A Novel Lysophosphatidic Acid Acyltransferase of Escherichia coli Produces Membrane Phospholipids with a cis-vaccenoyl Group and Is Related to Flagellar Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Construction of YihG- and PlsC-Expression Plasmids
2.3. JC201 Complementation Assay
2.4. Total PL Extraction and Analysis by Electrospray Ionization Tandem Mass Spectrometry (ESI–MS/MS)
2.5. Analysis of the sn-1 and sn-2 Fatty Acyl Groups of PLs
2.6. Motility Assay in Soft Agar Plates
2.7. Microscopic Observation of Swimming Cells
2.8. Flagellin Preparation and Analysis by Western Blot Analysis
2.9. Transmission Electron Microscope (TEM) Observation of the Flagellar Structures
3. Results
3.1. Identification of YihG in E. coli as an SlPlsC4 Ortholog
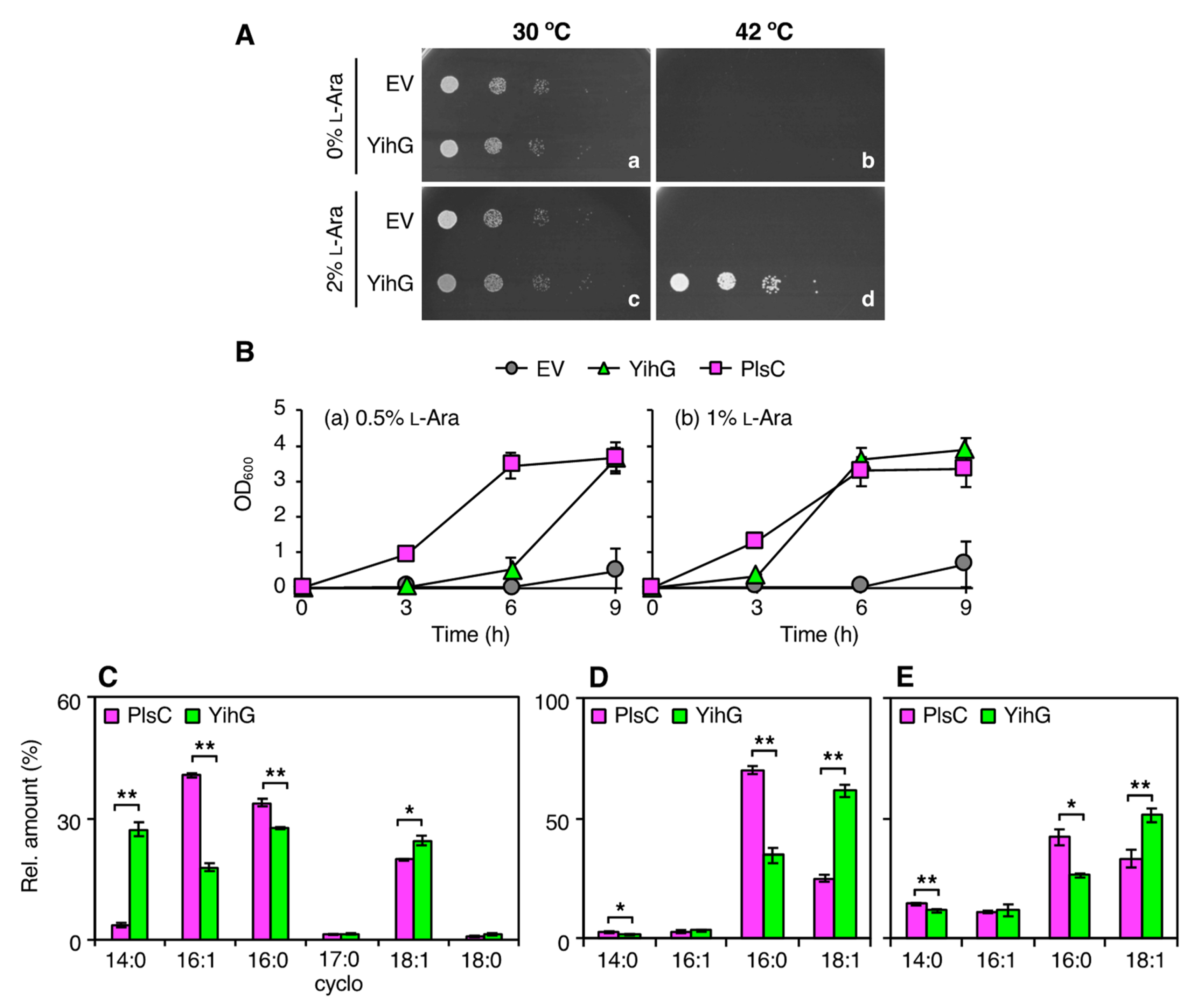
3.2. Overexpression of YihG in an E. coli plsC Mutant Allows its Growth at Non-Permissive Temperatures
3.3. In vivo Substrate Specificity of YihG is Different from that of PlsC
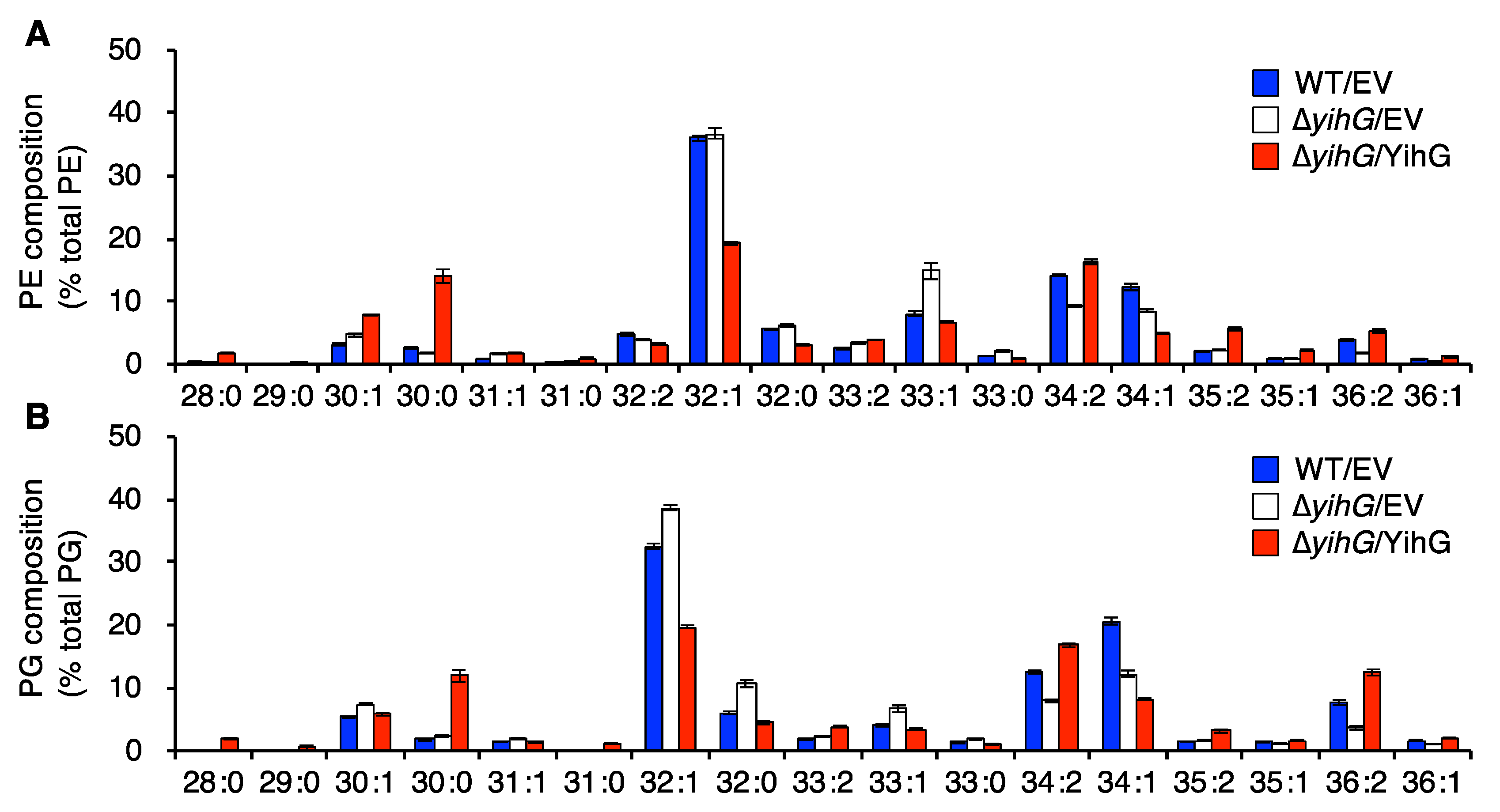
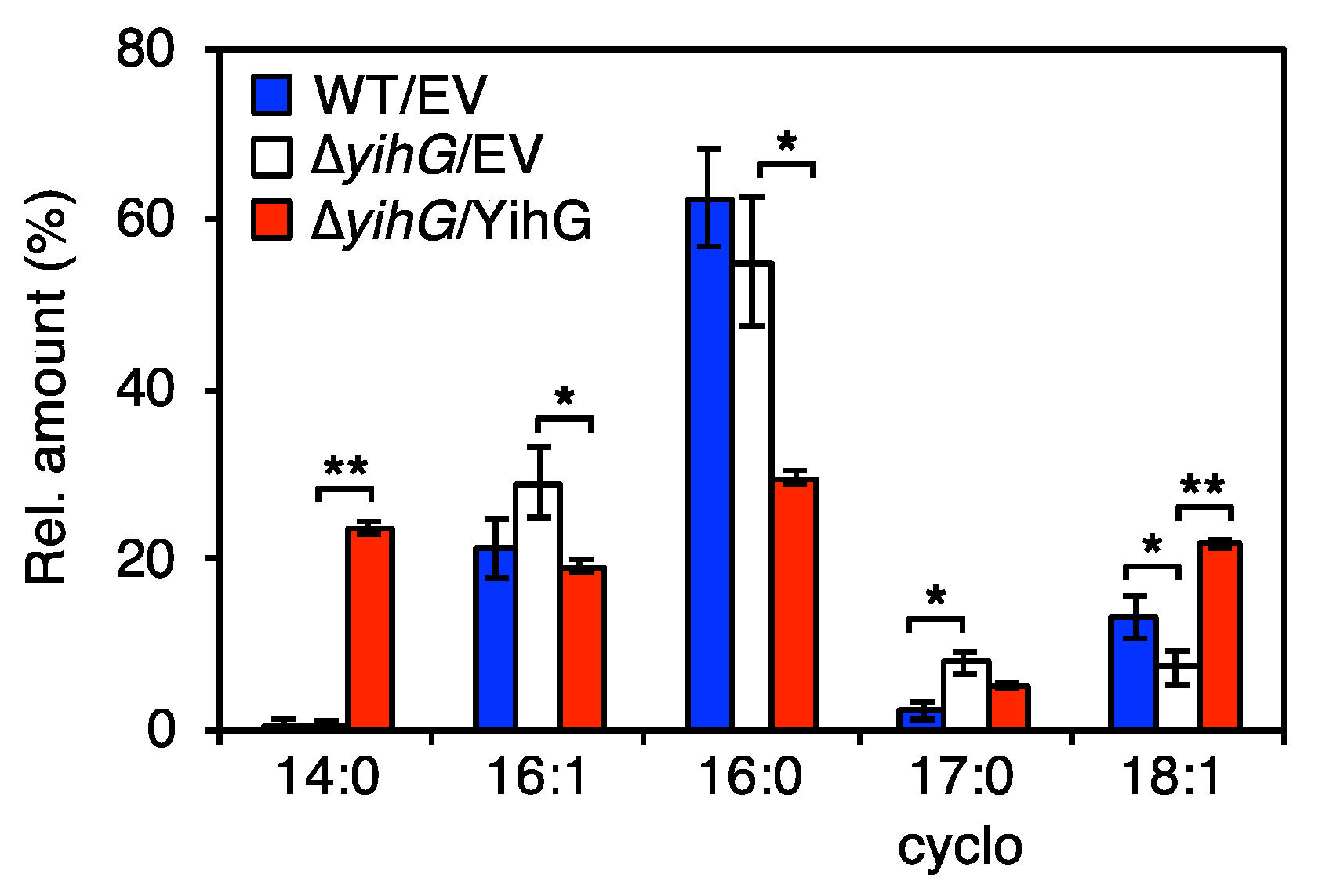
3.4. Deletion of Endogenous YihG Affects Membrane PL Composition
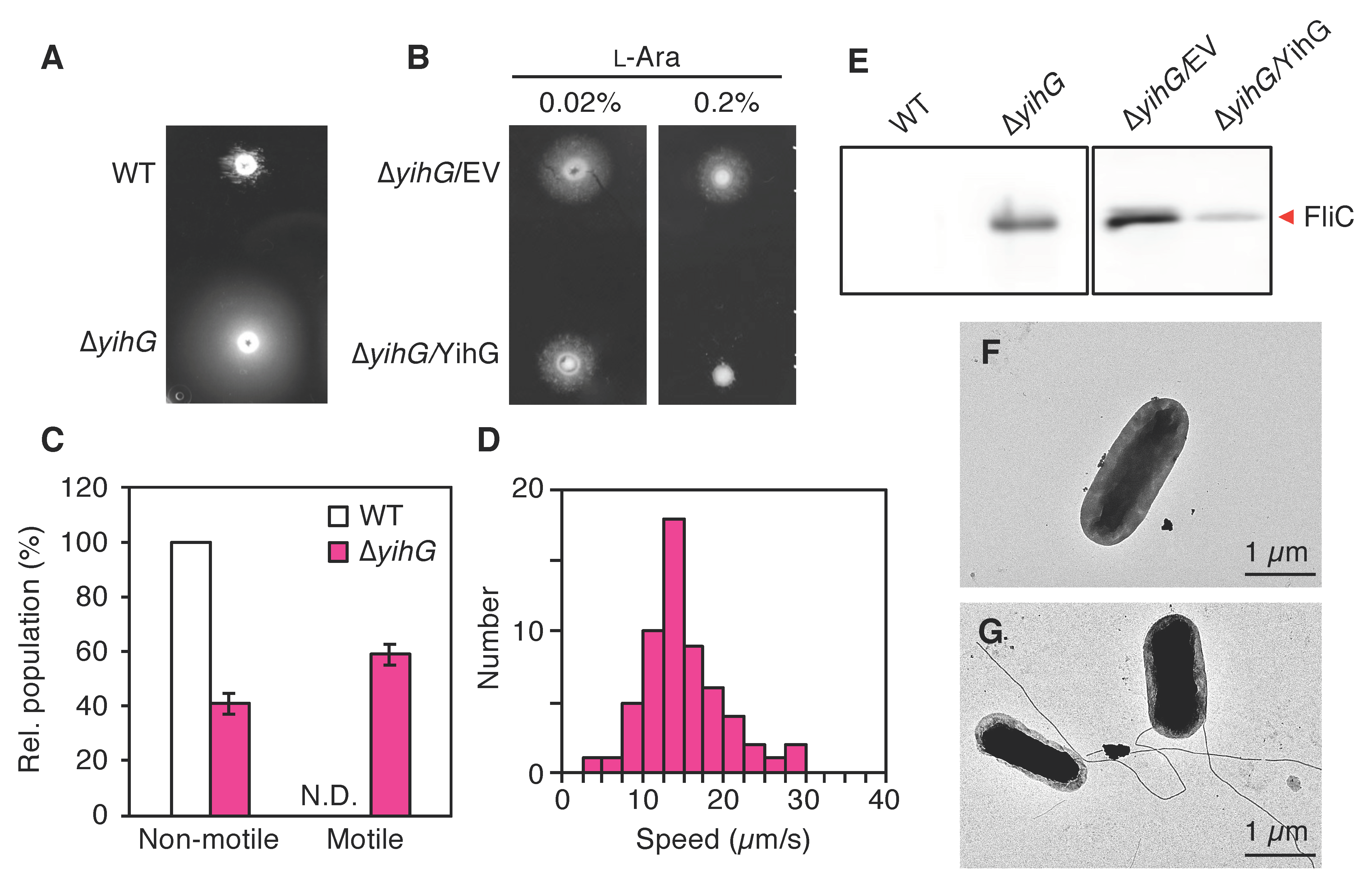
3.5. The Deletion of Endogenous YihG Causes Enhanced Swimming Motility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornell, B.A.; Separovic, F. Membrane thickness and acyl chain length. Biochim. Biophys. Acta Biomembr. 1983, 733, 189–193. [Google Scholar] [CrossRef]
- Lewis, B.A.; Engelman, D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 1983, 166, 211–217. [Google Scholar] [CrossRef]
- Mykytczuk, N.C.S.; Trevors, J.T.; Leduc, L.G.; Ferroni, G.D. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog. Biophys. Mol. Biol. 2007, 95, 60–82. [Google Scholar] [CrossRef]
- Mostofian, B.; Zhuang, T.; Cheng, X.; Nickels, J.D. Branched-chain fatty acid content modulates structure, fluidity, and phase in model microbial cell membranes. J. Phys. Chem. B 2019, 123, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, E.; Shibuya, S.; Hirai, Y.; Matsushita, O.; Tomochika, K.; Kanemasa, Y. Adaptational changes of fatty acid composition and the physical state of membrane lipids following the change of growth temperature in Yersinia enterocolitica. Microbiol. Immunol. 