Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Materials and Extraction
2.2. Profile of Bioactive Compounds
2.3. Determination of Antioxidant and Enzyme Inhibitory Effects
2.4. Statistical Analysis
2.5. In Silico Docking Calculations
3. Results
3.1. Bioactive Compounds
3.2. Antioxidant Assays
3.3. Enzymatic Inhibitory Effects
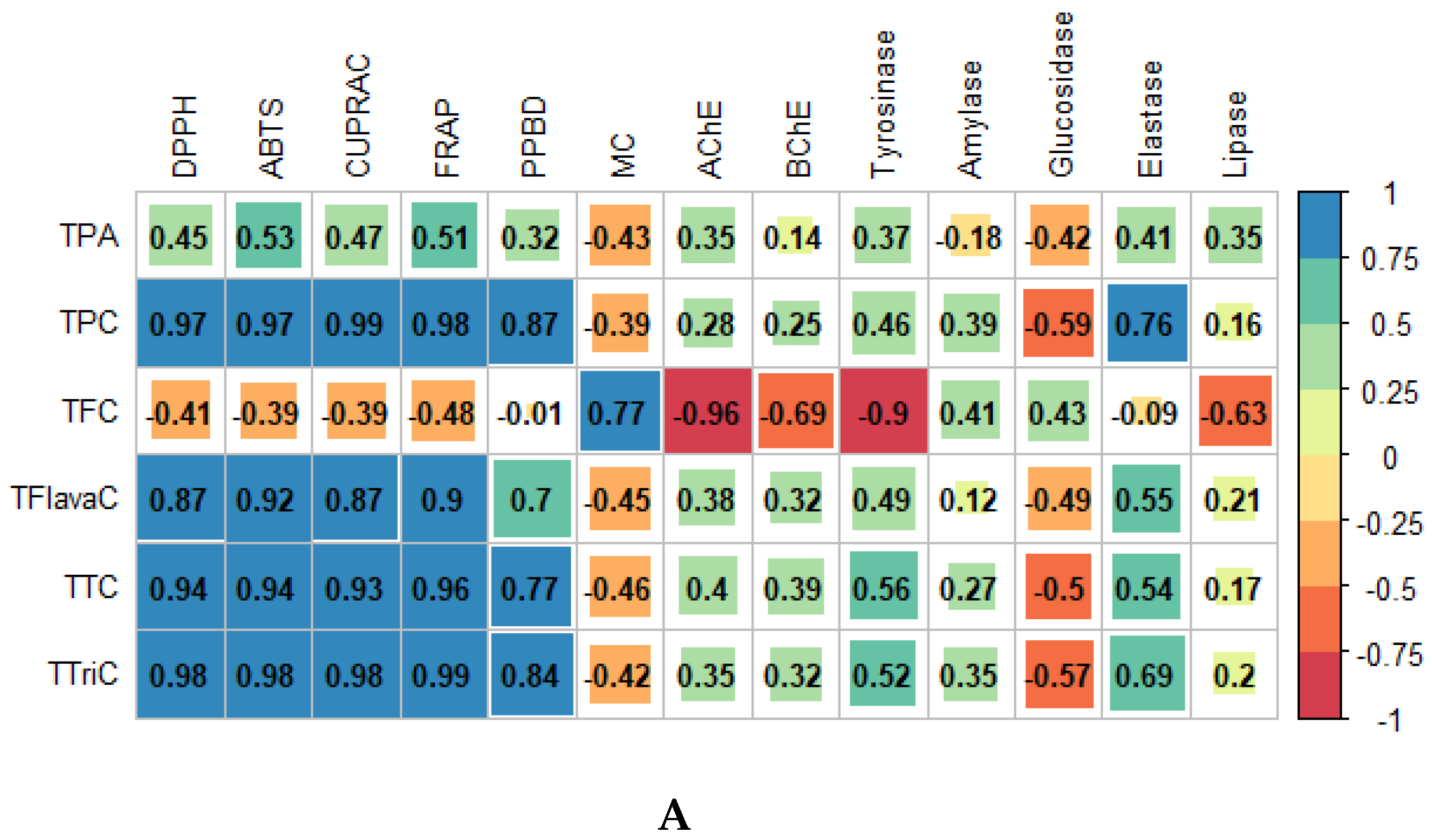
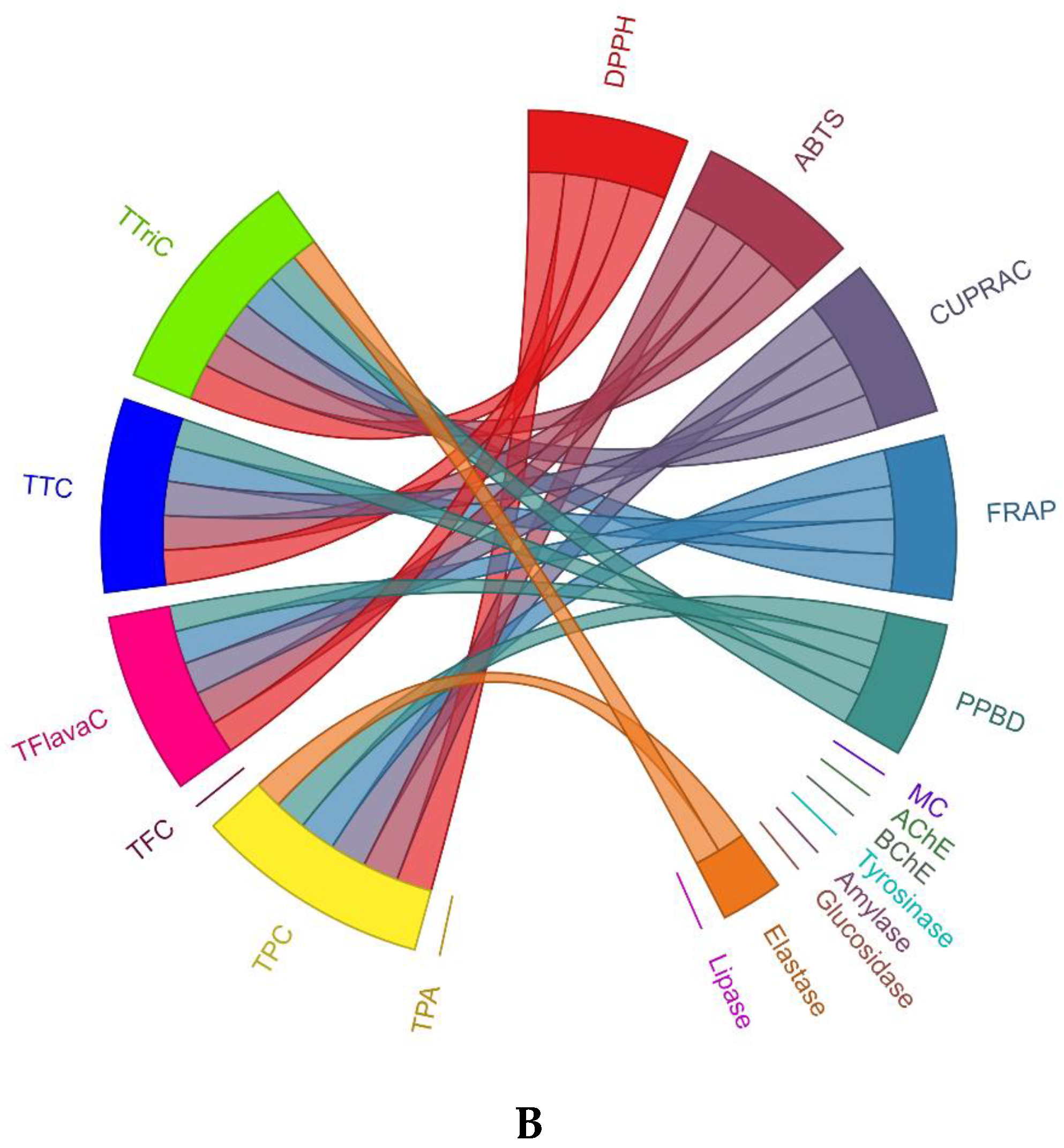
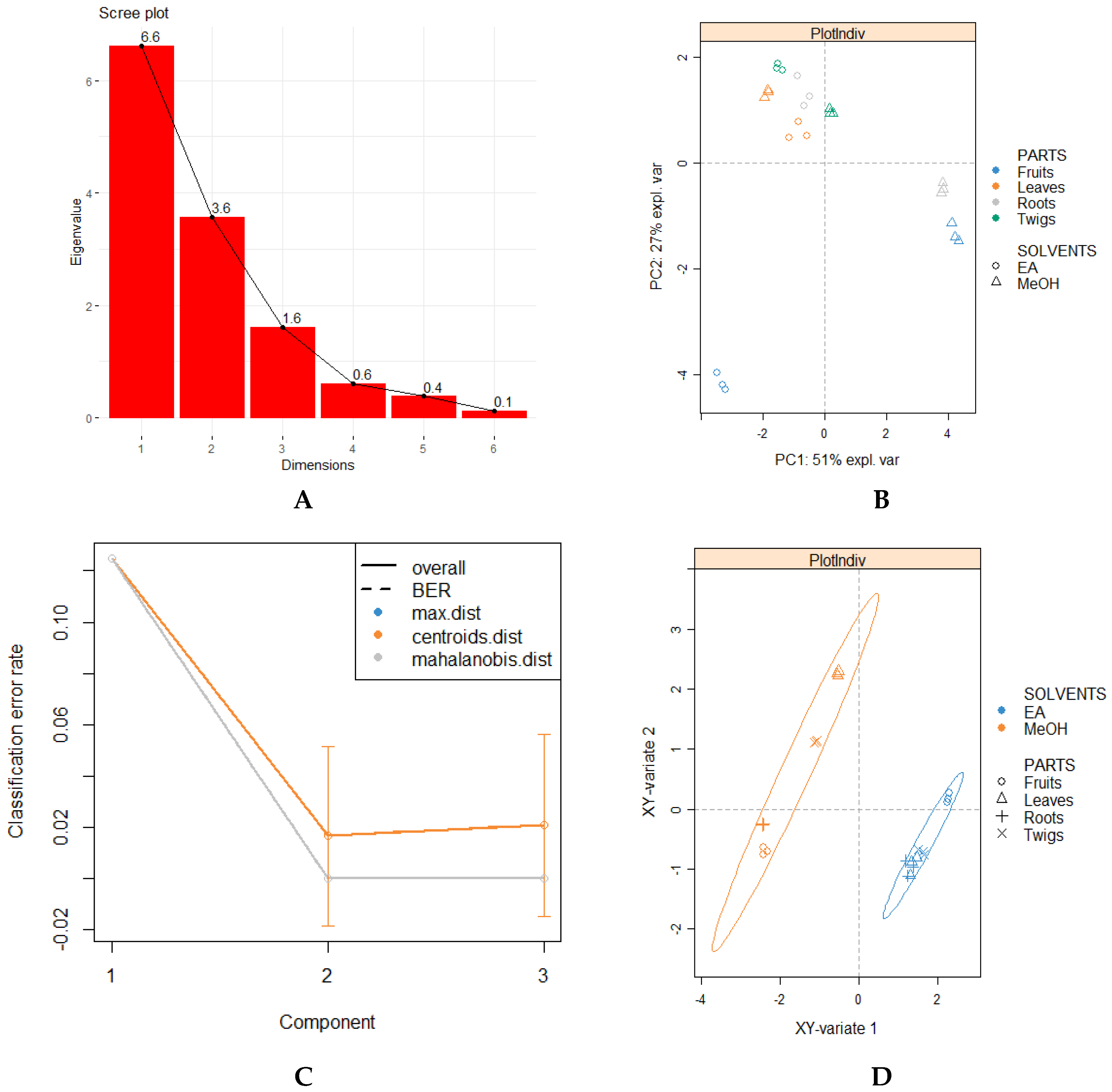
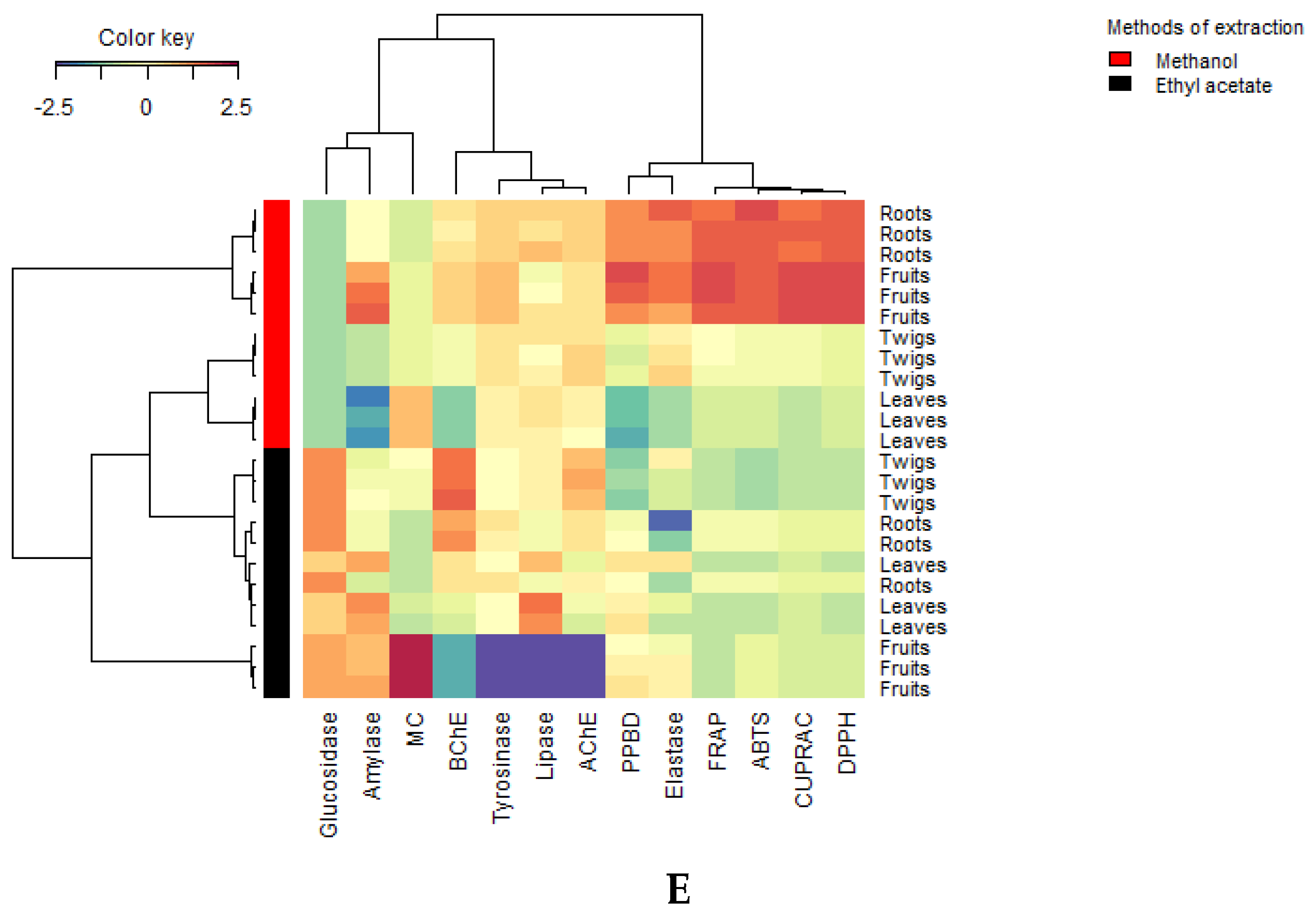
3.4. Multivariate Analysis
3.5. In Silico Docking
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Allkin, B. Useful Plants – Medicines. At least 28,187 plant species are currently recorded as being of medicinal use. In State of the World’s Plants 2017; Willis, K.J., Ed.; Royal Botanic Gardens, Kew: London, UK, 2017. [Google Scholar]
- Black, R. Species Count Put at 8.7 Million. Available online: https://www.bbc.com/news/science-environment-14616161 (accessed on 25 August 2019).
- Dasgupta, S. How Many Plant Species Are There in the World? Scientists NoW Have an Answer. Available online: https://news.mongabay.com/2016/05/many-plants-world-scientists-may-now-answer/ (accessed on 25 August 2019).
- Trager, R. In Situ with Frances Arnold. Available online: https://www.chemistryworld.com/culture/frances-arnold-i-wanted-to-become-an-engineer-of-the-biological-world/3008732.article (accessed on 25 August 2019).
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Mahomoodally, F.M.; Zengin, G.; Jeewon, R.; Nazurally, N.; Rengasamy, R.R.K.; Albuquerque, R.D.D.G.; Shunmugiah, P.K. Ethnopharmacology, phytochemistry, and global distribution of mangroves―A comprehensive review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef]
