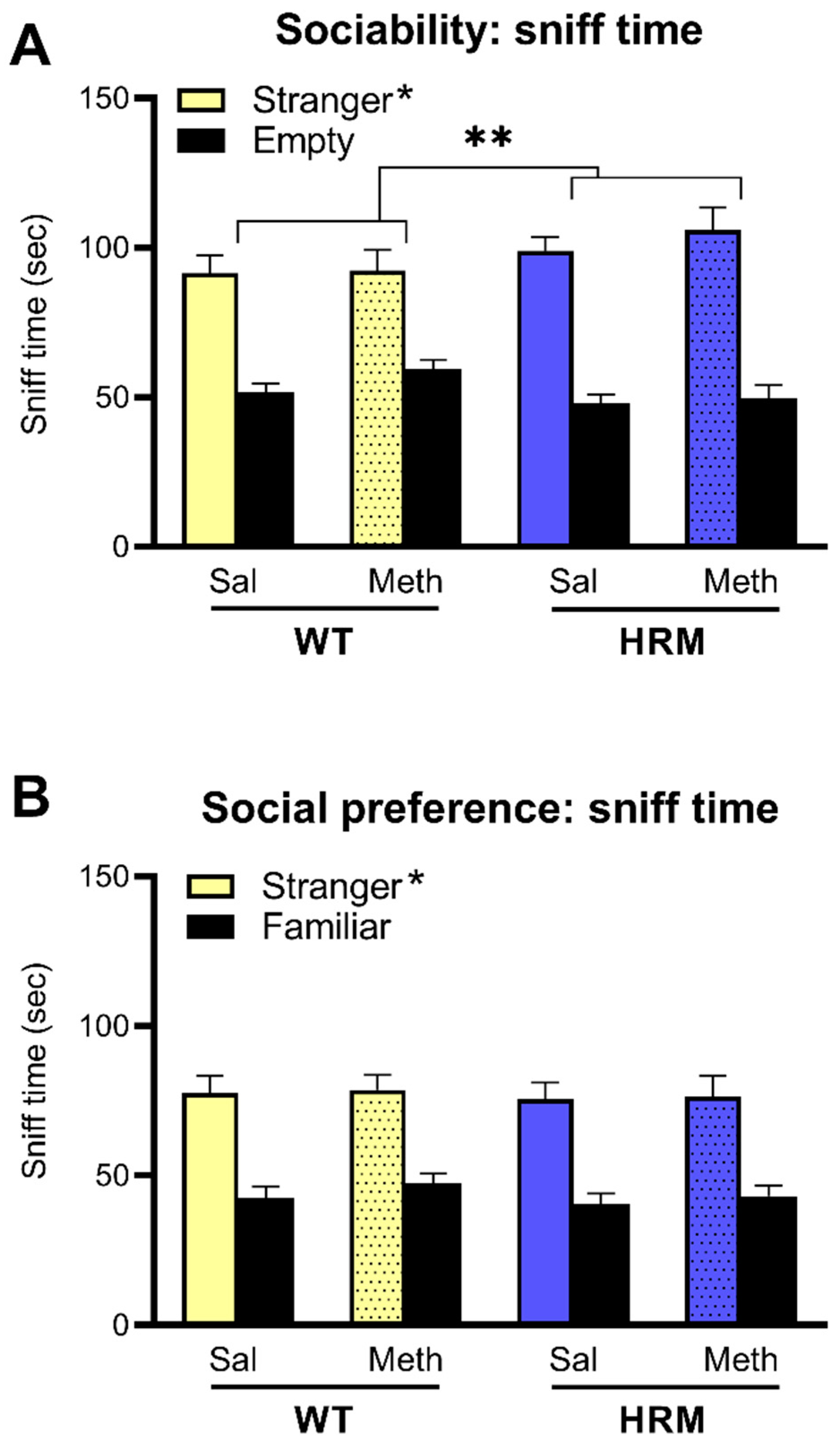
3.5. Effect of Meth Pretreatment on Sociability and Social Preference in WT and HRM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- D’Arcangelo, G. Reelin in the Years: Controlling Neuronal Migration and Maturation in the Mammalian Brain. Adv. Neurosci. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Lakatosova, S.; Ostatníková, D. Reelin and its complex involvement in brain development and function. Int. J. Biochem. Cell Boil. 2012, 44, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, A.; Auta, J.; Davis, J.M.; Dwivedi, Y.; Grayson, D.R.; Impagnatiello, F.; Pandey, G.; Pesold, C.; Sharma, R.; Uzunov, D.; et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch. Gen. Psychiatry 2000, 57, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Zhao, L.; Trotter, J.H.; Rusiana, I.; Peters, M.M.; Li, Q.; Donaldson, E.; Banko, J.L.; Keenoy, K.E.; Rebeck, G.W.; et al. Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J. Psychopharmacol. 2012, 27, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kubo, K.-I.; Nakajima, K. Reelin and Neuropsychiatric Disorders. Front. Cell. Neurosci. 2016, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Buret, L.; van den Buuse, M. Corticosterone treatment during adolescence induces down-regulation of reelin and NMDA receptor subunit GLUN2C expression only in male mice: Implications for schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 1221–1232. [Google Scholar] [CrossRef]
- Qiu, S.; Korwek, K.M.; Pratt-Davis, A.R.; Peters, M.; Bergman, M.Y.; Weeber, E.J. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol. Learn. Mem. 2006, 85, 228–242. [Google Scholar] [CrossRef]
- Costa, E. The heterozygote reeler mouse as a model for the development of a new generation of antipsychotics. Curr. Opin. Pharmacol. 2002, 2, 56–62. [Google Scholar] [CrossRef]
- Tueting, P.; Costa, E.; Dwivedi, Y.; Guidotti, A.; Impagnatiello, F.; Manev, R.; Pesold, C. The phenotypic characteristics of heterozygous reeler mouse. NeuroReport 1999, 10, 1329–1334. [Google Scholar] [CrossRef]
- Tueting, P.; Doueiri, M.; Guidotti, A.; Davis, J.; Costa, E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci. Biobehav. Rev. 2006, 30, 1065–1077. [Google Scholar] [CrossRef]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M.; Abi-Dargham, A. Dopamine as the wind of the psychotic fire: New evidence from brain imaging studies. J. Psychopharmacol. 1999, 13, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Gil, R.; Krystal, J.; Baldwin, R.M.; Seibyl, J.P.; Bowers, M.; van Dyck, C.H.; Charney, D.S.; Innis, R.B.; Laruelle, M. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am. J. Psychiatry 1998, 155, 761–767. [Google Scholar] [PubMed]
- Breier, A.; Su, T.-P.; Saunders, R.; Carson, R.E.; Kolachana, B.S.; de Bartolomeis, A.; Weinberger, D.R.; Weisenfeld, N.; Malhotra, A.K.; Eckelman, W.C.; et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. USA 1997, 94, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q. J. Nucl. Med. 1998, 42, 211–221. [Google Scholar]
