Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Design
2.3. Biochemical Assays
2.4. Gelatin Zymography
2.5. Western Blot Assay
2.6. Liver Histology
2.7. Statistical Analysis
3. Results
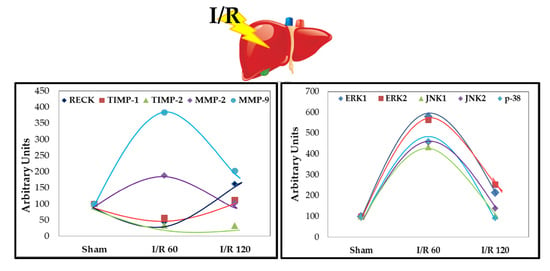
3.1. Transient Expression of Reck, Mmps, and Timps in a Rat Model of Hepatic I/R Injury
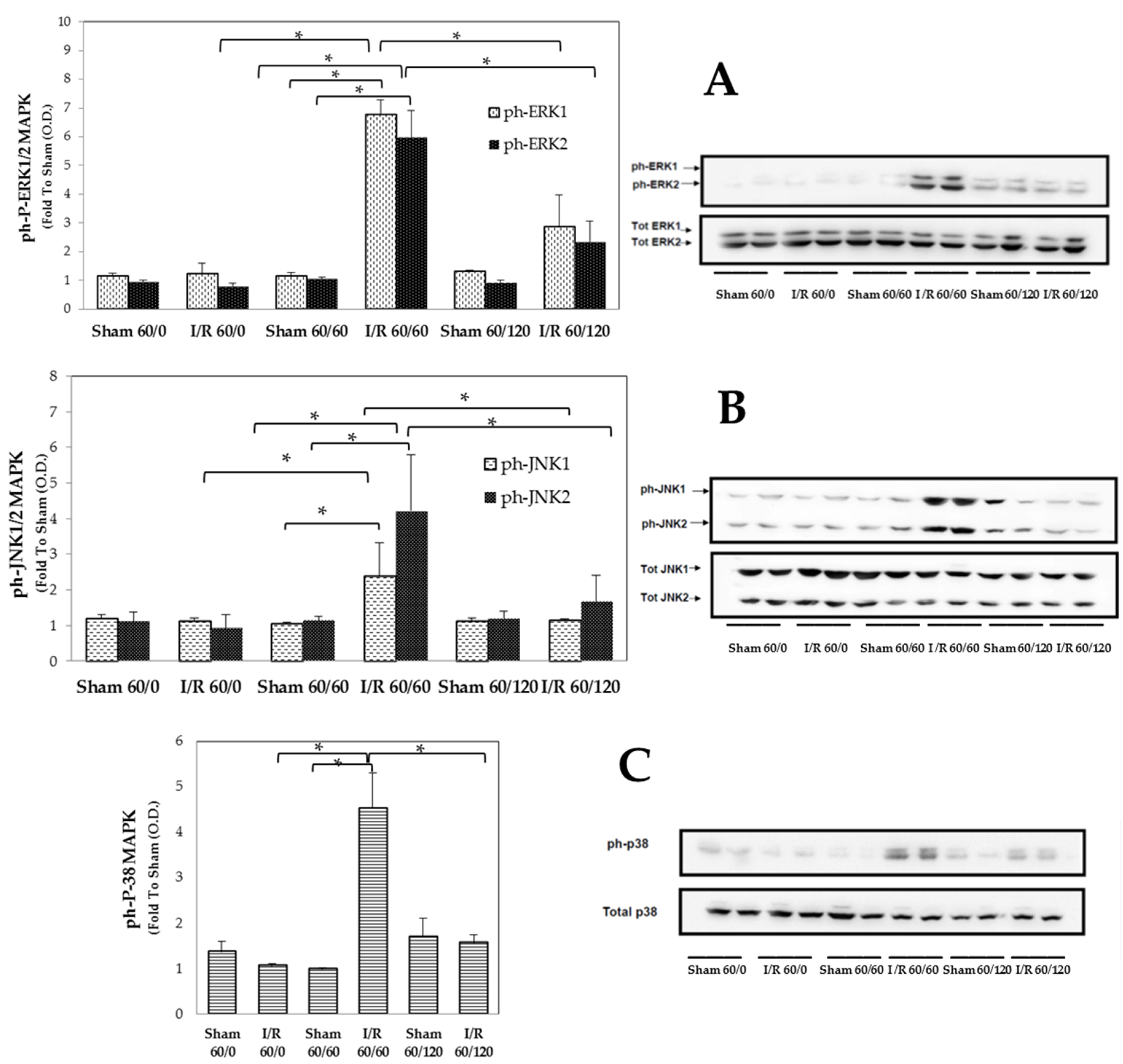
3.2. Transient Expression of Mapks under Hepatic I/R
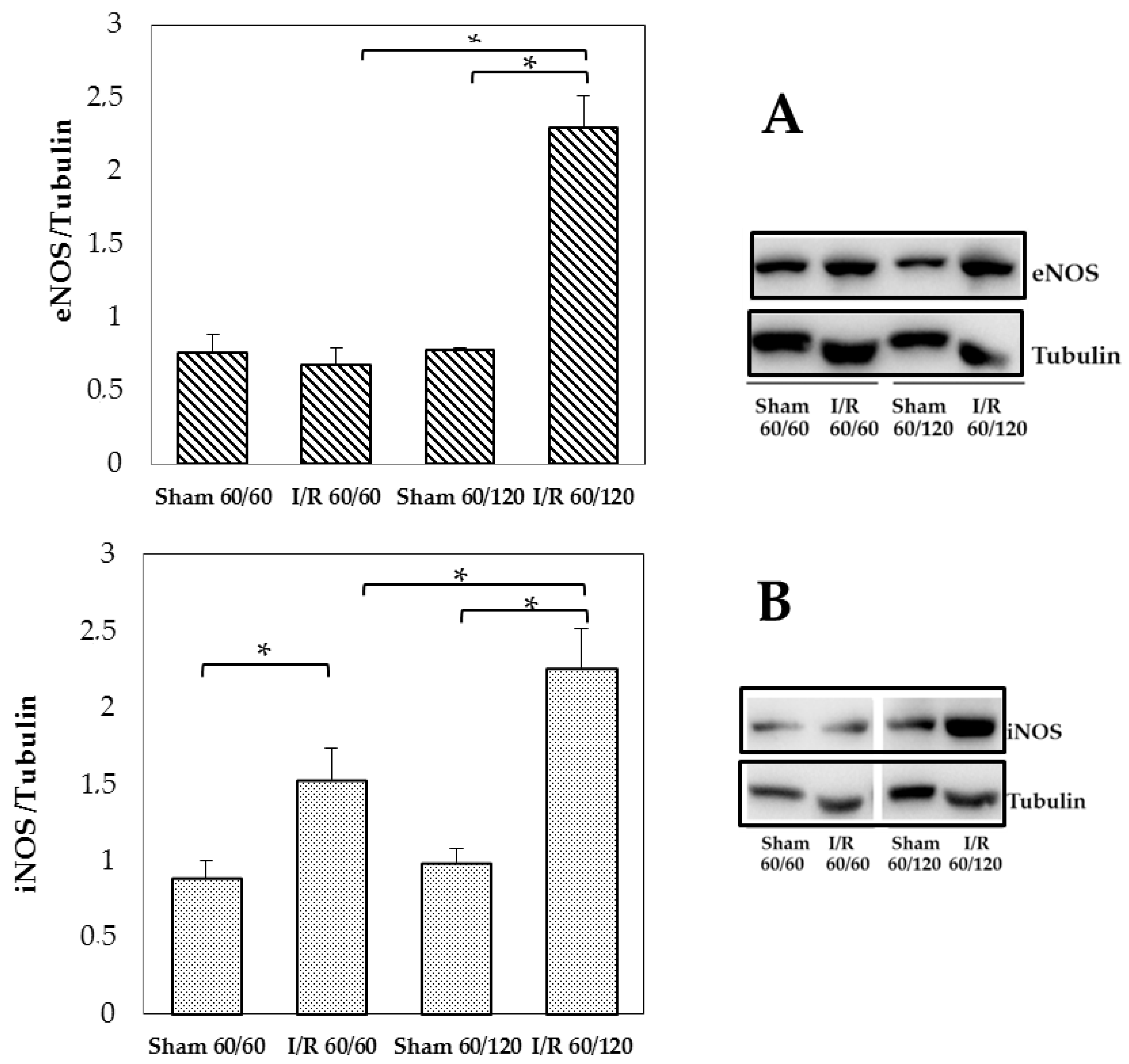
3.3. Changes in Enos and Inos Expression Under Hepatic I/R
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Niu, P.; Chen, K.; Xia, Y.; Yu, Q.; Liu, N.; Li, J.; Li, S.; Wu, L.; Feng, J.; et al. The liver protection of propylene glycol alginate sodium sulfate preconditioning against ischemia reperfusion injury: Focusing MAPK pathway activity. Sci. Rep. 2017, 7, 15175. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takeyoshi, I.; Yoshinari, D.; Matsumoto, K.; Morishita, Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery 2002, 131, 344–349. [Google Scholar] [CrossRef]
- Toledo-Pereyra, L.H.; Lopez-Neblina, F.; Toledo, A.H. Protein Kinases in Organ Ischemia and Reperfusion. J. Investig. Surg. 2008, 21, 215–226. [Google Scholar] [CrossRef]
- Viappiani, S.; Sariahmetoglu, M.; Schulz, R. The role of matrix metalloproteinase inhibitors in ischemia-reperfusion injury in the liver. Curr. Pharm. Des. 2006, 12, 2923–2934. [Google Scholar] [CrossRef]
- Khandoga, A.; Kessler, J.S.; Hanschen, M.; Burggraf, D.; Reichel, C.; Hamann, G.F.; Enders, G.; Krombach, F. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J. Leukoc. Boil. 2006, 79, 1295–1305. [Google Scholar] [CrossRef]
- Palladini, G.; Ferrigno, A.; Rizzo, V.; Boncompagni, E.; Richelmi, P.; Freitas, I.; Perlini, S.; Vairetti, M. Lobe-Specific Heterogeneity and Matrix Metalloproteinase Activation after Ischemia/Reperfusion Injury in Rat Livers. Toxicol. Pathol. 2012, 40, 722–730. [Google Scholar] [CrossRef]
- Coito, A.J. Leukocyte transmigration across endothelial and extracellular matrix protein barriers in liver ischemia/reperfusion injury. Curr. Opin. Organ Transplant. 2011, 16, 34–40. [Google Scholar] [CrossRef]
