LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Husbandry
2.3. Cell Culture
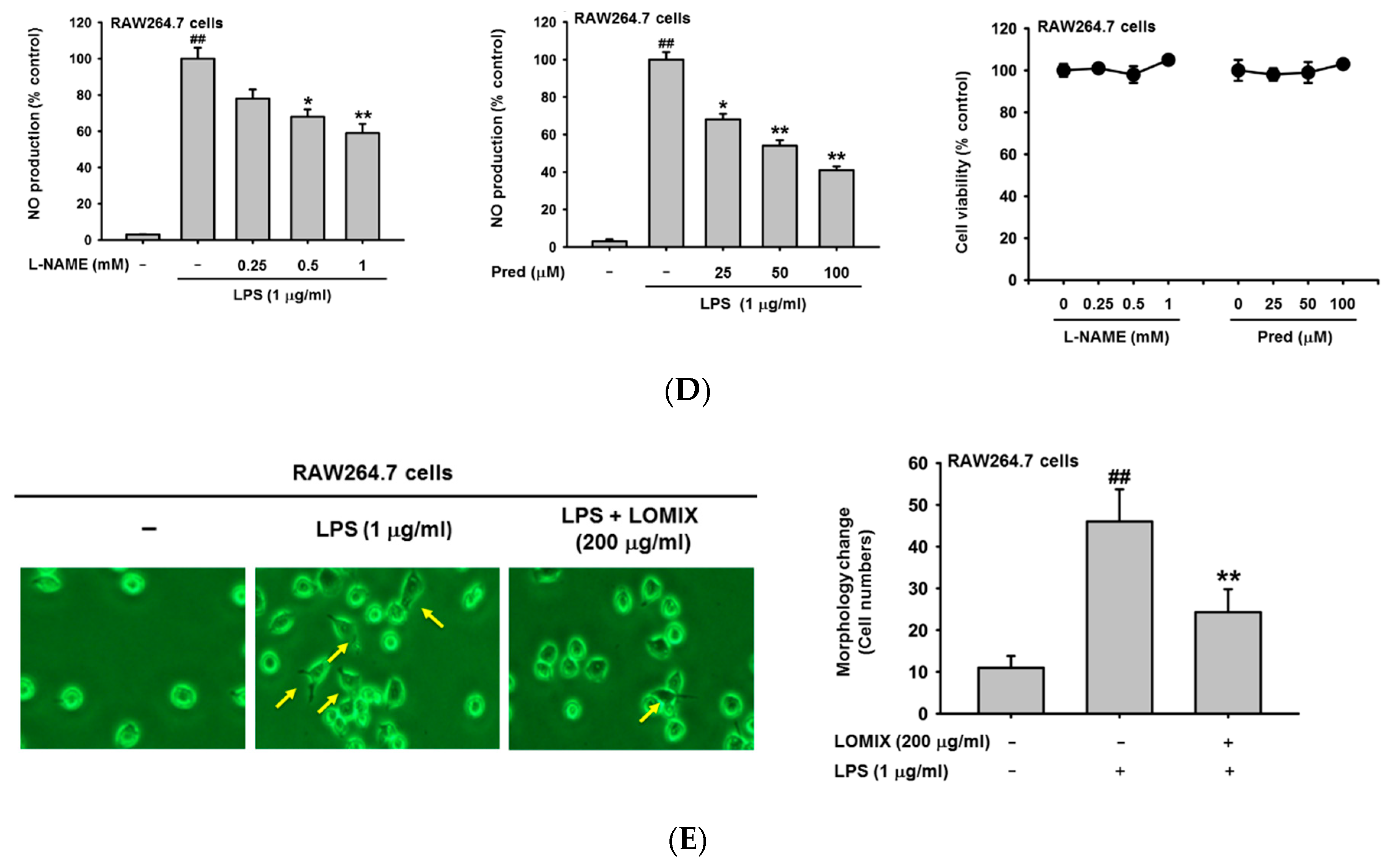
2.4. Cell Viability Assay
2.5. NO Production Assay
2.6. Quantitative Real-Time PCR and Semiquantitative RT-PCR
2.7. Luciferase Reporter Gene Assay
2.8. Western Blot Analysis
2.9. In Vitro Kinase Assay
2.10. HCl/EtOH-Induced Gastritis in Mice
2.11. DSS-Induced Colitis in Mice
2.12. LPS/D-GalN-Induced Hepatitis in Mice
2.13. In Vivo Acute Toxicity Test
2.14. Statistical Analysis
3. Results and Discussion
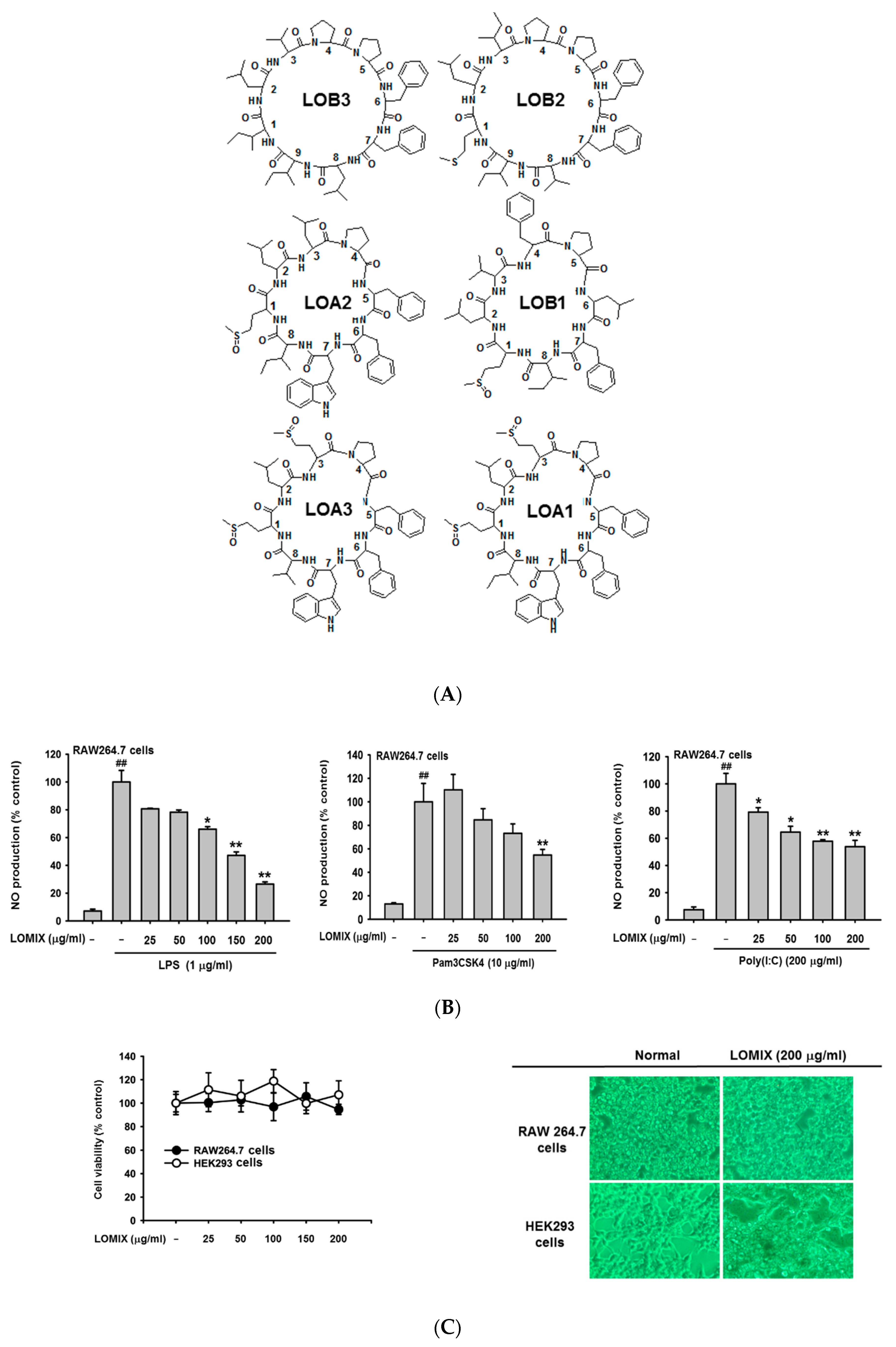
3.1. LOMIX Has Anti-Inflammatory Activity in Macrophages
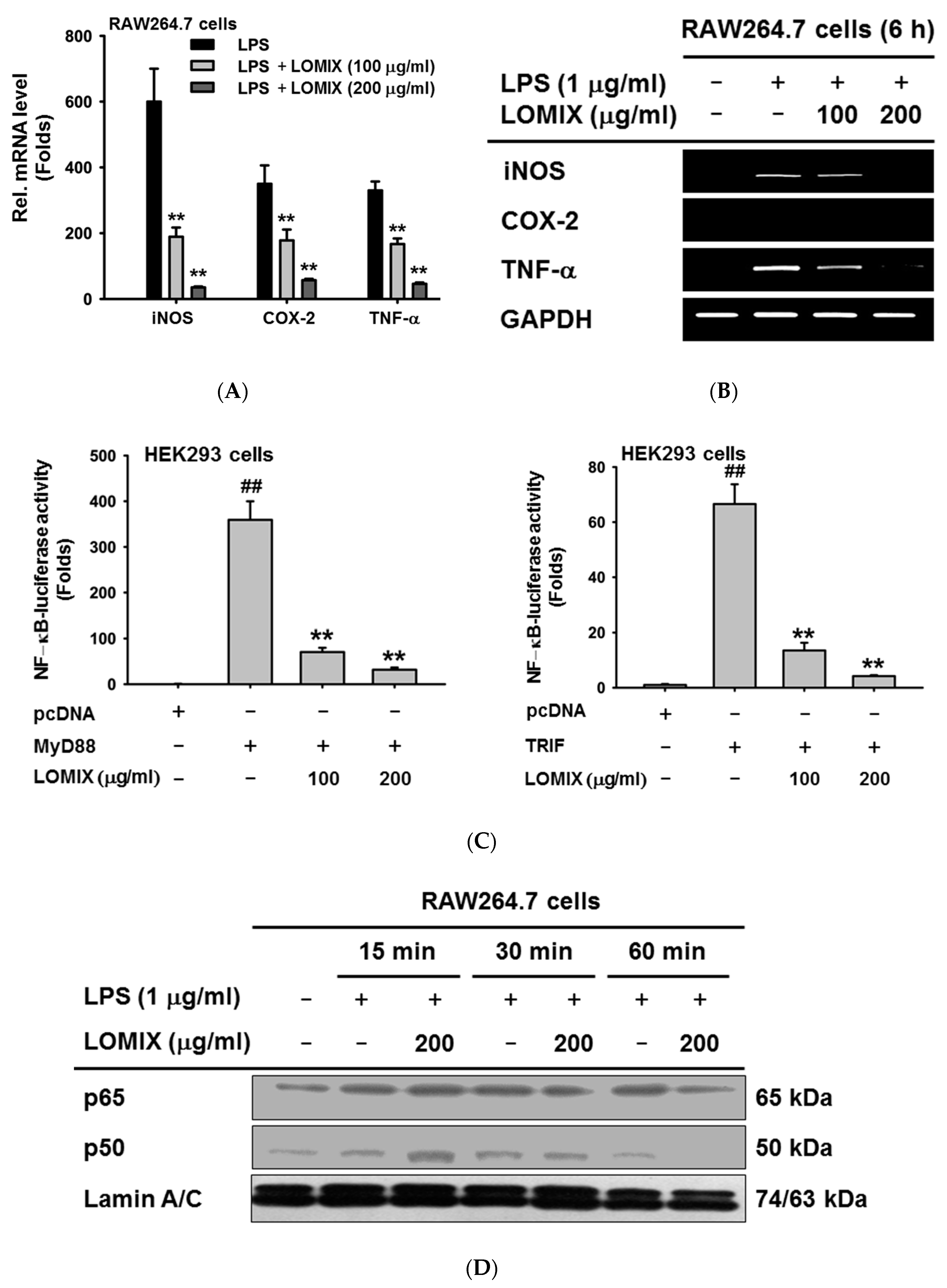
3.2. LOMIX Suppresses mRNA Expression of Inflammatory Genes by Inhibiting NF-κB
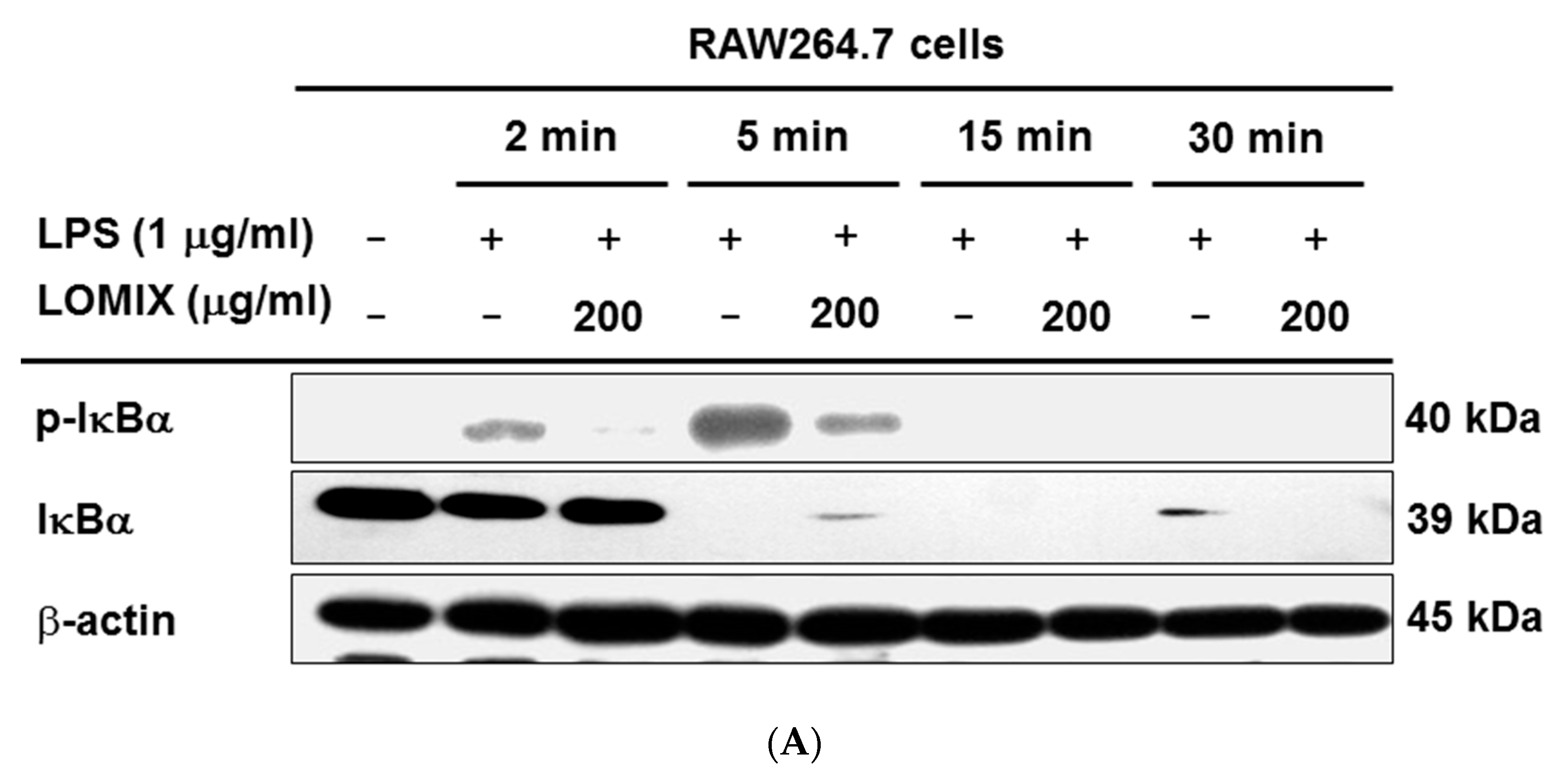
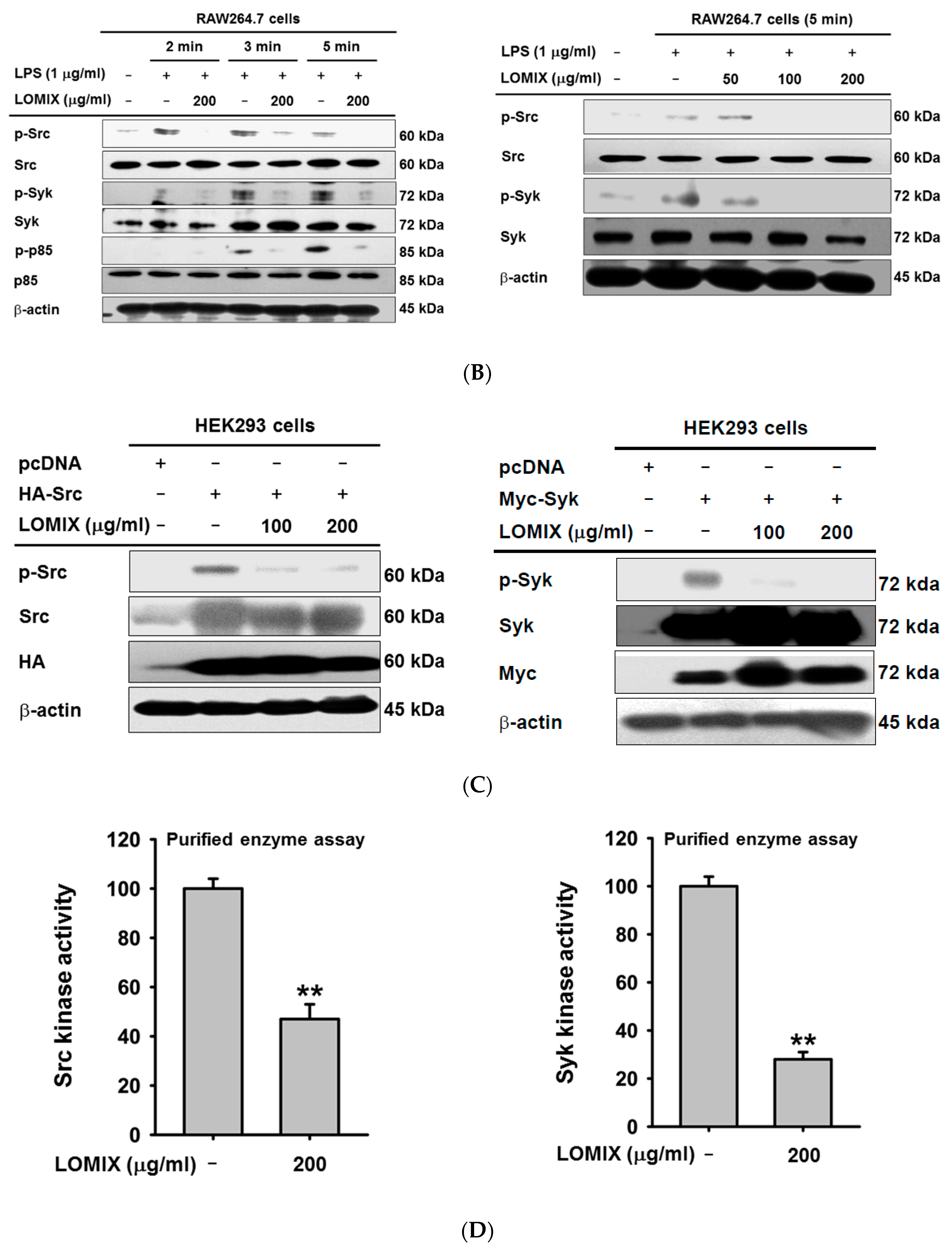
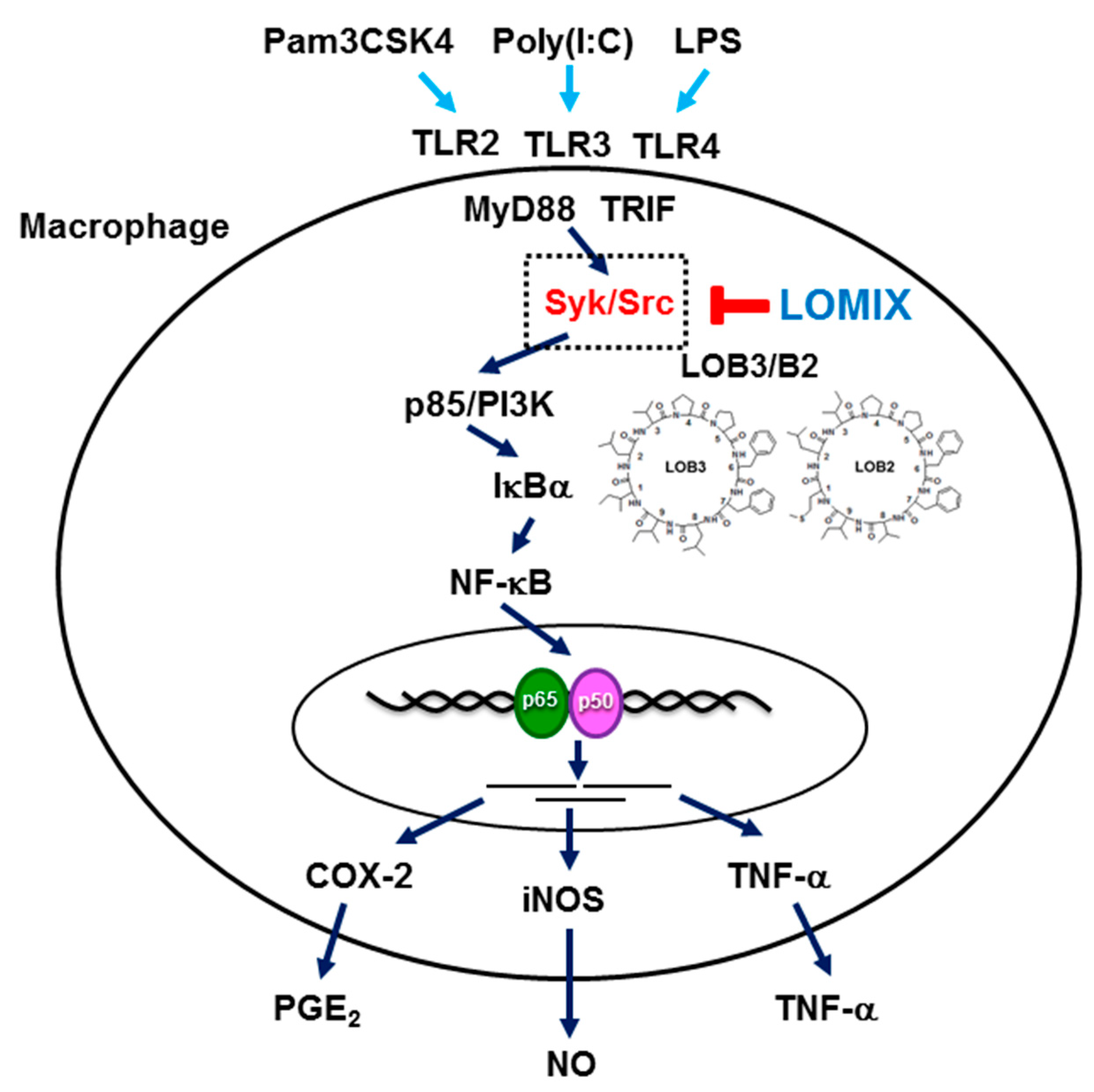
3.3. LOMIX Suppresses the NF-κB Signaling Pathway
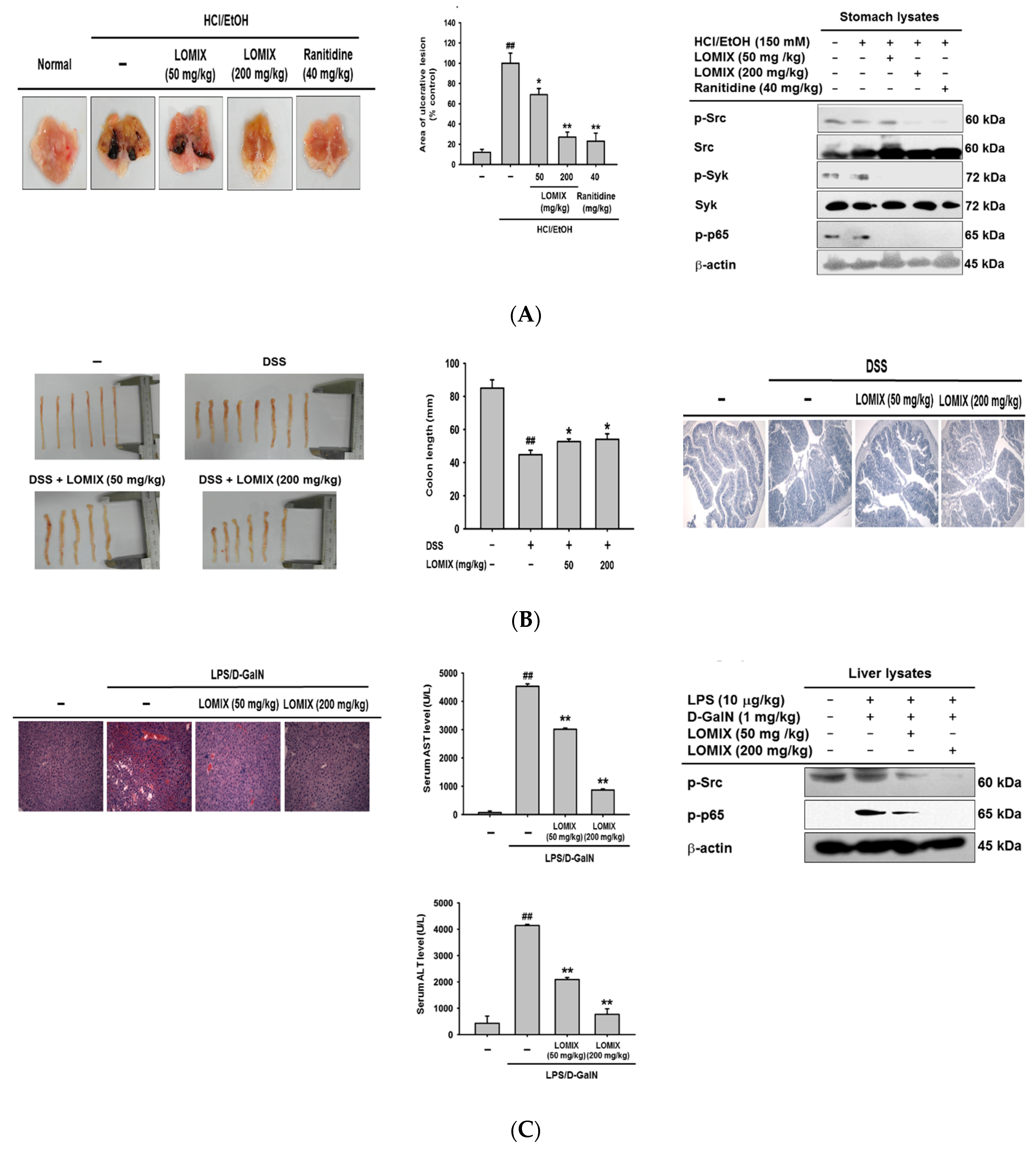
3.4. LOMIX Ameliorates Gastritis Symptoms in a Mouse Model of Gastritis, a DSS-Induced Mouse Model of Colitis, and an LPS/D-GalN-Induced Mouse Model of Hepatitis
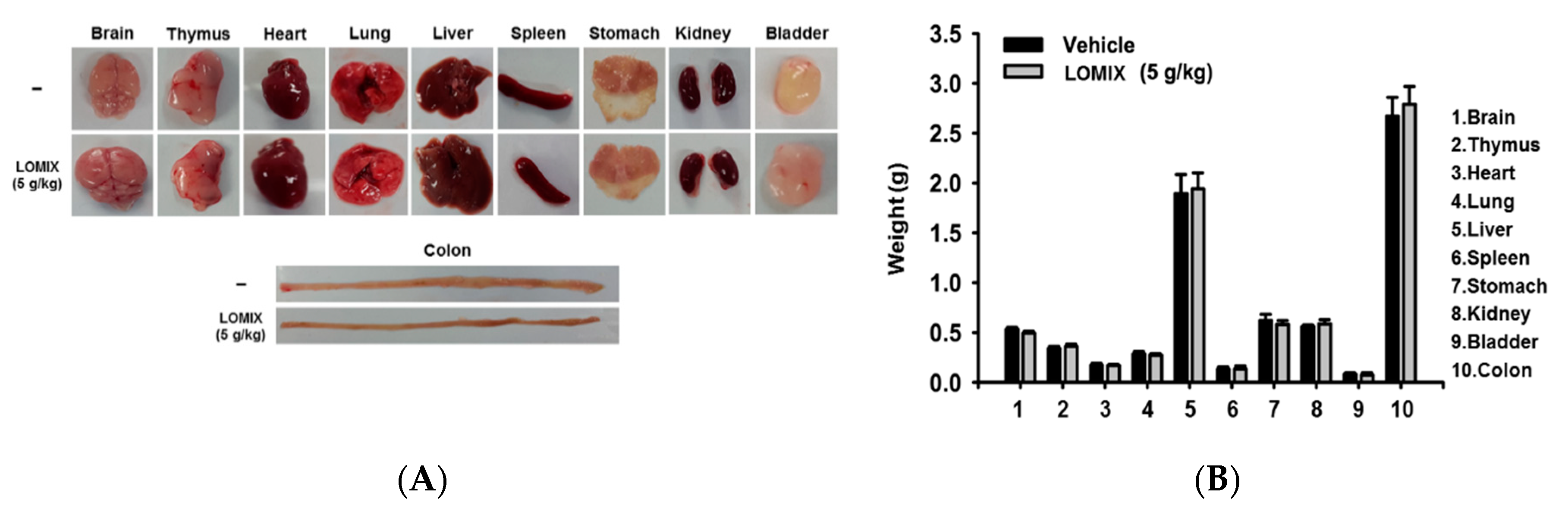
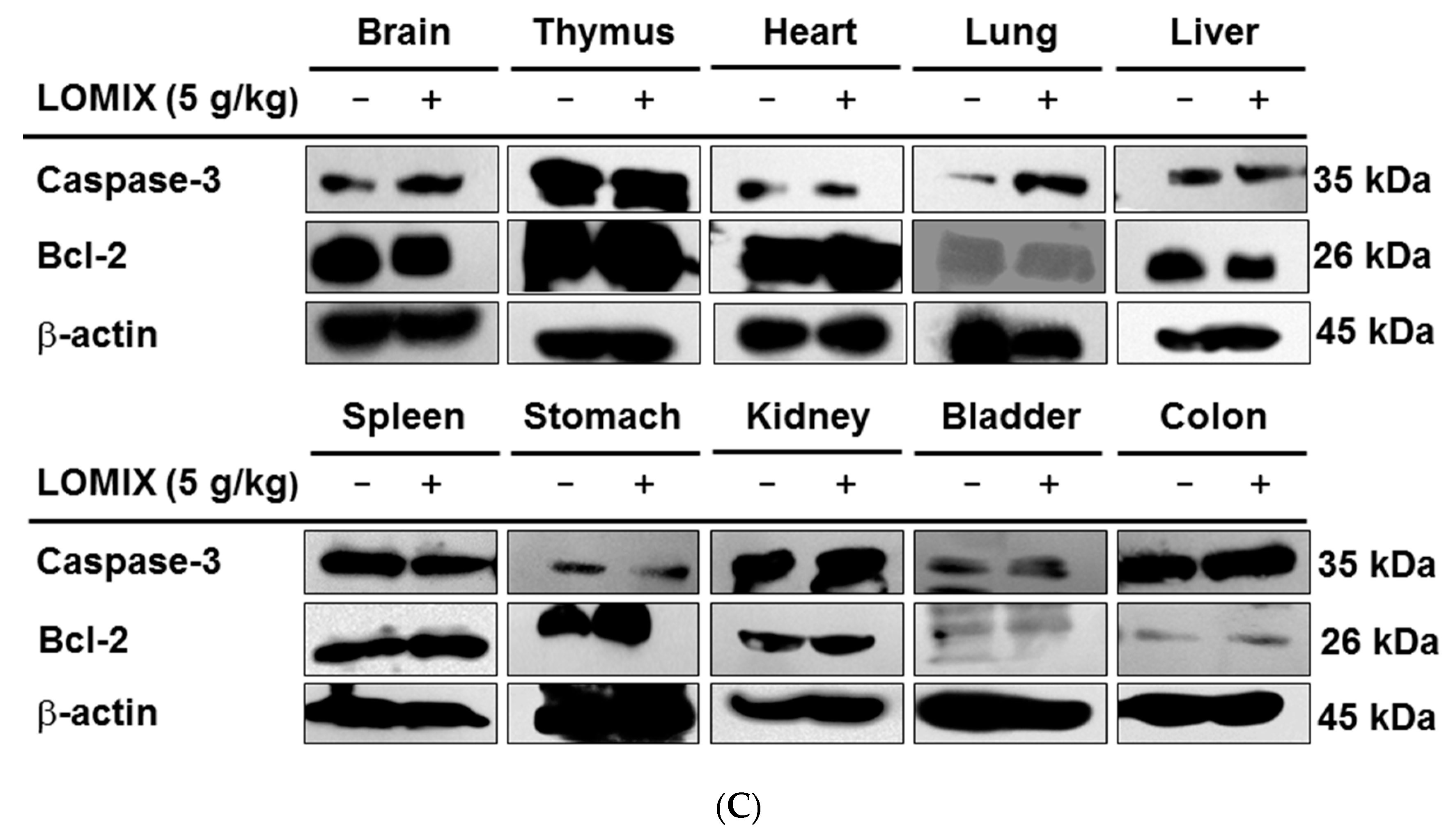
3.5. In Vivo Acute Toxicity of LOMIX