1991, 35, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Annous, B.A.; Becker, L.A.; Bayles, D.O.; Labeda, D.P.; Wilkinson, B.J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997, 63, 3887–3894. [Google Scholar] [CrossRef]
- Allen, E.E.; Facciotti, D.; Bartlett, D.H. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at high pressure and low temperature. Appl. Environ. Microbiol. 1999, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, C.; Sahm, K.; Jorgensen, B.B. Psychrophilic sulfate-reducing bacteria isolated from permanently cold Arctic marine sediments: Description of Desulfofrigus oceanense gen. nov., sp. nov., Desulfofrigus fragile sp. nov., Desulfofaba gelida gen. nov., sp. nov., Desulfotalea psychrophila gen. nov., sp. nov. and Desulfotalea arctica sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 1631–1643. [Google Scholar]
- Kawamoto, J.; Kurihara, T.; Yamamoto, K.; Nagayasu, M.; Tani, Y.; Mihara, H.; Hosokawa, M.; Baba, T.; Sato, S.B.; Esaki, N. Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. J. Bacteriol. 2009, 191, 632–640. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, X.; Ou, H.Y.; Gai, Y.; Wang, F. Role and regulation of fatty acid biosynthesis in the response of Shewanella piezotolerans WP3 to different temperatures and pressures. J. Bacteriol. 2009, 191, 2574–2584. [Google Scholar] [CrossRef]
- Poerschmann, J.; Spijkerman, E.; Langer, U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb. Ecol. 2004, 48, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, I.; Hirota, K.; Iwata, H.; Akutsu, M.; Kusumoto, K.; Morita, N.; Ezura, Y.; Okuyama, H.; Matsuyama, H. Temperature and nutrient availability control growth rate and fatty acid composition of facultatively psychrophilic Cobetia marina strain L-2. Arch. Microbiol. 2004, 181, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Drouin, P.; Prévost, D.; Antoun, H. Physiological adaptation to low temperatures of strains of Rhizobium leguminosarum bv. viciae associated with Lathyrus spp. FEMS Microbiol. Ecol. 2000, 32, 111–120. [Google Scholar] [PubMed]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [PubMed]