- Hamada, T.; Fondevila, C.; Busuttil, R.W.; Coito, A.J. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2007, 47, 186–198. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Medina, C.; Ledwidge, M.; Radomski, M.W.; Gilmer, J.F. Nitric oxide-matrix metaloproteinase-9 interactions: Biological and pharmacological significance. Biochim. Biophys. Acta 2014, 1843, 603–617. [Google Scholar] [CrossRef]
- Hamada, T.; Duarte, S.; Tsuchihashi, S.; Busuttil, R.W.; Coito, A.J. Inducible Nitric Oxide Synthase Deficiency Impairs Matrix Metalloproteinase-9 Activity and Disrupts Leukocyte Migration in Hepatic Ischemia/Reperfusion Injury. Am. J. Pathol. 2009, 174, 2265–2277. [Google Scholar] [CrossRef]
- Oh, J.; Takahashi, R.; Kondo, S.; Mizoguchi, A.; Adachi, E.; Sasahara, R.M.; Nishimura, S.; Imamura, Y.; Kitayama, H.; Alexander, D.B.; et al. The Membrane-Anchored MMP Inhibitor RECK is a Key Regulator of Extracellular Matrix Integrity and Angiogenesis. Cell 2001, 107, 789–800. [Google Scholar] [CrossRef]
- Chang, C.-K.; Hung, W.-C.; Chang, H.-C. The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo. J. Cell. Mol. Med. 2008, 12, 2781–2789. [Google Scholar] [CrossRef]
- Lee, H.N.; Mitra, M.; Bosompra, O.; Corney, D.C.; Johnson, E.L.; Rashed, N.; Ho, L.D.; Coller, H.A. RECK isoforms have opposing effects on cell migration. Mol. Boil. Cell 2018, 29, 1825–1838. [Google Scholar] [CrossRef]
- Meng, N.; Li, Y.; Zhang, H.; Sun, X.-F. RECK, a novel matrix metalloproteinase regulator. Histol. Histopathol. 2008, 23, 1003–1010. [Google Scholar]
- Ferrigno, A.; Palladini, G.; Bianchi, A.; Rizzo, V.; Di Pasqua, L.G.; Perlini, S.; Richelmi, P.; Vairetti, M. Lobe-Specific Heterogeneity in Asymmetric Dimethylarginine and Matrix Metalloproteinase Levels in a Rat Model of Obstructive Cholestasis. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ferrigno, A.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Richelmi, P.; Vairetti, M. Oxygen tension-independent protection against hypoxic cell killing in rat liver by low sodium. Eur. J. Histochem. 2017, 61, 61. [Google Scholar] [CrossRef]