- Karimulla, B.; Kumar, K. Antidiabetic and antihyperlipidemic activity of bark of Bruguiera gymnorhiza on streptozotocin induced diabetic rats. Asian J. Pharm. Clin. Res. 2011, 1, 4–7. [Google Scholar]
- Krishnamoorthy, M.; Sasikumar, J.M.; Shamna, R.; Pandiarajan, C.; Sofia, P.; Nagarajan, B. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J. Pharmacol. 2011, 43, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Seepana, R.; Perumal, K.; Kada, N.M.; Chatragadda, R.; Raju, M.; Annamalai, V. Evaluation of antimicrobial properties from the mangrove Rhizophora apiculata and Bruguiera gymnorhiza of Burmanallah coast, South Andaman, India. J. Coast. Life Med. 2016, 4, 475–478. [Google Scholar] [CrossRef]
- Barik, R.; Sarkar, R.; Biswas, P.; Bera, R.; Sharma, S.; Nath, S.; Karmakar, S.; Sen, T. 5,7-dihydroxy-2-(3-hydroxy-4, 5-dimethoxy-phenyl)-chromen-4-one-a flavone from Bruguiera gymnorhiza displaying anti-inflammatory properties. Indian J. Pharmacol. 2016, 48, 304–311. [Google Scholar] [CrossRef]
- Banerjee, D.; Chakrabarti, S.; Hazra, A.K.; Banerjee, S.; Ray, J.; Mukherjee, B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr. J. Biotechnol. 2008, 7, 6. [Google Scholar]
- Haq, M.; Sani, W.; Hossain, A.; Taha, R.M.; Monneruzzaman, K.M. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorhiza. J. Med. Plant Res. 2011, 5, 4112–4118. [Google Scholar]