- Zhang, Y.M.; Rock, C.O. Thematic review series: Glycerolipids. Acyltransferases in bacterial glycerophospholipid synthesis. J. Lipid Res. 2008, 49, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Rock, C.O. Phosphatidic acid synthesis in bacteria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 495–502. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef]
- Lightner, V.A.; Larson, T.J.; Tailleur, P.; Kantor, G.D.; Raetz, C.R.H.; Bell, R.M.; Modrich, P. Membrane phospholipid synthesis in Escherichia coli. Cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1980, 255, 9413–9420. [Google Scholar]
- Green, P.R.; Merrill, A.H.; Bell, R.M. Membrane phospholipid synthesis in Escherichia coli. Purification, reconstitution, and characterization of sn-glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1981, 256, 11151–11159. [Google Scholar]
- Lu, Y.J.; Zhang, Y.M.; Grimes, K.D.; Qi, J.; Lee, R.E.; Rock, C.O. Acyl-phosphates initiate membrane phospholipid synthesis in Gram-positive pathogens. Mol. Cell 2006, 23, 765–772. [Google Scholar] [CrossRef]
- Coleman, J. Characterization of the Escherichia coli gene for 1-acyl-sn-glycerol-3-phosphate acyltransferase (plsC). MGG Mol. Gen. Genet. 1992, 232, 295–303. [Google Scholar] [CrossRef]
- Robertson, R.M.; Yao, J.; Gajewski, S.; Kumar, G.; Martin, E.W.; Rock, C.O.; White, S.W. A two-helix motif positions the lysophosphatidic acid acyltransferase active site for catalysis within the membrane bilayer. Nat. Struct. Mol. Biol. 2017, 24, 666–671. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Wu, Y.; Zhao, S.; Bao, J.; Luo, Y.; Li, D. Structural insights into the committed step of bacterial phospholipid biosynthesis. Nat. Commun. 2017, 8, 1691. [Google Scholar] [CrossRef]
- Shih, G.C.; Kahler, C.M.; Swartley, J.S.; Rahman, M.M.; Coleman, J.; Carlson, R.W.; Stephens, D.S. Multiple lysophosphatidic acid acyltransferases in Neisseria meningitidis. Mol. Microbiol. 1999, 32, 942–952. [Google Scholar] [CrossRef]
