IL-22 Promotes IFN-γ-Mediated Immunity against Histoplasma capsulatum Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fungal Strain and Culture
2.3. Animals, Treatments and Infection
2.4. MiceTtreatments
2.5. Survival and Sample Collection
2.6. Determination of Fungal Burden
2.7. Flow Cytometry
2.8. Histopathological Analysis
2.9. Quantification of Cytokines by ELISA and NO2− by Griess Reaction
2.10. Quantification of LTB4 by HPLC/MS/MS
2.11. qRT PCR
2.12. Statistical Analysis
3. Results
3.1. IL-22 Is Critical for Host Resistance to H. capsulatum Infection
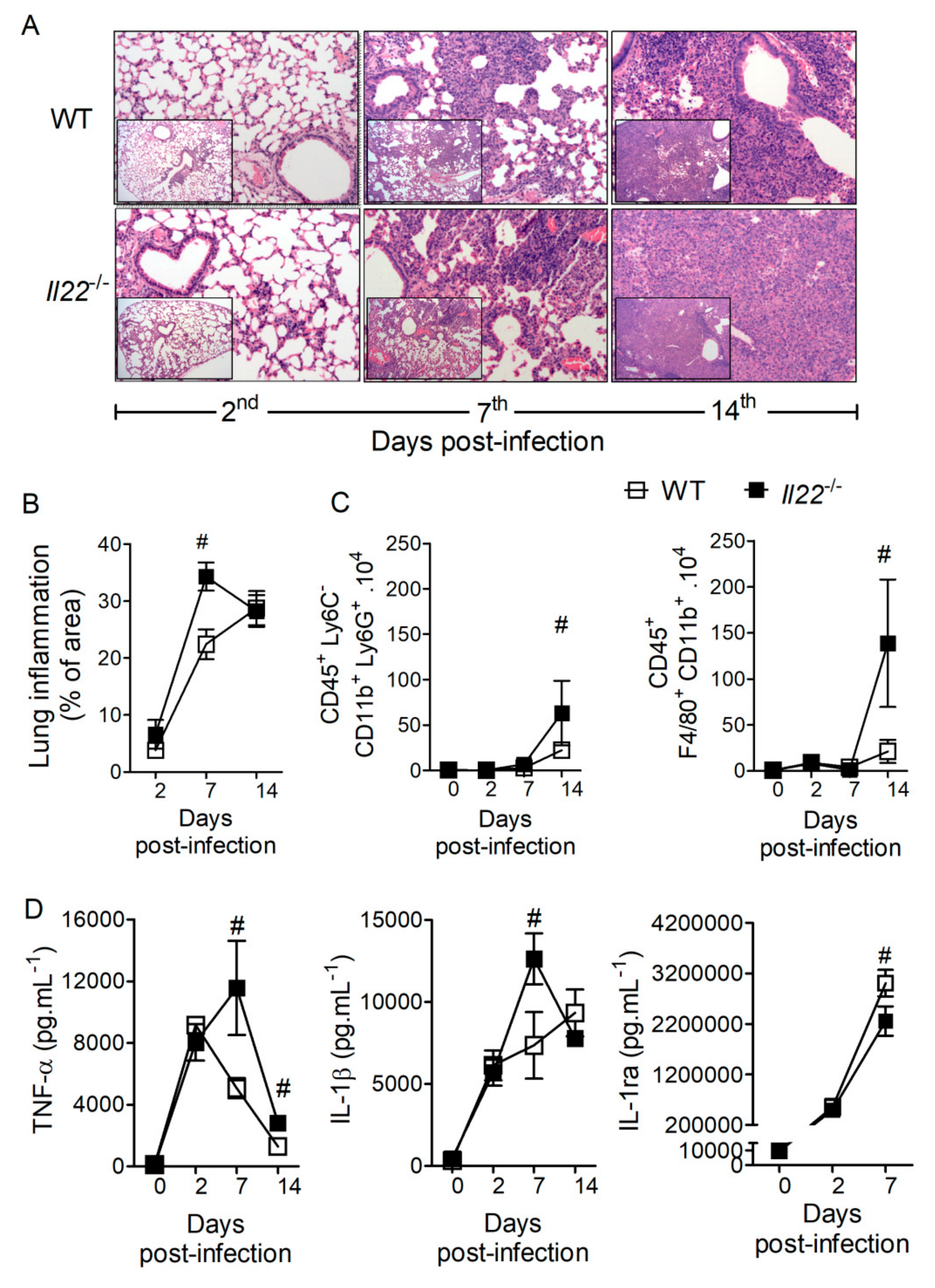
3.2. IL-22 Regulates Lung Inflammation during H. capsulatum Infection
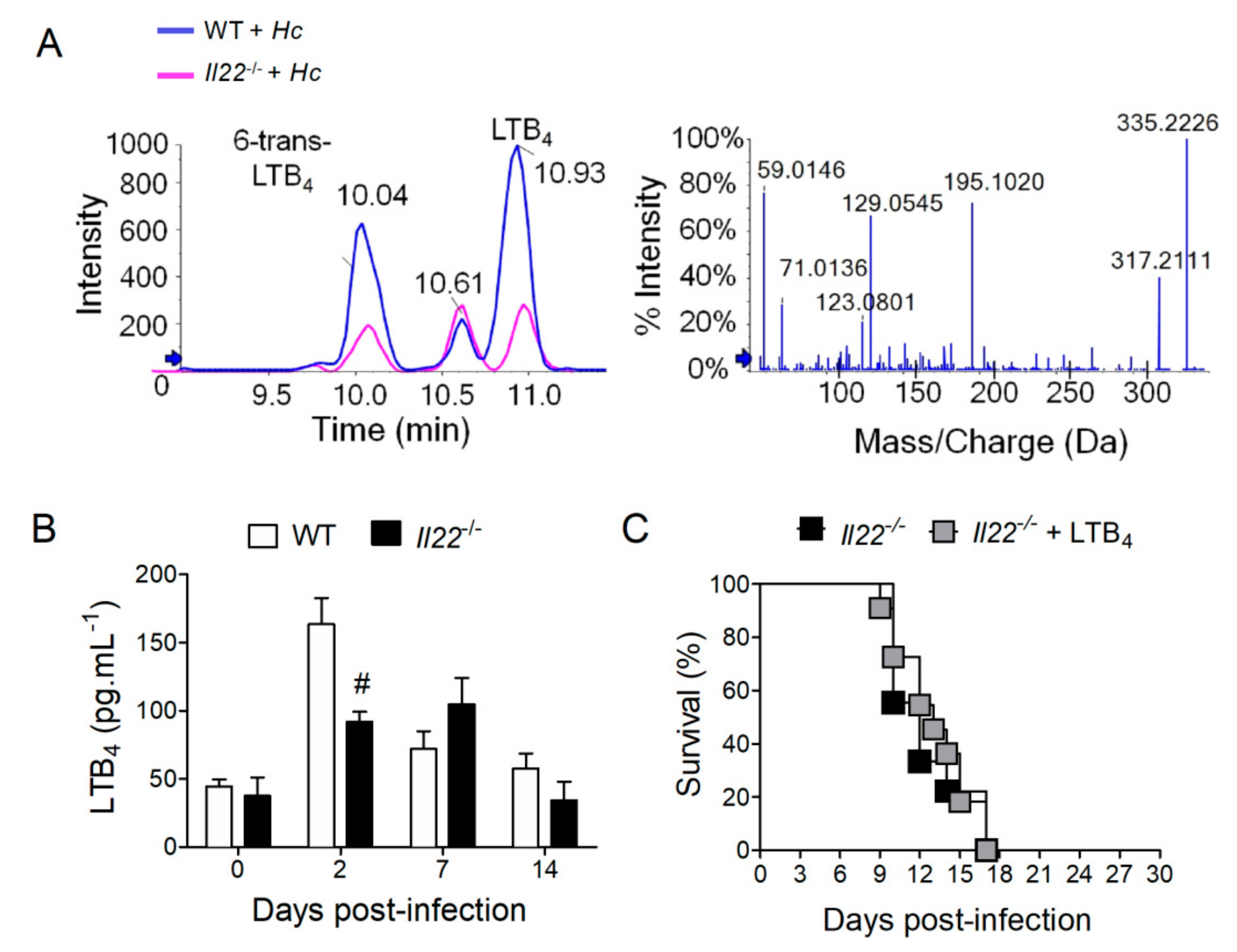
3.3. IL-22 Operates Independently of LTB4 and AMPs to Protect against H. capsulatum Infection
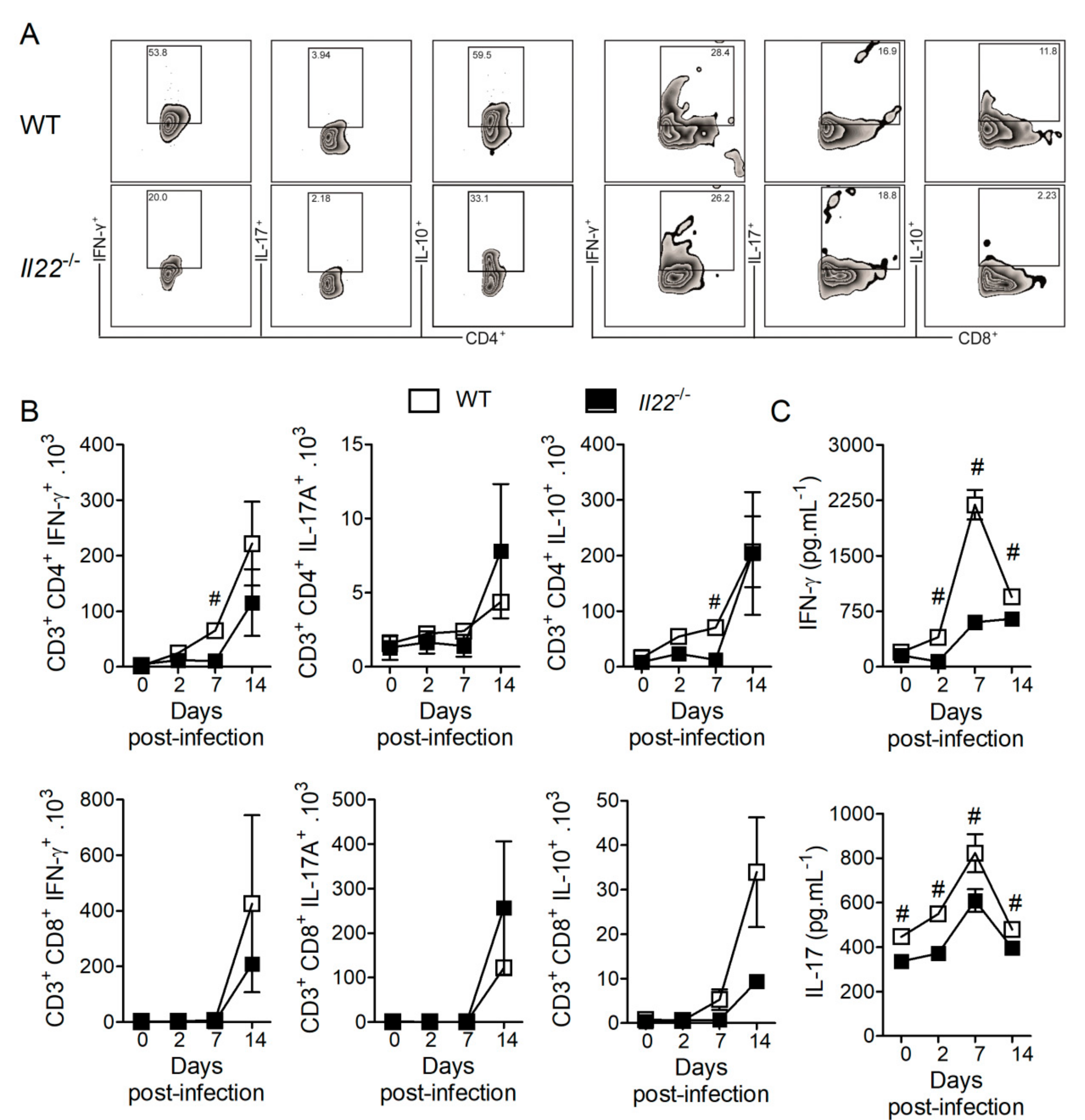
3.4. IL-22 Deficiency Impacts T Lymphocyte Responses against H. capsulatum
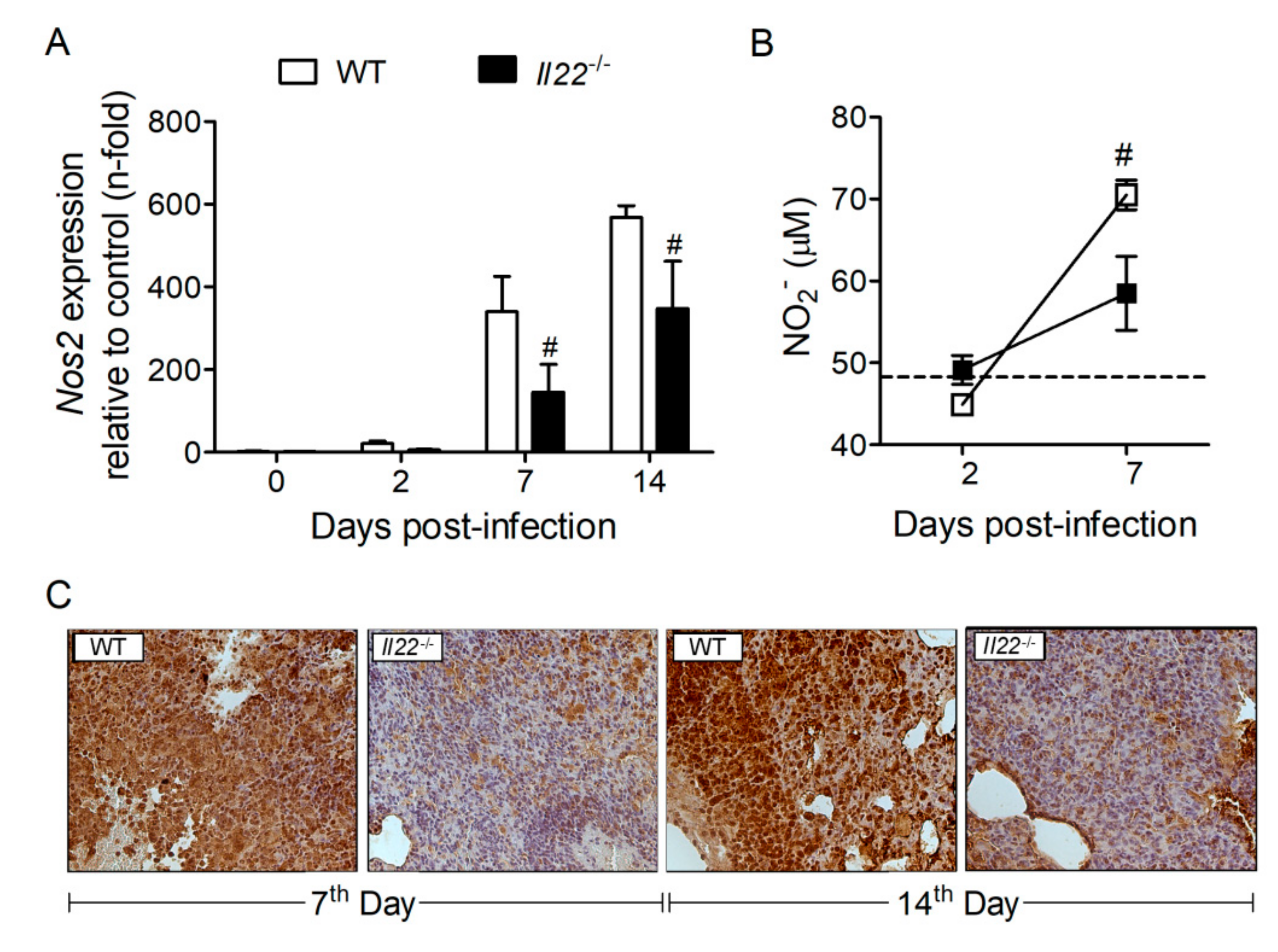
3.5. IL-22 Deficiency Impacts Nitric Oxide (NO) Production in the Lungs of H. capsulatum-Infected mMice
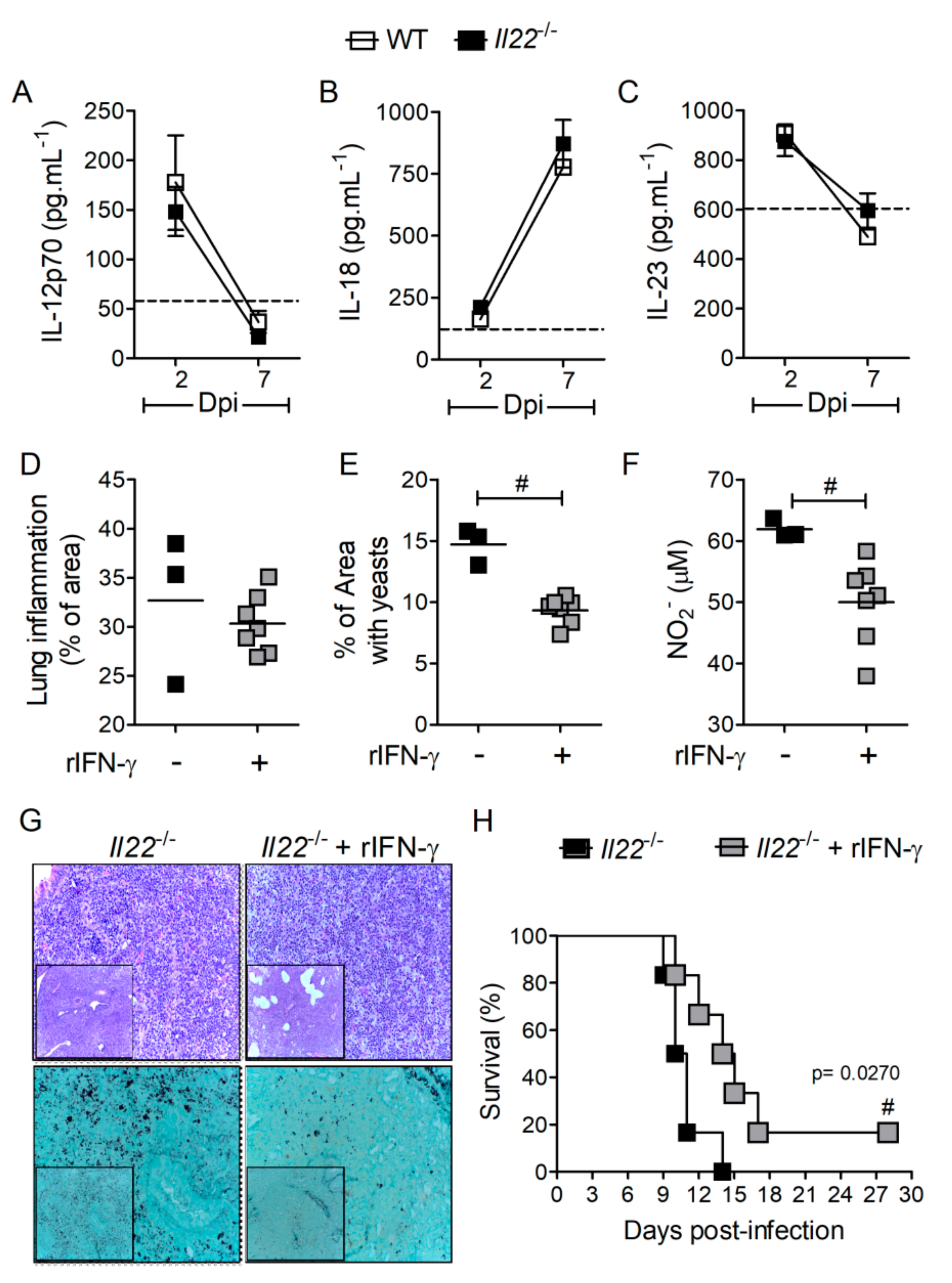
3.6. IFN-γ Treatment Rescues Survival of Il22−/− Mice during H. capsulatum Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antinori, S. Histoplasma capsulatum: More widespread than previously thought. Am. J. Trop. Med. Hyg. 2014, 90, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Tobón, A.; Restrepo, A.; Telles, F.D.Q.; Nucci, M. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 2011, 49, 1–14. [Google Scholar] [CrossRef]
- Horwath, M.A.; Fecher, R.; Deepe, G.S. Histoplasma capsulatum, lung infection and immunity. Futur. Microbiol. 2015, 10, 967–975. [Google Scholar] [CrossRef]
- Goodwin, A.R.; Shapiro, J.L.; Thurman, G.H.; Thurman, S.S.; Prez, R.M.D. Disseminated histoplasmosis: Clinical and pathologic correlations. Md. Med. J. 1980, 59, 1–33. [Google Scholar]