- Reddy, A.; Grace, J.R. Anticancer activity of methanolic extracts of selected mangrove plants. Int. J. Pharm. Sci. Rev. Res. 2016, 7, 3852. [Google Scholar]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid. Based Complement. Altern. Med. 2011, 2011, 578092. [Google Scholar] [CrossRef] [PubMed]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Uysal, S.; Aktumsek, A. A phytochemical study on Potentilla anatolica: An endemic Turkish plant. Ind. Crops Prod. 2015, 76, 1001–1007. [Google Scholar] [CrossRef]
- Vladimir-Knezevic, S.; Blazekovic, B.; Stefan, M.B.; Alegro, A.; Koszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Ferreira, O.; Pinho, S.P. Solubility of flavonoids in pure solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Karim, M.A.; Islam, M.A.; Islam, M.M.; Rahman, M.S.; Sultana, S.; Biswas, S.; Hosen, M.J.; Mozumder, K.; Rahman, M.M.; Hasan, M.N. Evaluation of antioxidant, anti-hemolytic, cytotoxic effects and anti-bacterial activity of selected mangrove plants (Bruguiera gymnorhiza and Heritiera littoralis) in Bangladesh. Clin. Phytoscience 2020, 6, 8. [Google Scholar] [CrossRef]
- Hemm, M.R.; Rider, S.D.; Ogas, J.; Murry, D.J.; Chapple, C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004, 38, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafezi, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Chandrasekaran, A.; Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Dailey, A.; Vuong, Q. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Aparadh, V.T.; Naik, V.V.; Karadge, B.A. Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Ann. di Bot. 2012, 2, 49–56. [Google Scholar]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Lancet, T. Global Burden of Disease Study 2013. Available online: https://www.thelancet.com/gbd/2013 (accessed on 30 August 2019).
