Large-Scale Differential Gene Expression Transcriptomic Analysis Identifies a Metabolic Signature Shared by All Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Median-of-Medians Calculation
2.2. PMS Calculation
2.3. Cell Lines and Cell Culture
2.4. RNA Preparation and RT-PCR Analysis
2.5. Analysis of Different Databases
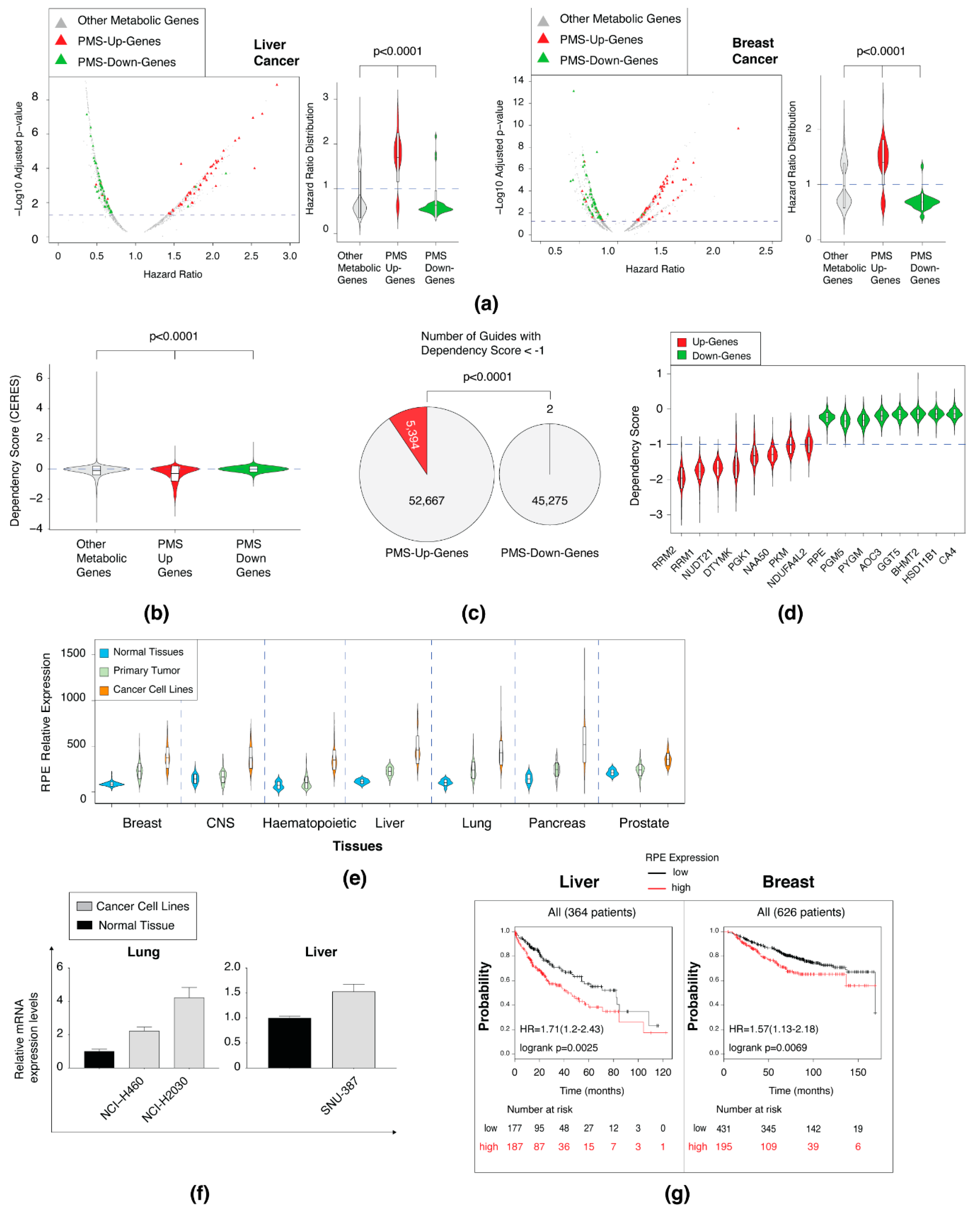
2.6. Determining the Correlation between the PMS Gene Set and Patient Outcomes
2.7. Statistical Analysis and Graphs
3. Results
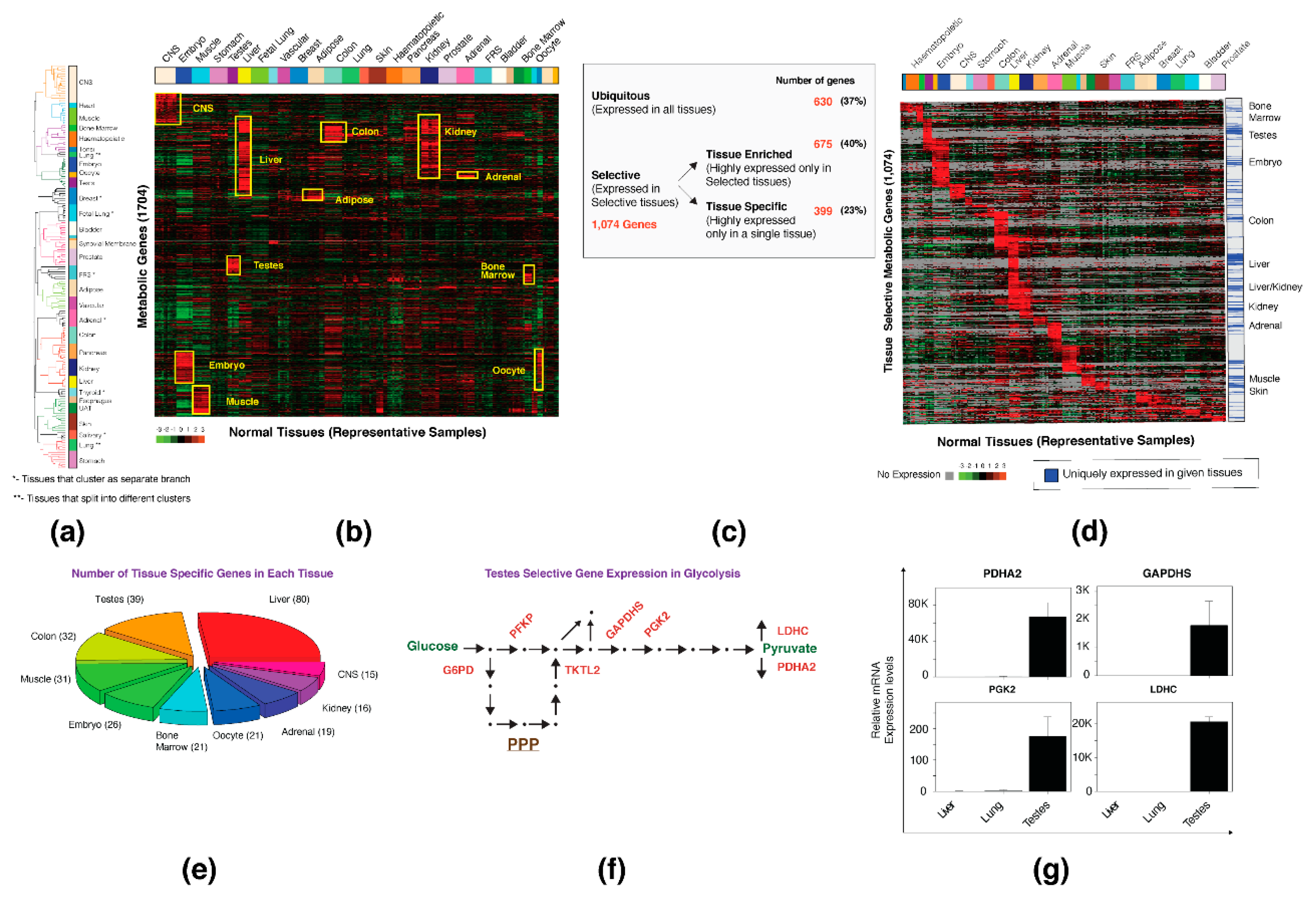
3.1. Normal Tissues Demonstrate a Tissue-Specific Metabolic Gene Expression Pattern
3.2. Cell Transformation Is Accompanied by a Loss of the Tissue-Specific Expression Profile
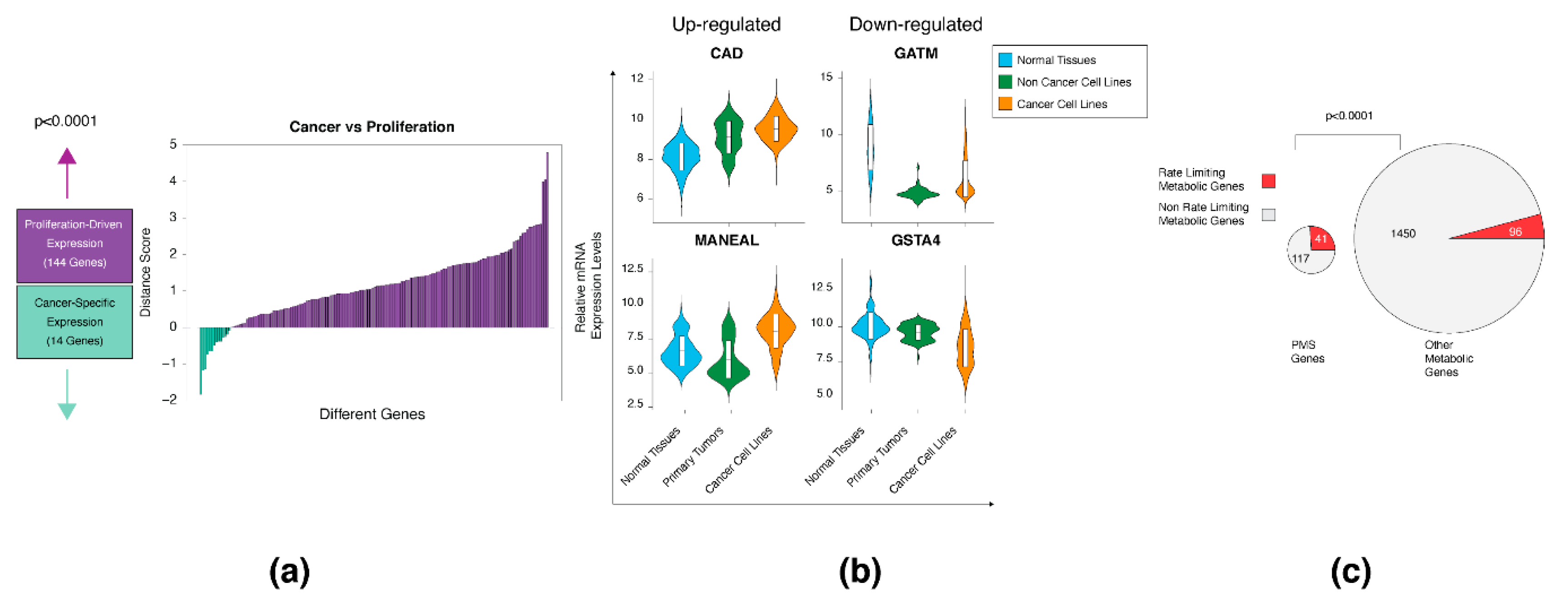
3.3. The Proliferation and Cancer-Specific Signature
3.4. The PMS Gene Set Is Enriched in Rate-Limiting Enzymes
3.5. The Upregulated PMS Gene Set Is Enriched in Essential Genes
3.6. Mapping the PMS Gene in Selected Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 174, 1034–1035. [Google Scholar]
- Waks, A.G.; Waks, A.G.; Winer, E.P.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Lee, N.; Kim, D. Cancer Metabolism: Fueling More than Just Growth. Mol. Cells 2016, 39, 847–854. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Tennant, D.A. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef]