- McKinsey, D.S.; Spiegel, R.A.; Hutwagner, L.; Stanford, J.; Driks, M.R.; Brewer, J.; Gupta, M.R.; Smith, D.L.; O’Connor, M.C.; Dall, L. Prospective study of histoplasmosis in patients infected with human immunodeficiency virus: Incidence, risk factors, and pathophysiology. Clin. Infect. Dis. 1997, 24, 1195–1203. [Google Scholar] [CrossRef]
- Heninger, E.; Hogan, L.H.; Karman, J.; Macvilay, S.; Hill, B.; Woods, J.P.; Sandor, M. Characterization of the Histoplasma capsulatum-Induced Granuloma1. J. Immunol. 2006, 177, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Deepe, G.S. Evolution of the Primary Immune Response to Histoplasma capsulatum in Murine Lung. Infect. Immun. 1998, 66, 1473–1481. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Clustering of Pattern Recognition Receptors for Fungal Detection. PloS Pathog. 2014, 10, 1–3. [Google Scholar] [CrossRef]
- Sorgi, C.A.; Secatto, A.; Fontanari, C.; Turato, W.M.; Belangér, C.; Medeiros, A.I.; Kashima, S.; Marleau, S.; Covas, D.T.; Bozza, P.T.; et al. Histoplasma capsulatum Cell Wall β-Glucan Induces Lipid Body Formation through CD18, TLR2, and Dectin-1 Receptors: Correlation with Leukotriene B4 Generation and Role in HIV-1 Infection. J. Immunol. 2009, 182, 4025–4035. [Google Scholar] [CrossRef]
- Funk, C. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Pereira, P.A.T.; Assis, P.A.; Prado, M.K.B.; Ramos, S.G.; Aronoff, D.M.; Paula-Silva, F.; Sorgi, C.A.; Faccioli, L.H. Prostaglandins D2 and E2 have opposite effects on alveolar macrophages infected with Histoplasma capsulatum[S]. J. Lipid Res. 2017, 59, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Secatto, A.; Rodrigues, L.C.; Serezani, C.; Ramos, S.G.; Dias-Baruffi, M.; Faccioli, L.H.; Medeiros, A.I. 5-Lipoxygenase Deficiency Impairs Innate and Adaptive Immune Responses during Fungal Infection. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.K.B.; Locachevic, G.A.; Zoccal, K.F.; Paula-Silva, F.; Fontanari, C.; Ferreira, J.C.; Pereira, P.A.T.; Gardinassi, L.G.; Ramos, S.G.; Sorgi, C.A.; et al. Leukotriene B4 is essential for lung host defence and alpha-defensin-1 production during Achromobacter xylosoxidans infection. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.I.; Sá-Nunes, A.; Turato, W.M.; Secatto, A.; Frantz, F.G.; Sorgi, C.A.; Serezani, C.; Deepe, G.S.; Faccioli, L.H. Leukotrienes are potent adjuvant during fungal infection: Effects on memory T cells. J. Immunol. 2008, 181, 8544–8551. [Google Scholar] [CrossRef]
- Secatto, A.; Soares, E.M.; Locachevic, G.A.; Assis, P.A.; Paula-Silva, F.; Serezani, C.; Medeiros, A.I.; Faccioli, L.H. The Leukotriene B4/BLT1 Axis Is a Key Determinant in Susceptibility and Resistance to Histoplasmosis. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef][Green Version]
- Schnizlein-Bick, C.; Durkin, M.; Kohler, S.; Connolly, P.; Lemonte, A.; Garringer, T.; Goldberg, J.; Smedema, M.; Brizendine, E.; Wheat, L.J. Effects of CD4 and CD8 T lymphocyte depletion on the course of histoplasmosis following pulmonary challenge. Med. Mycol. 2003, 41, 189–197. [Google Scholar] [CrossRef]
- Lin, J.-S.; Wu-Hsieh, B.A.-Y. Functional T cells in primary immune response to histoplasmosis. Int. Immunol. 2004, 16, 1663–1673. [Google Scholar] [CrossRef]
- Allendoerfer, R.; Deepe, G.S. Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect. Immun. 1997, 65, 2564–2569. [Google Scholar] [CrossRef]
- Deepe, G.S.; Gibbons, R.S. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J. Infect. Dis. 2009, 200, 142–151. [Google Scholar] [CrossRef]
- Allendoerfer, R.; Deepe, G.S. Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J. Immunol. 2000, 165, 2657–2664. [Google Scholar] [CrossRef]