- Cullinane, M.; Baysse, C.; Morrissey, J.P.; O’Gara, F. Identification of two lysophosphatidic acid acyltransferase genes with overlapping function in Pseudomonas fluorescens. Microbiology 2005, 151, 3071–3080. [Google Scholar] [CrossRef]
- Baysse, C.; Cullinane, M.; Dénervaud, V.; Burrowes, E.; Dow, J.M.; Morrissey, J.P.; Tam, L.; Trevors, J.T.; O’Gara, F. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 2005, 151, 2529–2542. [Google Scholar] [CrossRef]
- Aygun-Sunar, S.; Bilaloglu, R.; Goldfine, H.; Daldal, F. Rhodobacter capsulatus OlsA is a bifunctional enzyme active in both ornithine lipid and phosphatidic acid biosynthesis. J. Bacteriol. 2007, 189, 8564–8574. [Google Scholar] [CrossRef]
- Sato, S.; Kawamoto, J.; Sato, S.B.; Watanabe, B.; Hiratake, J.; Esaki, N.; Kurihara, T. Occurrence of a bacterial membrane microdomain at the cell division site enriched in phospholipids with polyunsaturated hydrocarbon chains. J. Biol. Chem. 2012, 287, 24113–24121. [Google Scholar] [CrossRef]
- Yokoyama, F.; Kawamoto, J.; Imai, T.; Kurihara, T. Characterization of extracellular membrane vesicles of an Antarctic bacterium, Shewanella livingstonensis Ac10, and their enhanced production by alteration of phospholipid composition. Extremophiles 2017, 21, 723–731. [Google Scholar] [CrossRef]
- Tokunaga, T.; Watanabe, B.; Sato, S.; Kawamoto, J.; Kurihara, T. Synthesis and functional assessment of a novel fatty acid probe, ω-ethynyl eicosapentaenoic acid analog, to analyze the in vivo behavior of eicosapentaenoic acid. Bioconjug. Chem. 2017, 28, 2077–2085. [Google Scholar] [CrossRef]
- Kawai, S.; Kawamoto, J.; Ogawa, T.; Kurihara, T. Development of a regulatable low-temperature protein expression system using the psychrotrophic bacterium, Shewanella livingstonensis Ac10, as the host. Biosci. Biotechnol. Biochem. 2019, 83, 2153–2162. [Google Scholar] [CrossRef]
- Cho, H.N.; Kasai, W.; Kawamoto, J.; Esaki, N.; Kurihara, T. Characterization of 1-acyl-sn-glycerol-3-phosphate acyltransferase from a polyunsaturated fatty acid-producing bacterium, Shewanella livingstonensis Ac10. Trace Nutr. Res. 2012, 29, 92–99. [Google Scholar]