- Rauf, A.; Jehan, N. Natural products as potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors & Activators; InTech: Rijeka, Croatia, 2017; pp. 165–177. [Google Scholar]
- Pandey, S.; Sree, A.; Sethi, D.; Kumar, C.; Kakollu, S.; Chowdhury, L.; Dash, S. A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microb. Cell Fact. 2014, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Kumar, V.; Mal, M.; Houghton, P. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Tocco, G.; Fais, A.; Meli, G.; Begala, M.; Podda, G.; Fadda, M.B.; Corda, M.; Attanasi, O.; Filippone, P.; Berretta, S. PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol–coumarin derivatives: Preliminary evaluation of their inhibitory activity on mushroom tyrosinase. Bioorg. Med. Chem. Lett. 2009, 19, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Gani, S.; Zainudin, B.; Abdullah, N. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar]
- Martinez-Gonzalez, A.I.; Diaz-Sanchez, A.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of alpha-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of flavonoids with alpha-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Ahmed, A. History of diabetes mellitus. Saudi Med. J. 2002, 23, 373–378. [Google Scholar]
- Aschner, P. Global Guideline for Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 104, 1–52. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Liu, F.; Hsieh, S.; Lin, C.; Liou, M.; Wang, C.; Huang, C.; Liu, G.; Lin, J. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: A randomized, double-blind, placebo-controlled pilot trial. PLoS ONE 2019, 14, e0221199. [Google Scholar] [CrossRef]
- Doukyu, N.; Ogino, H. Organic solvent-tolerant enzymes. Biochem. Eng. J. 2010, 48, 270–282. [Google Scholar] [CrossRef]
- Carcelli, M.; Rogolino, D.; Bartoli, J.; Pala, N.; Compari, C.; Ronda, N.; Bacciottini, F.; Incerti, M.; Fisicaro, E. Hydroxyphenyl thiosemicarbazones as inhibitors of mushroom tyrosinase and antibrowning agents. Food Chem. 2019, 303, 125310. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. Modifying skin pigmentation–approaches through intrinsic biochemistry and exogenous agents. Drug Discov. Today 2008, 5, e189–e199. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Nazir, Y.; Ashraf, Z.; Rafique, H.; Afzal, S.; Mumtaz, A.; Hassan, M.; Ali, A.; Afzal, K.; Yousuf, M.R. Synthesis, computational studies, tyrosinase inhibitory kinetics and antimelanogenic activity of hydroxy substituted 2-[(4-acetylphenyl) amino]-2-oxoethyl derivatives. J. Enzyme Inhib. Med. Chem. 2019, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844. [Google Scholar] [CrossRef]
- Slominski, A.T.; Paus, R.; Mihm, M. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: Selective review and hypothesis. Anticancer Res. 1998, 18, 3709–3715. [Google Scholar] [PubMed]
- Wu, L.; Chen, C.; Cheng, C.; Dai, H.; Ai, Y.; Lin, C.; Chung, Y. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Yusufzai, S.; Khan, M.S.; Sulaiman, O.; Osman, H.; Lamjin, D.N. Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer’s disease. Chem. Cent. J. 2018, 12, 128. [Google Scholar] [CrossRef]
- Yeomans, K.A.; Golder, P.A. The Guttman-Kaiser Criterion as a predictor of the number of common factors. J. R. Stat. Soc. Ser. D Stat. 1982, 31, 221–229. [Google Scholar] [CrossRef]
- Lê Cao, K.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [PubMed]
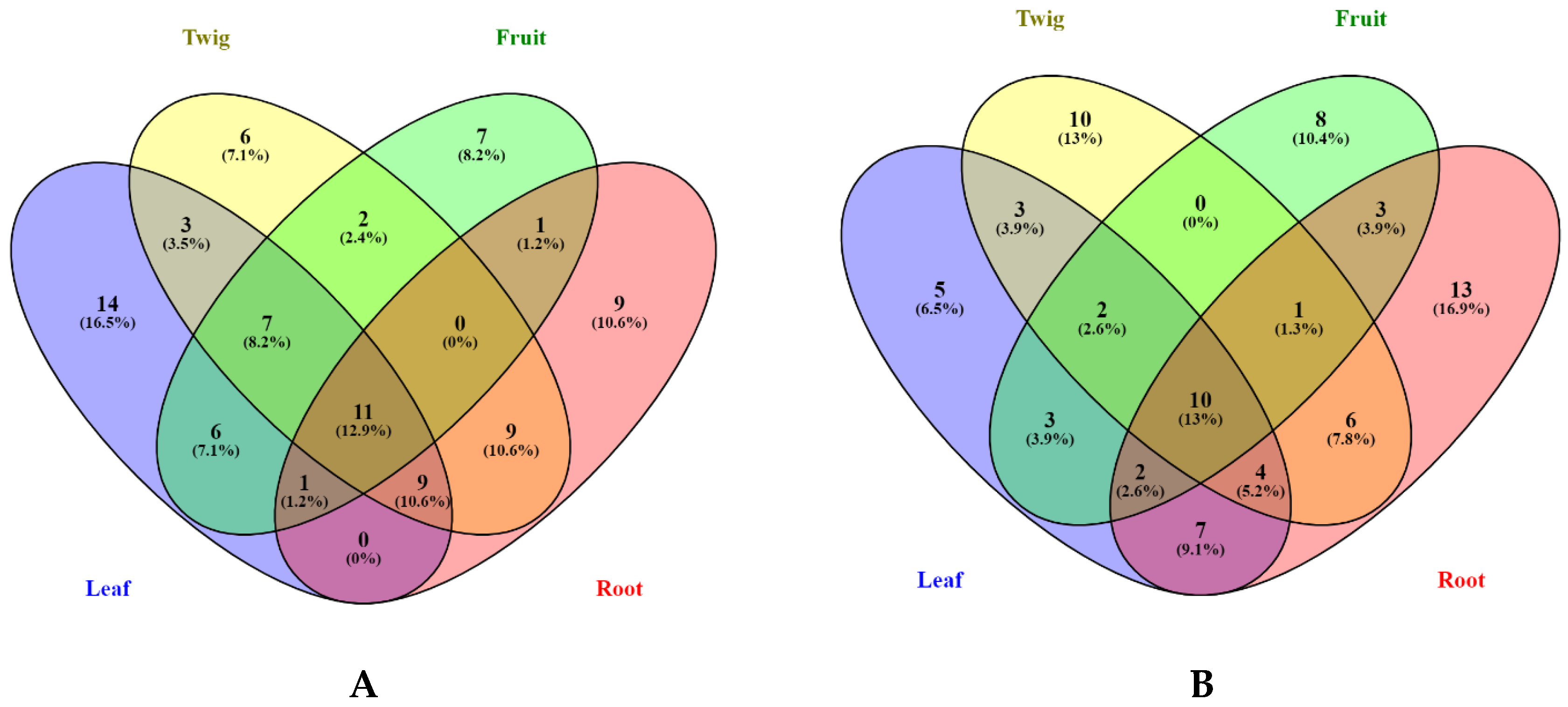

| Compound Name | MeOH | Ethyl Acetate |
|---|---|---|
| Quinic acid | + | + |
| Citric acid | + | - |
| Brugierol | + | + |