- Deepe, G.S.; McGuinness, M. Interleukin-1 and host control of pulmonary histoplasmosis. J. Infect. Dis. 2006, 194, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Deepe, G.S. Interleukin-12 Neutralization Alters Lung Inflammation and Leukocyte Expression of CD80, CD86, and Major Histocompatibility Complex Class II in Mice Infected with Histoplasma capsulatum. Infect. Immun. 2000, 68, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.T.; Otero, G.C.; Wu-Hsieh, B.A.-Y.; Howard, D.H. Expression of inducible nitric oxide synthase by stimulated macrophages correlates with their antihistoplasma activity. Infect. Immun. 1994, 62, 1478–1479. [Google Scholar] [CrossRef]
- Zhou, P.; Sieve, M.C.; Bennett, J.; Kwon-Chung, K.J.; Tewari, R.P.; Gazzinelli, R.T.; Sher, A.; A Seder, R. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J. Immunol. 1995, 155, 785–795. [Google Scholar]
- Kroetz, D.N.; Deepe, G.S. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 2011, 58, 112–117. [Google Scholar] [CrossRef]
- Deepe, G.S.; Gibbons, R.S. TNF-α Antagonism Generates a Population of Antigen-Specific CD4+CD25+ T Cells That Inhibit Protective Immunity in Murine Histoplasmosis1. J. Immunol. 2008, 180, 1088–1097. [Google Scholar] [CrossRef]
- Peng, J.-K.; Lin, J.-S.; Kung, J.T.; Finkelman, F.D.; Wu-Hsieh, B.A.-Y. The combined effect of IL-4 and IL-10 suppresses the generation of, but does not change the polarity of, type-1 T cells in Histoplasma infection. Int. Immunol. 2004, 17, 193–205. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef]
- Hansson, M.; Silverpil, E.; Lindén, A.; Glader, P. Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm. Res. 2013, 62, 561–569. [Google Scholar] [CrossRef]
- Malhotra, N.; Yoon, J.; Castillo, J.M.L.; Galand, C.; Archer, N.; Miller, L.S.; Geha, R.S. IL-22 derived from γδ T cells restricts Staphylococcus aureus infection of mechanically injured skin. J. Allergy Clin. Immunol. 2016, 138, 1098–1107. [Google Scholar] [CrossRef]