- Ogawa, T.; Tanaka, A.; Kawamoto, J.; Kurihara, T. Purification and characterization of 1-acyl-sn-glycerol-3-phosphate acyltransferase with a substrate preference for polyunsaturated fatty acyl donors from the eicosapentaenoic acid-producing bacterium Shewanella livingstonensis Ac10. J. Biochem. 2018, 164, 33–39. [Google Scholar] [CrossRef]
- Toyotake, Y.; Cho, H.N.; Kawamoto, J.; Kurihara, T. A novel 1-acyl-sn-glycerol-3-phosphate O-acyltransferase homolog for the synthesis of membrane phospholipids with a branched-chain fatty acyl group in Shewanella livingstonensis Ac10. Biochem. Biophys. Res. Commun. 2018, 500, 704–709. [Google Scholar] [CrossRef]
- Coleman, J. Characterization of Escherichia coli cells deficient in 1-acyl-sn-glycerol-3-phosphate acyltransferase activity. J. Biol. Chem. 1990, 265, 17215–17221. [Google Scholar]
- Cao, G.J.; Pogliano, J.; Sarkar, N. Identification of the coding region for a second poly(A) polymerase in Escherichia coli. Proc. Natl. Acad. Sci. USA 1996, 93, 11580–11585. [Google Scholar] [CrossRef]
- Mohanty, B.K.; Kushner, S.R. Residual polyadenylation in poly(A) polymerase I (pcnB) mutants of Escherichia coli does not result from the activity encoded by the f310 gene. Mol. Microbiol. 1999, 34, 1109–1119. [Google Scholar] [CrossRef]
- Heath, R.J.; Rock, C.O. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J. Bacteriol. 1998, 180, 1425–1430. [Google Scholar] [CrossRef]
- Yamashita, A.; Nakanishi, H.; Suzuki, H.; Kamata, R.; Tanaka, K.; Waku, K.; Sugiura, T. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 1202–1215. [Google Scholar] [CrossRef]
- Babu, V.M.P.; Itsko, M.; Baxter, J.C.; Schaaper, R.M.; Sutton, M.D. Insufficient levels of the nrdAB-encoded ribonucleotide reductase underlie the severe growth defect of the Δhda E. coli strain. Mol. Microbiol. 2017, 104, 377–399. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Dole, V.P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Investig. 1956, 35, 150–154. [Google Scholar] [CrossRef]
- Dole, V.P.; Meinertz, H. Microdetermination of long-chain fatty acids in plasma and tissues. J. Biol. Chem. 1960, 235, 2595–2599. [Google Scholar]