- Mastrocola, R.; Penna, C.R.R.; Tullio, F.; Femminò, S.; Nigro, D.; Chiazza, F.; Serpe, L.; Collotta, D.; Alloatti, G.; Cocco, M.; et al. Pharmacological Inhibition of NLRP3 Inflammasome Attenuates Myocardial Ischemia/Reperfusion Injury by Activation of RISK and Mitochondrial Pathways. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Cursio, R.; Filippa, N.; Miele, C.; Van Obberghen, E.; Gugenheim, J. Involvement of protein kinase B and mitogen-activated protein kinases in experimental normothermic liver ischaemia–reperfusion injury. BJS 2006, 93, 752–761. [Google Scholar] [CrossRef]
- Yan, K.H.; Lee, L.M.; Yan, S.H.; Huang, H.C.; Li, C.C.; Lin, H.T.; Chen, P.S. Tomadine inhibits invasion of human lung adenocarcinoma cell A549 by reducing matrix metalloproteinases expression. Chem. Biol. Interact. 2013, 203, 580–587. [Google Scholar] [CrossRef]
- Bahgat, D.M.R.; Shahin, R.M.H.; Makar, N.N.; Aziz, A.O.A.; Hunter, S.S. Reversion-Inducing-Cysteine-Rich Protein With Kazal Motifs (RECK) Gene Single Nucleotide Polymorphism With Hepatocellular Carcinoma: A Case-Control Study. J. Clin. Lab. Anal. 2014, 30, 36–40. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, K.Y.; Lee, Y.M. Downregulation of a tumor suppressor RECK by hypoxia through recruitment of HDAC1 and HIF-1α to reverse HRE site in the promoter. Biochim. Biophys. Acta 2010, 1803, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Zheng, M.; Chen, J.; Chen, H.; Liu, N. Effects of treadmill exercise on cerebral angiogenesis and MT1-MMP expression after cerebral ischemia in rats. Brain Behav. 2018, 8, e01079. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Boil. 2015, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Hamada, T.; Kuriyama, N.; Busuttil, R.W.; Coito, A.J. TIMP-1 deficiency leads to lethal partial hepatic ischemia and reperfusion injury. Hepatol. 2012, 56, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Cursio, R.; Mari, B.; Louis, K.; Rostagno, P.; Saint-Paul, M.-C.; Giudicelli, J.; Bottero, V.; Anglard, P.; Yiotakis, A.; Dive, V.; et al. Rat liver injury following normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB J. 2001, 16, 1–24. [Google Scholar] [CrossRef]