- Zindl, C.L.; Lai, J.-F.; Lee, Y.K.; Maynard, C.L.; Harbour, S.N.; Ouyang, W.; Chaplin, D.; Weaver, C.T. IL-22–producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 12768–12773. [Google Scholar]
- Res, P.C.M.; Piskin, G.; De Boer, O.J.; Van Der Loos, C.M.; Teeling, P.; Bos, J.D.; Teunissen, M.B.M. Overrepresentation of IL-17A and IL-22 Producing CD8 T Cells in Lesional Skin Suggests Their Involvement in the Pathogenesis of Psoriasis. PLoS ONE 2010, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Thakar, M.S.; Ouyang, W.; Malarkannan, S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2012, 6, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Functional Biology of the IL-22-IL-22R Pathway in Regulating Immunity and Inflammation at Barrier Surfaces. Adv. Immunol. 2010, 107, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.; Hole, C.R.; Yano, J.; Fidel, P.L.; Wormley, F.L. Characterization of IL-22 and antimicrobial peptide production in mice protected against pulmonary Cryptococcus neoformans infection. Microbiology 2014, 160, 1440–1452. [Google Scholar] [CrossRef]
- Gessner, M.A.; Werner, J.L.; Lilly, L.M.; Nelson, M.P.; Metz, A.E.; Dunaway, C.W.; Chan, Y.R.; Ouyang, W.; Brown, G.D.; Weaver, C.T.; et al. Dectin-1-Dependent Interleukin-22 Contributes to Early Innate Lung Defense against Aspergillus fumigatus. Infect. Immun. 2011, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; De Luca, A.; Puccetti, M.; Jaeger, M.; Mencacci, A.; Oikonomou, V.; Pariano, M.; Garlanda, C.; Moretti, S.; Bartoli, A.; et al. Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra. Cell Host Microbe 2015, 18, 198–209. [Google Scholar] [CrossRef]
- Sahaza, J.H.; Suárez-Alvarez, R.; Estrada-Bárcenas, D.A.; Pérez-Torres, A.; Taylor, M.L. Profile of cytokines in the lungs of BALB/c mice after intra-nasal infection with Histoplasma capsulatum mycelial propagules. Comp. Immunol. Microbiol. Infect. Dis. 2015, 41, 1–9. [Google Scholar] [CrossRef]