| Gallocatechin (Casuarin, Gallocatechol) | + | + |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | + | - |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | + | + |
| Procyanidin B isomer 1 | + | - |
| Procyanidin B isomer 2 | + | - |
| 3-O-(4-Coumaroyl) quinic acid | + | + |
| Catechin | + | + |
| Epigallocatechin (Epigallocatechol) | + | + |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | + | - |
| 3-O-Feruloylquinic acid | + | - |
| Ampelopsin (Ampeloptin, Dihydromyricetin) | + | + |
| Procyanidin B isomer 3 | + | - |
| Vanillin | + | + |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | + | - |
| Epicatechin | + | + |
| 4-O-(4-coumaroyl) quinic acid | + | - |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | + | - |
| 4-Coumaric acid | + | + |
| Antiarol (3,4,5-Trimethoxyphenol) | - | - |
| Loliolide or Isololiolide | + | + |
| 4-O-Feruloylquinic acid | + | - |
| Riboflavin | - | - |
| Indole-3-lactic acid | + | - |
| Ferulic acid | + | - |
| Loliolide or Isololiolide | + | + |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | + | + |
| Sinapic acid (Sinapinic acid) | - | - |
| Myricetin-3-O-rutinoside | + | + |
| Cinchonain I isomer 1 | + | + |
| Theaflavin | + | - |
| Dihydrokaempferol (Aromadendrin, Katuranin) | - | - |
| Cinchonain I isomer 2 | + | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 1 | + | + |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | + | - |
| Rutin (Quercetin-3-O-rutinoside) | + | - |
| Myricetin (Cannabisetin, Myricetol, 3,3′,4′,5,5′,7-hexahydroflavone) | - | - |
| Azelaic acid | + | + |
| Methoxy-trihydroxy(iso)flavone | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | + | - |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | + | + |
| Dihydroxy-trimethoxy(iso)flavone | + | + |
| Quercetin (3,3′,4′,5,7-Penthahyroxyflavone) | - | - |
| Naringenin (4′,5,7-Trihydroxyflavanone) | + | + |
| Sebacic acid | + | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 2 | + | + |
| Bruguierol A | + | + |
| Juniperic acid (16-hydroxyhexadecanoic acid) | ||
| Lupeol caffeate | + | + |
| Lupeol coumarate | + | + |
| Compound Name | MeOH | Ethyl Acetate |
|---|---|---|
| Quinic acid | + | + |
| Brugierol | + | + |
| Gallocatechin | - | - |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | + | + |
| Catechol | - | - |
| Genistic acid (2,5-Dihydroxybenzoic acid) | - | - |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | + | - |
| Procyanidin B isomer 1 | - | - |
| 3-O-(4-Coumaroyl) quinic acid | + | - |
| Catechin | + | - |
| Epigallocatechin | - | - |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | + | - |
| Dihydroxybenzoic acid isomer | - | - |
| Caffeic acid | + | + |
| 3-O-Feruloylquinic acid | + | - |
| Procyanidin B | + | - |
| Vanillin | - | + |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | + | - |
| Epicatechin | + | - |
| 4-O-(4-Coumaroyl) quinic acid | + | - |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | + | - |
| 4-Coumaric acid | + | + |
| Antiarol (3,4,5-Trimethoxyphenol) | + | + |
| Loliolide or Isololiolide | + | + |
| 4-O-Feruloylquinic acid | + | - |
| Riboflavin | + | - |
| Cinchonain I isomer 1 | + | + |
| Ferulic acid | + | + |
| Taxifolin (Didydroquercetin) | - | + |
| Loliolide or Isololiolide | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 1 | + | - |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer | - | - |
| Isoferulic acid | + | + |
| Dihydroxy-methoxy(iso)flavone-O-hexoside | + | - |
| Quercetin-O-dirhamnosylhexoside | + | - |
| Myricetin-3-O-rutinoside | + | + |
| Cinchonain I isomer 2 | + | + |
| Kaempferol-O-dirhamnosylhexoside | + | - |
| Cinchonain I isomer 3 | + | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 1 | + | + |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 2 | + | - |