- Weidenauer, A.; Bauer, M.; Sauerzopf, U.; Bartova, L.; Praschak-Rieder, N.; Sitte, H.H.; Kasper, S.; Willeit, M. Making Sense of: Sensitization in Schizophrenia. Int. J. Neuropsychopharmacol. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Ballmaier, M.; Zoli, M.; Leo, G.; Agnati, L.F.; Spano, P. Preferential alterations in the mesolimbic dopamine pathway of heterozygous reeler mice: An emerging animal-based model of schizophrenia. Eur. J. Neurosci. 2002, 15, 1197–1205. [Google Scholar] [CrossRef]
- Varela, M.; Lage, S.; Caruncho, H.; Cadavid, M.; Loza, M.; Brea, J. Reelin influences the expression and function of dopamine D2 and serotonin 5-HT2A receptors: A comparative study. Neuroscience 2015, 290, 165–174. [Google Scholar] [CrossRef]
- Michetti, C.; Romano, E.; Altabella, L.; Caruso, A.; Castelluccio, P.; Bedse, G.; Gaetani, S.; Canese, R.; Laviola, G.; Scattoni, M.L. Mapping Pathological Phenotypes in Reelin Mutant Mice. Front. Pediatr. 2014, 2, 95. [Google Scholar] [CrossRef][Green Version]
- van den Buuse, M.; Halley, P.; A Hill, R.; Labots, M.; Martin, S. Altered N-methyl-d-aspartate receptor function in reelin heterozygous mice: Male–female differences and comparison with dopaminergic activity. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2012, 37, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive Effects of Methamphetamine: A Critical Review and Meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Vearrier, D.; Greenberg, M.I.; Miller, S.N.; Okaneku, J.T.; Haggerty, D.A. Methamphetamine: History, Pathophysiology, Adverse Health Effects, Current Trends, and Hazards Associated with the Clandestine Manufacture of Methamphetamine. Disease 2012, 58, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, Z.; A Rikani, A.; Choudhry, A.M.; Tariq, S.; Zakaria, F.; Anwar, S.; Laghari, M.H.; Haider, K.; Shafiq, A.A.; Mobassarah, N.J. Pharmacology, neurobiology, and neurotoxicity of methamphetamine. El Mednifico J. 2014, 2, 15. [Google Scholar] [CrossRef]
- Miyazaki, M.; Noda, Y.; Mouri, A.; Kobayashi, K.; Mishina, M.; Nabeshima, T.; Yamada, K. Role of convergent activation of glutamatergic and dopaminergic systems in the nucleus accumbens in the development of methamphetamine psychosis and dependence. Int. J. Neuropsychopharmacol. 2013, 16, 1341–1350. [Google Scholar] [CrossRef]
- Weidenauer, A.; Bauer, M.; Sauerzopf, U.; Bartova, L.; Nics, L.; Pfaff, S.; Philippe, C.; Berroterán-Infante, N.; Pichler, V.; Meyer, B.M.; et al. On the relationship of first-episode psychosis to the amphetamine-sensitized state: A dopamine D2/3 receptor agonist radioligand study. Transl. Psychiatry 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Grant, K.M.; le van, T.D.; Wells, S.M.; Li, M.; Stoltenberg, S.F.; Gendelman, H.E.; Carlo, G.; Bevins, R.A. Methamphetamine-Associated Psychosis. J. Neuroimmune Pharmacol. 2011, 7, 113–139. [Google Scholar] [CrossRef]