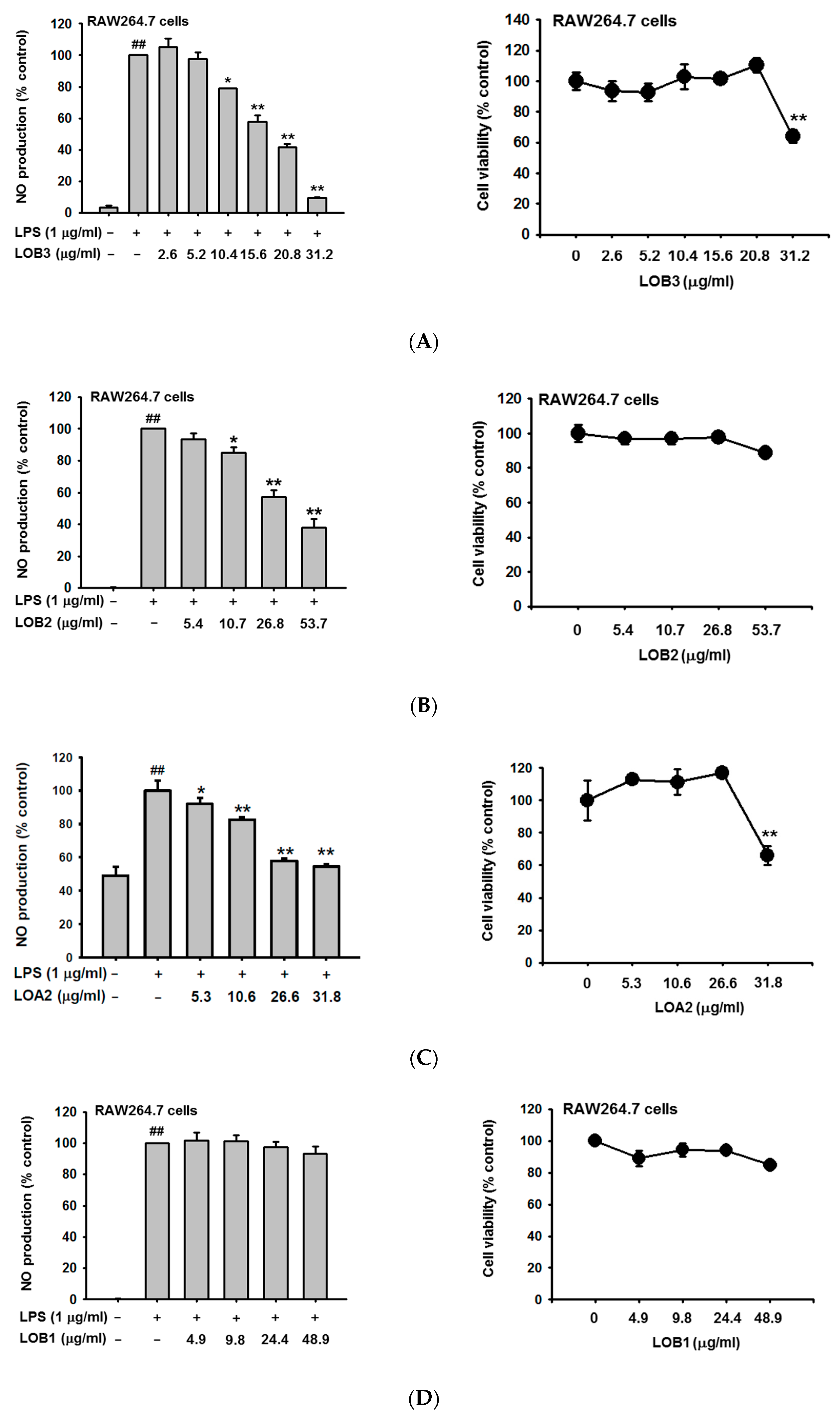
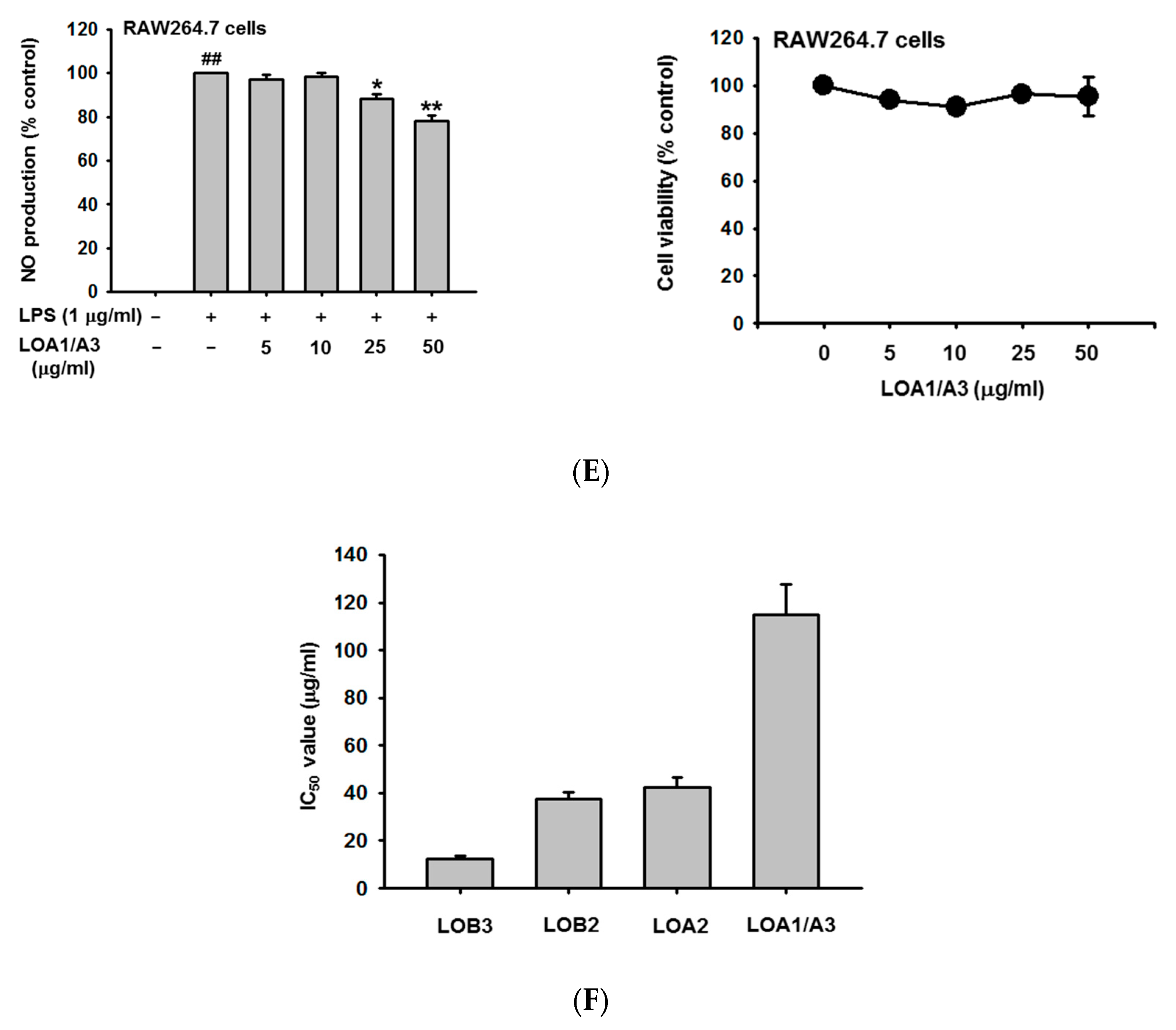
3.6. Anti-Inflammatory Effect of Each Linusorb in LOMIX
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Regulatory Roles of the Caspase-11 Non-Canonical Inflammasome in Inflammatory Diseases. Immune Netw. 2018, 18, e41. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, J.; Kim, E.; Kim, S.H.; Seo, D.B.; Kim, J.H.; Shin, S.S.; Cho, J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J. Ginseng Res. 2018, 42, 496–503. [Google Scholar] [CrossRef]
- Yu, T.; Yi, Y.S.; Yang, Y.; Oh, J.; Jeong, D.; Cho, J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediat. Inflamm. 2012, 2012, 979105. [Google Scholar] [CrossRef]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Hossain, F.M.A.; Choi, J.Y.; Uyangaa, E.; Park, S.O.; Eo, S.K. The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation. Immune Netw. 2019, 19, e31. [Google Scholar] [CrossRef]
- Abarca-Buis, R.F.; Martinez-Jimenez, A.; Vera-Gomez, E.; Contreras-Figueroa, M.E.; Garciadiego-Cazares, D.; Paus, R.; Robles-Tenorio, A.; Krotzsch, E. Mechanisms of epithelial thickening due to IL-1 signalling blockade and TNF-alpha administration differ during wound repair and regeneration. Differentiation 2018, 99, 10–20. [Google Scholar] [CrossRef]