- Kroetz, D.N.; Deepe, G.S. CCR5 Dictates the Equilibrium of Proinflammatory IL-17+and Regulatory Foxp3+T Cells in Fungal Infection. J. Immunol. 2010, 184, 5224–5231. [Google Scholar] [CrossRef]
- Medeiros, A.I.; Sá-Nunes, A.; Soares, E.G.; Peres, C.M.; Silva, C.; Faccioli, L.H. Blockade of Endogenous Leukotrienes Exacerbates Pulmonary Histoplasmosis. Infect. Immun. 2004, 72, 1637–1644. [Google Scholar] [CrossRef]
- Locachevic, G.A.; Pereira, P.A.T.; Secatto, A.; Fontanari, C.; Galvão, A.F.; Prado, M.K.B.; Zoccal, K.F.; Petta, T.; De Moraes, L.A.B.; Ramos, S.G.; et al. Erythropoietin Exacerbates Inflammation and Increases the Mortality of Histoplasma capsulatum-Infected Mice. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Yamamoto, N.; Shibamori, M.; Ogura, M.; Seko, Y.; Kikuchi, M. Effects of Intranasal Administration of Recombinant Murine Interferon-γ on Murine Acute Myocarditis Caused by Encephalomyocarditis Virus. Circulation 1998, 97, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Clemons, K.V.; Lutz, J.E.; Stevens, D.A. Efficacy of Recombinant Gamma Interferon for Treatment of Systemic Cryptococcosis in SCID Mice. Antimicrob. Agents Chemother. 2001, 45, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J. Immunol. 1988, 140, 4342–4347. [Google Scholar] [PubMed]
- Souza, C.O.S.; Espíndola, M.S.; Fontanari, C.; Prado, M.K.B.; Frantz, F.G.; Rodrigues, V.; Gardinassi, L.G.; Faccioli, L.H. CD18 Regulates Monocyte Hematopoiesis and Promotes Resistance to Experimental Schistosomiasis. Front. Immunol. 2018, 9, 9–24. [Google Scholar] [CrossRef]
- Tristão, F.S.M.; Rocha, F.A.; Carlos, D.; Ketelut-Carneiro, N.; Souza, C.O.S.; Milanezi, C.M.; Silva, J.S. Th17-Inducing Cytokines IL-6 and IL-23 Are Crucial for Granuloma Formation during Experimental Paracoccidioidomycosis. Front. Immunol. 2017, 8, 949–962. [Google Scholar] [CrossRef]