- Ito, T.; Gong, C.; Kawamoto, J.; Kurihara, T. Development of a versatile method for targeted gene deletion and insertion by using the pyrF gene in the psychrotrophic bacterium, Shewanella livingstonensis Ac10. J. Biosci. Bioeng. 2016, 122, 645–651. [Google Scholar] [CrossRef]
- Nishiyama, M. High-pressure microscopy for tracking dynamic properties of molecular machines. Biophys. Chem. 2017, 231, 71–78. [Google Scholar] [CrossRef]
- Nishiyama, M.; Arai, Y. Tracking the movement of a single prokaryotic cell in extreme environmental conditions. In The Bacterial Flagellum. Methods in Molecular Biology; Minamino, T., Namba, K., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1593, pp. 175–184. [Google Scholar]
- Nishiyama, M.; Kojima, S. Bacterial motility measured by a miniature chamber for high-pressure microscopy. Int. J. Mol. Sci. 2012, 13, 9225–9239. [Google Scholar] [CrossRef]
- Furuno, M.; Atsumi, T.; Yamada, T.; Kojima, S.; Nishioka, N.; Kawagishi, I.; Homma, M. Characterization of polar-flagellar-length mutants in Vibrio alginolyticus. Microbiology 1997, 143, 1615–1621. [Google Scholar] [CrossRef]
- Wood, T.K.; González Barrios, A.F.; Herzberg, M.; Lee, J. Motility influences biofilm architecture in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 361–367. [Google Scholar] [CrossRef]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef]
- Kojima, S. Dynamism and regulation of the stator, the energy conversion complex of the bacterial flagellar motor. Curr. Opin. Microbiol. 2015, 28, 66–71. [Google Scholar] [CrossRef]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef]
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of motility—A proposed history of motility systems in the tree of life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef]
- Sakai, T.; Inoue, Y.; Terahara, N.; Namba, K.; Minamino, T. A triangular loop of domain D1 of FlgE is essential for hook assembly but not for the mechanical function. Biochem. Biophys. Res. Commun. 2018, 495, 1789–1794. [Google Scholar] [CrossRef]
- Barker, C.S.; Prüß, B.M.; Matsumura, P. Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J. Bacteriol. 2004, 186, 7529–7537. [Google Scholar] [CrossRef]