- Kim, E.; Yi, Y.S.; Son, Y.J.; Han, S.Y.; Kim, D.H.; Nam, G.; Hossain, M.A.; Kim, J.H.; Park, J.; Cho, J.Y. BIOGF1K, a compound K-rich fraction of ginseng, plays an antiinflammatory role by targeting an activator protein-1 signaling pathway in RAW264.7 macrophage-like cells. J. Ginseng Res. 2018, 42, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology 2019, 159, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembinska-Kiec, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Golabek, I.; Bartus, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Lee, J.O.; Choi, E.; Shin, K.K.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Hossain, M.A.; Kim, H.S.; Yi, Y.S.; Kim, D.; et al. Compound K, a ginsenoside metabolite, plays an antiinflammatory role in macrophages by targeting the AKT1-mediated signaling pathway. J. Ginseng Res. 2019, 43, 154–160. [Google Scholar] [CrossRef]
- Hong, Y.H.; Kim, D.; Nam, G.; Yoo, S.; Han, S.Y.; Jeong, S.G.; Kim, E.; Jeong, D.; Yoon, K.; Kim, S.; et al. Photoaging protective effects of BIOGF1K, a compound-K-rich fraction prepared from Panax ginseng. J. Ginseng Res. 2018, 42, 81–89. [Google Scholar] [CrossRef]