- Gururajan, A.; Manning, E.E.; Klug, M.; van den Buuse, M. Drugs of abuse and increased risk of psychosis development. Aust. New Zealand J. Psychiatry 2012, 46, 1120–1135. [Google Scholar] [CrossRef]
- Thirthalli, J.; Benegal, V. Psychosis among substance users. Curr. Opin. Psychiatry 2006, 19, 239–245. [Google Scholar] [CrossRef]
- Chen, C.-K.; Lin, S.-K.; Sham, P.C.; Ball, D.; Loh, E.-W.; Murray, R.M. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 136, 87–91. [Google Scholar] [CrossRef]
- Manning, E.E.; van den Buuse, M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front. Cell. Neurosci. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.E.; van den Buuse, M. Altered social cognition in male BDNF heterozygous mice and following chronic methamphetamine exposure. Behav. Brain Res. 2016, 305, 181–185. [Google Scholar] [CrossRef] [PubMed]
- O’Tuathaigh, C.M.P.; Kirby, B.; Moran, P.; Waddington, J.L. Mutant Mouse Models: Genotype-Phenotype Relationships to Negative Symptoms in Schizophrenia. Schizophr. Bull. 2009, 36, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.R.; Weber, M.; Qu, Y.; Light, G.A.; Braff, D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 2008, 199, 331–388. [Google Scholar] [CrossRef] [PubMed]
- van den Buuse, M. Modeling the Positive Symptoms of Schizophrenia in Genetically Modified Mice: Pharmacology and Methodology Aspects. Schizophr. Bull. 2009, 36, 246–270. [Google Scholar] [CrossRef]
- Tenn, C.C.; Fletcher, P.J.; Kapur, S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality like that of schizophrenia. Schizophr. Res. 2003, 64, 103–114. [Google Scholar] [CrossRef]
- Manning, E.E.; Halberstadt, A.L.; van den Buuse, M. BDNF-Deficient Mice Show Reduced Psychosis-Related Behaviors Following Chronic Methamphetamine. Int. J. Neuropsychopharmacol. 2015, 19. [Google Scholar] [CrossRef]
- Nadler, J.J.; Moy, S.S.; Dold, G.; Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.; Piven, J.; Magnuson, T.R.; Crawley, J.N.; et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- van den Buuse, M.; Becker, T.; Kwek, P.; Martin, S.; Ruimschotel, E.; Risbrough, V. Disruption of prepulse inhibition by 3,4-methylenedioxymethamphetamine (MDMA): Comparison between male and female wild-type and 5-HT1A receptor knockout mice. Int. J. Neuropsychopharmacol. 2011, 14, 856–861. [Google Scholar] [CrossRef]
- Powell, S.B.; Zhou, X.; Geyer, M.A. Prepulse inhibition and genetic mouse models of schizophrenia. Behav. Brain Res. 2009, 204, 282–294. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Minabe, Y.; Nakamura, K.; Suzuki, K.; Iwata, Y.; Sekine, Y.; Tsuchiya, K.J.; Sugihara, G.; Suda, S.; Takei, N.; et al. Disruption of reelin signaling attenuates methamphetamine-induced hyperlocomotion. Eur. J. Neurosci. 2007, 25, 3376–3384. [Google Scholar] [CrossRef] [PubMed]