| Rutin (Quercetin-3-O-rutinoside) | + | + |
| Apigenin-O-rhamnosylhexoside | + | - |
| Azelaic acid | + | + |
| Methoxy-trihydroxy(iso)flavone | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | + | + |
| Cinchonain I isomer 4 | + | + |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 3 | + | + |
| Dihydroxy-methoxy(iso)flavone | + | + |
| Dihydroxy-dimethoxy(iso)flavone | + | + |
| Dihydroxy-trimethoxy(iso)flavone | + | + |
| Quercetin (3,3′, 4′, 5, 7-Pentahydroxylflavone) | - | + |
| Naringenin (4′,5,7-Trihydroxyflavanone) | + | + |
| Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | + | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 2 | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-rhamnoside | + | + |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | + | + |
| Apigenin (4′,5,7-Trihydroxyflavone) | + | + |
| Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | + | + |
| Bruguierol A | + | + |
| Compound Name | MeOH | Ethyl Acetate |
|---|---|---|
| Quinic acid | + | + |
| Citric acid | + | - |
| Brugierol | + | - |
| Gallocatechin | + | + |
| Unidentified compound | - | - |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | + | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | + | - |
| Syringic acid-O-hexoside isomer 1 | + | - |
| Syringic acid-O-hexoside isomer 1 | + | - |
| Prodelphinidin C | + | - |
| Unidentified compound | - | - |
| Catechin | + | + |
| Epigallocatechin | + | + |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | + | - |
| Caffeic acid | + | + |
| Unidentified compound | - | + |
| Procyanidin B isomer 1 | + | + |
| Vanillin | + | + |
| Syringic acid | - | + |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | + | - |
| Procyanidin C | + | + |
| Epicatechin | + | + |
| 4-Coumaric acid | - | + |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | + | - |
| Antiarol (3,4,5-Trimethoxyphenol) | + | + |
| 3,4-Dihydro-3-hydroxy-7-methoxy-2H-1,5-benzodithiepine-6,9-dione | + | + |
| Cinchonain I isomer 1 | + | + |
| Epiafzelechin | + | + |
| Ferulic acid | + | + |
| Taxifolin (Dihydroquercetin) | + | + |
| Isoferulic acid | - | + |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | + | + |
| Procyanidin B isomer 2 | + | - |
| Trihydroxystilbene | + | + |
| Myricetin-3-O-rutinoside | + | - |
| Cinchonain I isomer 2 | + | + |
| Cinchonain I isomer 3 | + | + |
| Rutin (Quercetin-3-O-rutinoside) | + | + |
| Azelaic acid | + | + |
| Methoxy-trihydroxy(iso)flavone | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | - | + |
| Cinchonain I isomer 4 | + | + |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | + | + |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | - | + |
| Naringenin (4′,5,7-Trihydroxyflavanone) | - | + |
| 3-O-Methylellagic acid-4′-O-rhamnoside | + | - |
| Sebacic acid | + | + |
| Phloretin | + | + |
| Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | + | + |
| Norstictic acid | + | + |
| Methyl-trihydroxyxanthone | + | + |
| 16,17-Dihydroxy-9(11)-kauren-19-al or Steviol | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | + | + |
| Bruguierol A | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| Dihydroxy-methoxy-methylxanthone | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | + | + |
| Unidentified xanthone isomer 1 | + | + |
| Unidentified xanthone isomer 2 | + | + |
| Isopimar-7-en-15,16-diol or isomer | + | + |
| Isopimar-7-en-15,16-diol or isomer | + | + |
| Compound Name | MeOH | Ethyl Acetate |
|---|---|---|
| Quinic acid | + | + |
| Citric acid | + | + |
| Brugierol | + | + |