- Tamar, E.; Koler, M.; Vaknin, A. The role of motility and chemotaxis in the bacterial colonization of protected surfaces. Sci. Rep. 2016, 6, 19616. [Google Scholar] [CrossRef]
- Wang, X.; Wood, T.K. IS5 inserts upstream of the master motility operon flhDC in a quasi-Lamarckian way. ISME J. 2011, 5, 1517–1525. [Google Scholar] [CrossRef]
- Inoue, K.; Matsuzaki, H.; Matsumoto, K.; Shibuya, I. Unbalanced membrane phospholipid compositions affect transcriptional expression of certain regulatory genes in Escherichia coli. J. Bacteriol. 1997, 179, 2872–2878. [Google Scholar] [CrossRef]
- Lai, H.C.; Soo, P.C.; Wei, J.R.; Yi, W.C.; Liaw, S.J.; Horng, Y.T.; Lin, S.M.; Ho, S.W.; Swift, S.; Williams, P. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J. Bacteriol. 2005, 187, 3407–3414. [Google Scholar] [CrossRef]
- Amin, D.N.; Hazelbauer, G.L. Influence of membrane lipid composition on a transmembrane bacterial chemoreceptor. J. Biol. Chem. 2012, 287, 41697–41705. [Google Scholar] [CrossRef]
- Keller, R.; Ariöz, C.; Hansmeier, N.; Stenberg-Bruzell, F.; Burstedt, M.; Vikström, D.; Kelly, A.; Wieslander, Å.; Daley, D.O.; Hunke, S. The Escherichia coli Envelope Stress Sensor CpxA responds to changes in lipid bilayer properties. Biochemistry 2015, 54, 3670–3676. [Google Scholar] [CrossRef]
- Blanka, A.; Düvel, J.; Dötsch, A.; Klinkert, B.; Abraham, W.R.; Kaever, V.; Ritter, C.; Narberhaus, F.; Häussler, S. Constitutive production of c-di-GMP is associated with mutations in a variant of Pseudomonas aeruginosa with altered membrane composition. Sci. Signal. 2015, 8, ra36. [Google Scholar] [CrossRef]
- Fuller, R.S.; Kornberg, A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc. Natl. Acad. Sci. USA 1983, 80, 5817–5821. [Google Scholar] [CrossRef]
- Fuller, R.S.; Funnell, B.E.; Kornberg, A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell 1984, 38, 889–900. [Google Scholar] [CrossRef]
- Mizushima, T.; Tomura, A.; Shinpuku, T.; Miki, T.; Sekimizu, K. Loss of flagellation in dnaA mutants of Escherichia coli. J. Bacteriol. 1994, 176, 5544–5546. [Google Scholar] [CrossRef]