- Salinger, W.L.; Ladrow, P.; Wheeler, C. Behavioral Phenotype of the Reeler Mutant Mouse: Effects of Reln Gene Dosage and Social Isolation. Behav. Neurosci. 2003, 117, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Hammond, V.; So, E.; Gunnersen, J.; Valcanis, H.; Kalloniatis, M.; Tan, S.-S. Layer Positioning of Late-Born Cortical Interneurons Is Dependent on Reelin But Not p35 Signaling. J. Neurosci. 2006, 26, 1646–1655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tissir, F.; Goffinet, A.M. Reelin and brain development. Nat. Rev. Neurosci. 2003, 4, 496–505. [Google Scholar] [CrossRef]
- Vaswani, A.R.; Weykopf, B.; Hagemann, C.; Fried, H.-U.; Brüstle, O.; Blaess, S. Correct setup of the substantia nigra requires Reelin-mediated fast, laterally directed migration of dopaminergic neurons. eLife 2019, 8. [Google Scholar] [CrossRef]
- Robinson, T.E.; Jurson, P.A.; Bennett, J.A.; Bentgen, K.M. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: A microdialysis study in freely moving rats. Brain Res. 1988, 462, 211–222. [Google Scholar] [CrossRef]
- Podhorna, J.; Didriksen, M. The heterozygous reeler mouse: Behavioural phenotype. Behav. Brain Res. 2004, 153, 43–54. [Google Scholar] [CrossRef]
- Braff, D.L. Information Processing and Attention Dysfunctions in Schizophrenia. Schizophr. Bull. 1993, 19, 233–259. [Google Scholar] [CrossRef]
- Braff, D.L.; Grillon, C.; A Geyer, M. Gating and Habituation of the Startle Reflex in Schizophrenic Patients. Arch. Gen. Psychiatry 1992, 49, 206. [Google Scholar] [CrossRef]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; de la Casa, L.G. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef]
- Schroeder, A.; Buret, L.; A Hill, R.; van den Buuse, M. Gene–environment interaction of reelin and stress in cognitive behaviours in mice: Implications for schizophrenia. Behav. Brain Res. 2015, 287, 304–314. [Google Scholar] [CrossRef] [PubMed]
| Sex/Genotype | Pretreatment | Locomotor Hyperactivity | PPI | Social Behaviour |
|---|---|---|---|---|
| Male WT | Saline | 12 | 12 | 11 |
| Meth | 19 | 19 | 14 | |
| Male HRM | Saline | 11 | 12 | 12 |
| Meth | 14 | 15 | 11 | |
| Female WT | Saline | 12 | 12 | 10 |
| Meth | 12 | 12 | 12 | |
| Female HRM | Saline | 11 | 11 | 11 |
| Meth | 11 | 11 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hume, C.; Massey, S.; van den Buuse, M. The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia. Biomolecules 2020, 10, 940. https://doi.org/10.3390/biom10060940
Hume C, Massey S, van den Buuse M. The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia. Biomolecules. 2020; 10(6):940. https://doi.org/10.3390/biom10060940
Chicago/Turabian StyleHume, Camilla, Shelley Massey, and Maarten van den Buuse. 2020. "The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia" Biomolecules 10, no. 6: 940. https://doi.org/10.3390/biom10060940
APA StyleHume, C., Massey, S., & van den Buuse, M. (2020). The Effect of Chronic Methamphetamine Treatment on Schizophrenia Endophenotypes in Heterozygous Reelin Mice: Implications for Schizophrenia. Biomolecules, 10(6), 940. https://doi.org/10.3390/biom10060940