- Sorgi, C.A.; Peti, A.P.F.; Petta, T.; Meirelles, A.F.G.; Fontanari, C.; De Moraes, L.A.B.; Faccioli, L.H. Comprehensive high-resolution multiple-reaction monitoring mass spectrometry for targeted eicosanoid assays. Sci. Data 2018, 5, 180167–180179. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Witte, K.; Warszawska, K.; Sabat, R. Biology of interleukin-22. Semin. Immunopathol. 2010, 32, 17–31. [Google Scholar] [CrossRef]
- Deepe, G.S.; Gibbons, R.S. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J. Immunol. 2003, 171, 5353–5362. [Google Scholar] [CrossRef]
- Nakamura, L.T.; Wu-Hsieh, B.A.-Y.; Howard, D.H. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit intracellular growth of Histoplasma capsulatum. Infect. Immun. 1994, 62, 680–684. [Google Scholar] [CrossRef]
- Rutz, S.; Eidenschenk, C.; Ouyang, W. IL-22, not simply a Th17 cytokine. Immunol. Rev. 2013, 252, 116–132. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.-C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.G.; Talbot, J.; Peres, R.S.; Franca, R.F.D.O.; Ferreira, S.H.; Ryffel, B.; Alves-Filho, J.C.; Figueiredo, F.; Cunha, F.Q.; Cunha, F.Q. Joint production of IL-22 participates in the initial phase of antigen-induced arthritis through IL-1β production. Arthritis Res. 2015, 17, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Eidenschenk, C.; Ota, N.; Wong, K.; Lohmann, U.; Kühl, A.A.; Wang, X.; Manzanillo, P.; Li, Y.; Rutz, S.; et al. Interleukin-22 Induces Interleukin-18 Expression from Epithelial Cells during Intestinal Infection. Immun. 2015, 42, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Karow, M.; Flavell, R.A. Interleukin-22 but Not Interleukin-17 Provides Protection to Hepatocytes during Acute Liver Inflammation. Immun. 2007, 27, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hong, F.; Radaeva, S.; Gao, B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell. Mol. Immunol. 2004, 1, 43–49. [Google Scholar]