- Jeon, H.W.; Lee, K.-J.; Lee, S.H.; Kim, W.-H.; Lee, Y.M. Attenuated expression and function of the RECK tumor suppressor under hypoxic conditions is mediated by the MAPK signaling pathways. Arch. Pharmacal Res. 2011, 34, 137–145. [Google Scholar] [CrossRef]
- Deng, J.; Feng, J.; Liu, T.; Lu, X.; Wang, W.; Liu, N.; Lv, Y.; Liu, Q.; Guo, C.; Zhou, Y. Beraprost sodium preconditioning prevents inflammation, apoptosis, and autophagy during hepatic ischemia-reperfusion injury in mice via the P38 and JNK pathways. Drug Des. Dev. Ther. 2018, 12, 4067–4082. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Casillas-Ramírez, A.; Peralta, C. Mitogen Activated Protein Kinases in Steatotic and Non-Steatotic Livers Submitted to Ischemia-Reperfusion. Int. J. Mol. Sci. 2019, 20, 1785. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, S.-E.; Lee, Y.-S. SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury. Exp. Mol. Med. 2006, 38, 408–416. [Google Scholar] [CrossRef][Green Version]
- Yu, Q.; Wu, L.; Liu, T.; Li, S.; Feng, J.; Mao, Y.; Fan, X.; Guo, C.; Wu, J. Protective effects of levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are mediated by inhibition of the ERK/NF-κB pathway. Int. Immunopharmacol. 2019, 70, 435–445. [Google Scholar] [CrossRef]
- Li, J.; Billiar, T.R., IV. Determinants of nitric oxide protection and toxicity in liver. Am. J. Physiol. Liver Physiol. 1999, 276, G1069–G1073. [Google Scholar] [CrossRef]
- Isobe, M.; Katsuramaki, T.; Hirata, K.; Kimura, H.; Nagayama, M.; Matsuno, T. Beneficial Effects of Inducible Nitric Oxide Synthase Inhibitor on Reperfusion Injury in the Pig Liver. Transplantation 1999, 68, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Morisue, A.; Wakabayashi, G.; Shimazu, M.; Tanabe, M.; Mukai, M.; Matsumoto, K.; Kawachi, S.; Yoshida, M.; Yamamoto, S.; Kitajima, M. The role of nitric oxide after a short period of liver ischemia-reperfusion. J. Surg. Res. 2003, 109, 101–109. [Google Scholar] [CrossRef]