- Hong, Y.H.; Yi, Y.S.; Han, S.Y.; Aziz, N.; Kim, H.G.; Park, S.H.; Hossain, M.A.; Baik, K.S.; Choi, S.Y.; Lee, J.; et al. Morinda citrifolia noni water extract enhances innate and adaptive immune responses in healthy mice, ex vivo, and in vitro. Phytother Res. 2019, 33, 676–689. [Google Scholar] [CrossRef]
- Song, C.; Hong, Y.H.; Park, J.G.; Kim, H.G.; Jeong, D.; Oh, J.; Sung, G.H.; Hossain, M.A.; Taamalli, A.; Kim, J.H.; et al. Suppression of Src and Syk in the NF-kappaB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J. Ethnopharmacol. 2019, 235, 38–46. [Google Scholar] [CrossRef]
- Han, S.Y.; Yi, Y.S.; Jeong, S.G.; Hong, Y.H.; Choi, K.J.; Hossain, M.A.; Hwang, H.; Rho, H.S.; Lee, J.; Kim, J.H.; et al. Ethanol Extract of Lilium Bulbs Plays an Anti-Inflammatory Role by Targeting the IKK[Formula: See text]/[Formula: See text]-Mediated NF-[Formula: See text]B Pathway in Macrophages. Am. J. Chin. Med. 2018, 46, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Choi, S.; Lee, J.; Hong, Y.H.; Jeong, D.; Yoon, K.; Yoon, D.H.; Sung, G.H.; Lee, S.; Hong, S.; et al. Src Is a Prime Target Inhibited by Celtis choseniana Methanol Extract in Its Anti-Inflammatory Action. Evid. Based Complement. Alternat Med. 2018, 2018, 3909038. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, M.Y.; Cho, J.Y. Alisma canaliculatum ethanol extract suppresses inflammatory responses in LPS-stimulated macrophages, HCl/EtOH-induced gastritis, and DSS-triggered colitis by targeting Src/Syk and TAK1 activities. J. Ethnopharmacol. 2018, 219, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S.; Cho, J.Y.; Kim, D. Cerbera manghas methanol extract exerts anti-inflammatory activity by targeting c-Jun N-terminal kinase in the AP-1 pathway. J. Ethnopharmacol. 2016, 193, 387–396. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci 2018, 19, 3805. [Google Scholar] [CrossRef]
- Park, S.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Antioxidative and Antimelanogenesis Effect of Momordica charantia Methanol Extract. Evid. Based Complement. Alternat Med. 2019, 2019, 5091534. [Google Scholar] [CrossRef]
- Ahuja, A.; Jeong, D.; Kim, M.Y.; Cho, J.Y. Trichosanthes tricuspidata Lour. Methanol Extract Exhibits Anti-Inflammatory Activity by Targeting Syk, Src, and IRAK1 Kinase Activity. Evid. Based Complement. Alternat Med. 2019, 2019, 6879346. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Ploeger, D.T.; Hosper, N.A.; Schipper, M.; Koerts, J.A.; de Rond, S.; Bank, R.A. Cell plasticity in wound healing: Paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 2013, 11, 29. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lorz, L.R.; Yi, D.K.; Noh, J.K.; Yi, Y.S.; Cho, J.Y. Viburnum pichinchense methanol extract exerts anti-inflammatory effects via targeting the NF-kappaB and caspase-11 non-canonical inflammasome pathways in macrophages. J. Ethnopharmacol. 2019, 245, 112161. [Google Scholar] [CrossRef]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.S.; Kim, J.H.; et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Byeon, S.E.; Yi, Y.S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012, 2012, 512926. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Parray, F.Q.; Wani, M.L.; Malik, A.A.; Wani, S.N.; Bijli, A.H.; Irshad, I.; Nayeem Ul, H. Ulcerative colitis: A challenge to surgeons. Int. J. Prev. Med. 2012, 3, 749–763. [Google Scholar]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Corcoran, G.B.; Fix, L.; Jones, D.P.; Moslen, M.T.; Nicotera, P.; Oberhammer, F.A.; Buttyan, R. Apoptosis: Molecular control point in toxicity. Toxicol. Appl. Pharmacol. 1994, 128, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Young, L.W.; Arnison, P.G.; Gilding, E.; Reaney, M.J. Proposed systematic nomenclature for orbitides. J. Nat. Prod. 2015, 78, 645–652. [Google Scholar] [CrossRef] [PubMed]