- Radaeva, S.; Sun, R.; Pan, H.-N.; Hong, F.; Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004, 39, 1332–1342. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Nair, M.G.; Kirn, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef]
- Wang, C.; Gong, G.; Sheh, A.; Muthupalani, S.; Bryant, E.M.; Puglisi, A.D.; Holcombe, H.; Conaway, A.E.; Parry, N.; Bakthavatchalu, V.; et al. Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer. Mucosal Immunol. 2017, 10, 1504–1517. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, M.K.B.; Fontanari, C.; Souza, C.O.S.; Gardinassi, L.G.; Zoccal, K.F.; de Paula-Silva, F.W.G.; Peti, A.P.F.; Sorgi, C.A.; Meirelles, A.F.G.; Ramos, S.G.; et al. IL-22 Promotes IFN-γ-Mediated Immunity against Histoplasma capsulatum Infection. Biomolecules 2020, 10, 865. https://doi.org/10.3390/biom10060865
Prado MKB, Fontanari C, Souza COS, Gardinassi LG, Zoccal KF, de Paula-Silva FWG, Peti APF, Sorgi CA, Meirelles AFG, Ramos SG, et al. IL-22 Promotes IFN-γ-Mediated Immunity against Histoplasma capsulatum Infection. Biomolecules. 2020; 10(6):865. https://doi.org/10.3390/biom10060865
Chicago/Turabian StylePrado, Morgana K.B., Caroline Fontanari, Camila O.S. Souza, Luiz G. Gardinassi, Karina F. Zoccal, Francisco W.G. de Paula-Silva, Ana P.F. Peti, Carlos A. Sorgi, Alyne F.G. Meirelles, Simone G. Ramos, and et al. 2020. "IL-22 Promotes IFN-γ-Mediated Immunity against Histoplasma capsulatum Infection" Biomolecules 10, no. 6: 865. https://doi.org/10.3390/biom10060865
APA StylePrado, M. K. B., Fontanari, C., Souza, C. O. S., Gardinassi, L. G., Zoccal, K. F., de Paula-Silva, F. W. G., Peti, A. P. F., Sorgi, C. A., Meirelles, A. F. G., Ramos, S. G., Alves-Filho, J. C., & Faccioli, L. H. (2020). IL-22 Promotes IFN-γ-Mediated Immunity against Histoplasma capsulatum Infection. Biomolecules, 10(6), 865. https://doi.org/10.3390/biom10060865