- Cowled, P.A.; Khanna, A.; Laws, P.E.; Field, J.B.F.; Fitridge, R.A. Simvastatin Plus Nitric Oxide Synthase Inhibition Modulates Remote Organ Damage Following Skeletal Muscle Ischemia-Reperfusion Injury. J. Investig. Surg. 2008, 21, 119–126. [Google Scholar] [CrossRef] [PubMed]


| Sham 60/60 | I/R 60/60 | Sham 60/120 | I/R 60/120 | |
|---|---|---|---|---|
| AST | 243 ± 57 | 3444 ± 1062 | 198 ± 43 | 10,387 ± 1158 * |
| ALT | 66 ± 19 | 3830 ± 961 | 61 ± 28 | 9320 ± 1040 * |
| ALP | 431 ± 50 | 605 ± 51 | 417 ± 55 | 769 ± 29 * |
| Total Bilirubin | 0.13 ± 0.024 | 0.25 ± 0.070 | 0.12 ± 0.011 | 0.35 ± 0.043 |
| Direct Bilirubin | 0.045 ± 0.019 | 0.17 ± 0.032 | 0.04 ± 0.024 | 0.18 ± 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrigno, A.; Di Pasqua, L.G.; Palladini, G.; Berardo, C.; Verta, R.; Richelmi, P.; Perlini, S.; Collotta, D.; Collino, M.; Vairetti, M. Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways. Biomolecules 2020, 10, 747. https://doi.org/10.3390/biom10050747
Ferrigno A, Di Pasqua LG, Palladini G, Berardo C, Verta R, Richelmi P, Perlini S, Collotta D, Collino M, Vairetti M. Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways. Biomolecules. 2020; 10(5):747. https://doi.org/10.3390/biom10050747
Chicago/Turabian StyleFerrigno, Andrea, Laura G. Di Pasqua, Giuseppina Palladini, Clarissa Berardo, Roberta Verta, Plinio Richelmi, Stefano Perlini, Debora Collotta, Massimo Collino, and Mariapia Vairetti. 2020. "Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways" Biomolecules 10, no. 5: 747. https://doi.org/10.3390/biom10050747
APA StyleFerrigno, A., Di Pasqua, L. G., Palladini, G., Berardo, C., Verta, R., Richelmi, P., Perlini, S., Collotta, D., Collino, M., & Vairetti, M. (2020). Transient Expression of Reck Under Hepatic Ischemia/Reperfusion Conditions Is Associated with Mapk Signaling Pathways. Biomolecules, 10(5), 747. https://doi.org/10.3390/biom10050747