- Mizushima, T.; Koyanagi, R.; Katayama, T.; Miki, T.; Sekimizu, K. Decrease in expression of the master operon of flagellin synthesis in a dnaA46 mutant of Escherichia coli. Biol. Pharm. Bull. 1997, 20, 327–331. [Google Scholar] [CrossRef][Green Version]
- Speck, C.; Weigel, C.; Messer, W. ATP- and ADP-DnaA protein, a molecular switch in gene regulation. EMBO J. 1999, 18, 6169–6176. [Google Scholar] [CrossRef]
- Yung, B.Y.M.; Kornberg, A. Membrane attachment activates dnaA protein, the initiation protein of chromosome replication in Escherichia coli. Proc. Natl. Acad. Sci. USA 1988, 85, 7202–7205. [Google Scholar] [CrossRef]
- Castuma, C.E.; Crooke, E.; Kornberg, A. Fluid membranes with acidic domains activate DnaA, the initiator protein of replication in Escherichia coli. J. Biol. Chem. 1993, 268, 24665–24668. [Google Scholar]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyotake, Y.; Nishiyama, M.; Yokoyama, F.; Ogawa, T.; Kawamoto, J.; Kurihara, T. A Novel Lysophosphatidic Acid Acyltransferase of Escherichia coli Produces Membrane Phospholipids with a cis-vaccenoyl Group and Is Related to Flagellar Formation. Biomolecules 2020, 10, 745. https://doi.org/10.3390/biom10050745
Toyotake Y, Nishiyama M, Yokoyama F, Ogawa T, Kawamoto J, Kurihara T. A Novel Lysophosphatidic Acid Acyltransferase of Escherichia coli Produces Membrane Phospholipids with a cis-vaccenoyl Group and Is Related to Flagellar Formation. Biomolecules. 2020; 10(5):745. https://doi.org/10.3390/biom10050745
Chicago/Turabian StyleToyotake, Yosuke, Masayoshi Nishiyama, Fumiaki Yokoyama, Takuya Ogawa, Jun Kawamoto, and Tatsuo Kurihara. 2020. "A Novel Lysophosphatidic Acid Acyltransferase of Escherichia coli Produces Membrane Phospholipids with a cis-vaccenoyl Group and Is Related to Flagellar Formation" Biomolecules 10, no. 5: 745. https://doi.org/10.3390/biom10050745
APA StyleToyotake, Y., Nishiyama, M., Yokoyama, F., Ogawa, T., Kawamoto, J., & Kurihara, T. (2020). A Novel Lysophosphatidic Acid Acyltransferase of Escherichia coli Produces Membrane Phospholipids with a cis-vaccenoyl Group and Is Related to Flagellar Formation. Biomolecules, 10(5), 745. https://doi.org/10.3390/biom10050745