| Target | Sequence (5′ to 3′) | |
|---|---|---|
| Quantitative Real-Time PCR | ||
| iNOS | Forward | GGAGCCTTTAGACCTCAACAGA |
| Reverse | TGAACGAGGAGGGTGGTG | |
| COX-2 | Forward | GGGAGTCTGGAACATTGTGAA |
| Reverse | GCACATTGTAAGTAGGTGGACTGT | |
| TNF-α | Forward | TGCCTATGTCTCAGCCTCTTC |
| Reverse | GAGGCCATTTGGGAACTTCT | |
| GAPDH | Forward | CAATGAATACGGCTACAGCAAC |
| Reverse | AGGGAGATGCTCAGTGTTGG | |
| Semiquantitative RT-PCR | ||
| iNOS | Forward | CCCTTCCGAAGTTTCTGGCAGCAG |
| Reverse | GGCTGTCAGAGCCTCGTGGCTTTGG | |
| COX-2 | Forward | CACTACATCCTGACCCACTT |
| Reverse | ATGCTCCTGCTTGAGTATGT | |
| TNF-α | Forward | TTGACCTCAGCGCTGAGTTG |
| Reverse | CCTGTAGCCCACGTCGTAGC | |
| GAPDH | Forward | CACTCACGGCAAATTCAACGGCAC |
| Reverse | GACTCCACGACATACTCAGCAC | |
| Code | Linusorb a | MW | Quantity b | Amount (µg/mL) c |
|---|---|---|---|---|
| LOB3 | [1-9-NαC]-linusorb B3 | 1040.34 | 0.14 mg (22.95%) | 45.9 |
| LOB2 | [1-9-NαC]-linusorb B2 | 1074.37 | 0.16 mg (26.23%) | 52.5 |
| LOA2 | [1-8-NαC], [1-(Rs,Ss)-MetO]-linusorb A2 | 1064.34 | 0.05 mg (8.20%) | 16.4 |