| Gallocatechin | + | - |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | + | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | + | - |
| Syringic acid-O-hexoside isomer 1 | + | + |
| Syringic acid-O-hexoside isomer 2 | + | + |
| Catechin | + | - |
| Epigallocatechin | + | - |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | + | - |
| Caffeic acid | + | + |
| Vanillin | - | + |
| Procyanidin B | + | - |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | + | - |
| Syringic acid | + | + |
| Ehyl syringate | - | + |
| Procyanidin C | + | - |
| Epicatechin | + | - |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | + | - |
| 4-Coumaric acid | + | + |
| Antiarol (3,4,5-Trimethoxyphenol) | + | + |
| 3,4-Dihydro-3-hydroxy-7-methoxy-2H-1,5-benzodithiepine-6,9-dione | + | + |
| Riboflavin | + | - |
| Cinchonain I isomer 1 | + | - |
| Ferulic acid | + | + |
| Loliolide or Isololiolide | + | + |
| Coumarin | - | + |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | - | + |
| 3,5-Dimethoxy-4-hydroxycinnamaldehyde (Sinapyl aldehyde) | - | + |
| Isoquercitrin (Hirsutin, Quercetin-3-O-glucoside) | - | + |
| Cinchonain I isomer 2 | + | - |
| Cinchonain I isomer 3 | + | - |
| Rutin (Quercetin-3-O-rutinoside) | + | - |
| Azelaic acid | + | + |
| Methoxy-trihydroxy(iso)flavone | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | + | - |
| Cinchonain I isomer 4 | + | - |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | + | + |
| Dihydroxy-methoxy(iso)flavone | + | + |
| Dihydroxy-dimethoxy(iso)flavone | + | + |
| Dihydroxy-trimethoxy(iso)flavone | + | + |
| Naringenin (4′,5,7-Trihydroxyflavanone) | + | + |
| 16,17-Dihydroxy-9(11)-kauran-19-al | + | + |
| 16,17-Dihydroxy-9(11)-kauren-19-al or Steviol | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| Methyl 16α,17-dihydroxy-9(11)-kauren-19-oate or isomer | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | + | + |
| 16,17-Dihydroxy-9(11)-kauran-19-al isomer | + | + |
| Bruguierol A | + | + |
| Methyl 16α,17-dihydroxy-9(11)-kauren-19-oate or isomer | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | + | + |
| Isopimar-7-en-15,16-diol or isomer | + | + |
| Isopimar-7-en-15,16-diol or isomer | + | + |
| Lupeol caffeate | + | - |
| Lupeol coumarate | + | - |
| Yield (%) | Total Phenolic Acid (mg CAE/g) | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) | Total Flavanol (mg CE/g) | Total Tannin (mg CE/g) | Total Triterpenoid (mg OAE/g) | |
|---|---|---|---|---|---|---|---|
| BLM | 12.62 | 1.21 ± 0.08 d,e | 30.26 ± 0.25 f | 9.28 ± 0.09 c | 9.72 ± 0.17 e | 17.95 ± 0.35 e | 9.12 ± 0.30 c |
| BRM | 9.60 | 9.86 ± 0.37 a | 161.64 ± 1.45 b | 1.92 ± 0.07 f | 119.00 ± 1.49 b | 134.55 ± 5.97 b | 55.73 ± 6.48 a |
| BTM | 8.26 | 5.22 ± 0.23 b | 91.06 ± 0.98 c | 1.65 ± 0.16 f | 34.84 ± 0.68 d | 48.21 ± 1.71 d | 21.19 ± 1.63 b |
| BFM | 14.28 | 0.77 ± 0.11 e | 174.18 ± 4.27 a | 3.29 ± 0.05 e | 71.99 ± 2.46 d | 176.24 ± 3.10 a | 63.11 ± 3.27 a |
| BLE | 1.88 | 1.95 ± 0.73 d | 54.94 ± 0.88 e | 20.39 ± 0.43 b | 5.67 ± 0.01 f | 8.66 ± 1.25 f | 13.30 ± 0.12 c |
| BRE | 0.46 | 3.33 ± 0.38 c | 71.08 ± 0.39 d | 1.27 ± 0.07 f | 56.81 ± 1.26 c | 92.37 ± 2.10 c | 24.37 ± 0.42 b |
| BTE | 1.06 | 0.85 ± 0.05 e | 27.27 ± 0.43 f | 4.48 ± 0.08 d | 1.53 ± 0.04 g | 5.99 ± 0.55 f | 6.84 ± 0.30 c |
| BFE | 0.66 | 0.73 ± 0.15 e | 52.52 ± 0.37 e | 36.64 ± 0.59 a | 8.61 ± 0.09 e,f | 7.56 ± 0.60 f | 9.76 ± 0.11 c |
| DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Phosphomolybdenum (mmol TE/g) | Chelating Activity (mg EDTAE/g) | |
|---|---|---|---|---|---|---|
| BLM | 42.35 ± 0.36 d | 63.73 ± 0.58 e | 108.56 ± 1.63 g | 65.51 ± 0.81d | 0.69 ± 0.06 e | 33.84 ± 0.72 b |