| LOB1 | [1-8-NαC], [1-(Rs,Ss)-MetO]-linusorb B1 | 977.26 | 0.12 mg (19.67%) | 39.3 |
| LOA3 | [1-8-NαC], [1,3-(Rs,Ss)-MetO]-linusorb A3 | 1084.35 | 0.03 mg (4.92%) | 45.9 |
| LOA1 | [1-8-NαC], [1,3-(Rs,Ss)-MetO]-linusorb A1 | 1098.38 | 0.11 mg (18.03%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratan, Z.A.; Jeong, D.; Sung, N.Y.; Shim, Y.Y.; Reaney, M.J.T.; Yi, Y.-S.; Cho, J.Y. LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway. Biomolecules 2020, 10, 859. https://doi.org/10.3390/biom10060859
Ratan ZA, Jeong D, Sung NY, Shim YY, Reaney MJT, Yi Y-S, Cho JY. LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway. Biomolecules. 2020; 10(6):859. https://doi.org/10.3390/biom10060859
Chicago/Turabian StyleRatan, Zubair Ahmed, Deok Jeong, Nak Yoon Sung, Youn Young Shim, Martin J. T. Reaney, Young-Su Yi, and Jae Youl Cho. 2020. "LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway" Biomolecules 10, no. 6: 859. https://doi.org/10.3390/biom10060859
APA StyleRatan, Z. A., Jeong, D., Sung, N. Y., Shim, Y. Y., Reaney, M. J. T., Yi, Y.-S., & Cho, J. Y. (2020). LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway. Biomolecules, 10(6), 859. https://doi.org/10.3390/biom10060859