| BRM | 439.53 ± 1.52 b | 470.58 ± 7.68 a | 858.28 ± 20.92 b | 513.48 ± 19.84 b | 3.65 ± 0.02 b | 8.20 ± 0.92 e |
| BTM | 97.04 ± 0.35 c | 140.56 ± 0.20 c | 321.27 ± 2.63 c | 185.72 ± 3.03 c | 1.86 ± 0.13 d | 11.61 ± 0.79 d |
| BFM | 492.62 ± 5.31 a | 457.88 ± 8.32 b | 961.46 ± 11.18 a | 552.49 ± 8.71 a | 4.17 ± 0.31 a | 11.92 ± 0.38 d |
| BLE | 19.85 ± 0.57 e | 26.22 ± 1.02 f | 144.92 ± 2.05 f | 59.22 ± 0.91 d | 2.68 ± 0.15 c | 6.07 ± 0.92 f |
| BRE | 94.07 ± 0.25 c | 140.62 ± 0.10 c | 243.82 ± 1.75 d | 165.01 ± 3.64 c | 2.26 ± 0.13 c,d | 4.87 ± 0.62 f |
| BTE | 12.73 ± 0.26 f | 13.77 ± 1.25 g | 72.60 ± 1.28 h | 34.23 ± 1.01 e | 1.10 ± 0.07 e | 16.87 ± 0.76 c |
| BFE | 42.54 ± 0.50 d | 98.17 ± 2.08 d | 187.18 ± 4.07 e | 53.16 ± 0.44 d,e | 2.54 ± 0.23 c | 60.22 ± 0.05 a |
| AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) | Elastase Inhibition (mg CAE/g) | Lipase Inhibition (mg OE/g) | |
|---|---|---|---|---|---|---|---|
| BLM | 4.23 ± 0.15 c | 1.60 ± 0.09 d | 133.63 ± 0.43 d | 0.42 ± 0.02 d | na | 3.63 ± 0.03 c,d | 79.37 ± 2.29 b,c |
| BRM | 4.95 ± 0.02 a,b | 4.60 ± 0.04 b,c | 147.72 ± 0.86 b | 0.76 ± 0.01 b | na | 4.61 ± 0.06 a | 88.81 ± 4.54 b |
| BTM | 4.80 ± 0.12 a,b | 3.54 ± 0.04 c | 139.77 ± 0.26 c | 0.61 ± 0.01 c | na | 4.24 ± 0.08 a,b | 76.37 ± 4.29 b–d |
| BFM | 4.68 ± 0.08 b,c | 5.17 ± 0.11 b | 155.35 ± 0.29 a | 1.00 ± 0.05 a | na | 4.56 ± 0.10 a | 69.49 ± 8.22 c,d |
| BLE | 3.40 ± 0.41 d | 3.52 ± 1.05 c | 125.21 ± 1.59 e | 0.96 ± 0.02 a | 22.90 ± 0.81 c | 3.93 ± 0.25 b,c | 102.80 ± 7.25 a |
| BRE | 4.64 ± 0.24 b,c | 5.64 ± 0.72 a,b | 134.73 ± 3.29 d | 0.70 ± 0.03 b | 30.43 ± 0.07 a | 3.44 ± 0.29 d | 64.30 ± 1.92 d |
| BTE | 5.34 ± 0.17 a | 6.90 ± 0.10 a | 123.65 ± 0.39 e | 0.73 ± 0.04 b | 30.98 ± 0.01 a | 3.91 ± 0.19 b,c | 75.34 ± 1.35 c,d |
| BFE | na | 0.87 ± 0.03 d | 33.48 ± 0.43 f | 0.93 ± 0.04 a | 28.59 ± 0.08 b | 4.08 ± 0.09 b,c | na |
| Enzyme | Compound | Chemical Structure |
|---|---|---|
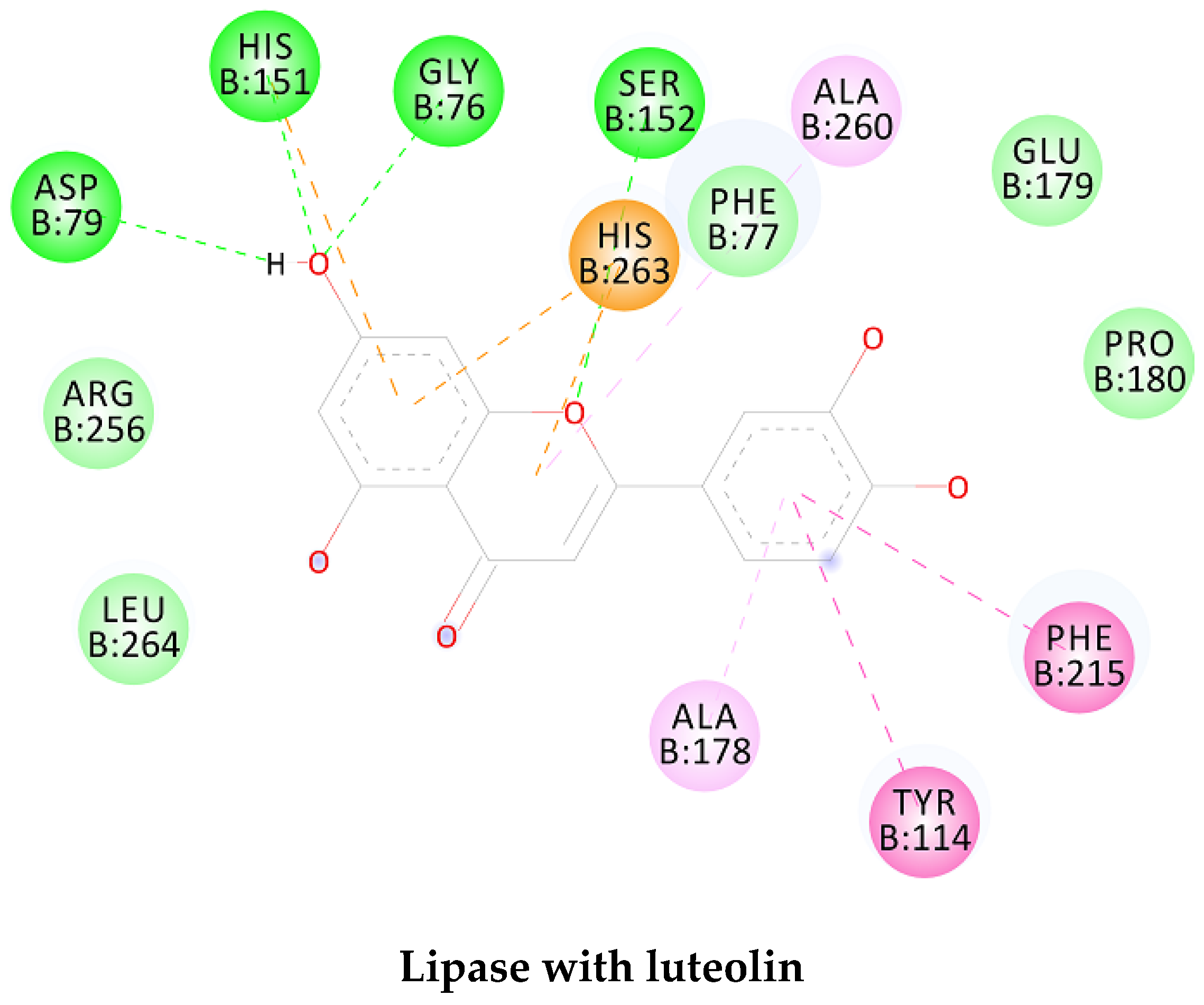
| Lipase | Luteolin | 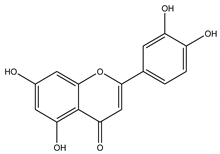 |
| Taxifolin | 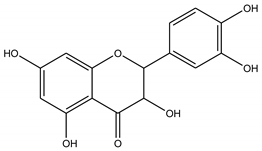 | |
| Bruguierol A |  | |
| Brugierol | 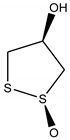 | |
| Tyrosinase | Azelaic acid | 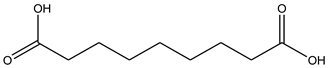 |
| Quinic acid | 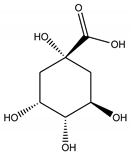 |
| Enzyme | Compounds | Binding Free Energy (kcal/mol) | Inhibition Constant (Ki) |
|---|---|---|---|
| Lipase | Luteolin | -8.49 | 600.10 nM |
| Taxifolin | -7.02 | 7.11 µM | |
| Bruguiera A | -7.14 | 5.83 µM | |
| Brugierol | -3.14 | 4.96 mM | |
| Tyrosinase | Azelaic acid | -5.35 | 120.45 µM |
| Quinic acid | -4.57 | 443.43 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Fawzi Mahomoodally, M. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. https://doi.org/10.3390/biom10050731
Bibi Sadeer N, Sinan KI, Cziáky Z, Jekő J, Zengin G, Jeewon R, Abdallah HH, Rengasamy KRR, Fawzi Mahomoodally M. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules. 2020; 10(5):731. https://doi.org/10.3390/biom10050731
Chicago/Turabian StyleBibi Sadeer, Nabeelah, Kouadio Ibrahime Sinan, Zoltán Cziáky, József Jekő, Gokhan Zengin, Rajesh Jeewon, Hassan H. Abdallah, Kannan R. R. Rengasamy, and Mohamad Fawzi Mahomoodally. 2020. "Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis" Biomolecules 10, no. 5: 731. https://doi.org/10.3390/biom10050731
APA StyleBibi Sadeer, N., Sinan, K. I., Cziáky, Z., Jekő, J., Zengin, G., Jeewon, R., Abdallah, H. H., Rengasamy, K. R. R., & Fawzi Mahomoodally, M. (2020). Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules, 10(5), 731. https://doi.org/10.3390/biom10050731