- Farber, S.; Diamond, L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 1948, 238, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Deberardinis, R.J. Metabolic dysregulation in monogenic disorders and cancer—Finding method in madness. Nat. Rev. Cancer 2015, 15, 440–448. [Google Scholar] [CrossRef]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, e201643300-1730. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Deberardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Nanda, C.S.; Venkateswaran, S.V.; Patani, N.; Yuneva, M. Defining a metabolic landscape of tumours: Genome meets metabolism. Br. J. Cancer 2020, 122, 136–149. [Google Scholar] [CrossRef]
- Shaul, Y.D.; Yuan, B.; Thiru, P.; Nutter-Upham, A.; McCallum, S.; Lanzkron, C.; Bell, G.W.; Sabatini, D.M. MERAV: A tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016, 44, D560–D566. [Google Scholar] [CrossRef]
- Shaul, Y.D.; Freinkman, E.; Comb, W.C.; Cantor, J.R.; Tam, W.L.; Thiru, P.; Kim, D.; Kanarek, N.; Pacold, M.E.; Chen, W.W.; et al. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell 2014, 158, 1094–1109. [Google Scholar] [CrossRef]
- Rosario, S.R.; Long, M.D.; Affronti, H.C.; Rowsam, A.M.; Eng, K.H.; Smiraglia, D.J. Pan-cancer analysis of transcriptional metabolic dysregulation using The Cancer Genome Atlas. Nat. Commun. 2018, 9, 5330. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Lanczky, A.; Nagy, Á.; Bottai, G.; Munkácsy, G.; Szabó, A.; Santarpia, L.; Györffy, B. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 2016, 160, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Srivastava, A.K.; Schwartz, C.E. Microarray data integration for genome-wide analysis of human tissue-selective gene expression. BMC Genom. 2010, 11 (Suppl. 2), S15. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Cheng, W.-C.; Chen, C.-R.; Shu, W.-Y.; Tsai, M.-L.; Huang, C.-L.; Hsu, I.C. Identification of Human Housekeeping Genes and Tissue-Selective Genes by Microarray Meta-Analysis. PLoS ONE 2011, 6, e22859. [Google Scholar] [CrossRef] [PubMed]
- Miki, R.; Kadota, K.; Bono, H.; Mizuno, Y.; Tomaru, Y.; Carninci, P.; Itoh, M.; Shibata, K.; Kawai, J.; Konno, H.; et al. Delineating developmental and metabolic pathways in vivo by expression profiling using the RIKEN set of 18,816 full-length enriched mouse cDNA arrays. Proc. Natl. Acad. Sci. USA 2001, 98, 2199–2204. [Google Scholar] [CrossRef]
- Su, A.I.; Wiltshire, T.; Batalov, S.; Lapp, H.; Ching, K.A.; Block, D.; Zhang, J.; Soden, R.; Hayakawa, M.; Kreiman, G.; et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 2004, 101, 6062–6067. [Google Scholar] [CrossRef]
- Borges, I.; Sena, I.; Azevedo, P.; Andreotti, J.; Almeida, V.; Paiva, A.; Santos, G.; Guerra, D.; Prazeres, P.; Mesquita, L.L.; et al. Lung as a Niche for Hematopoietic Progenitors. Stem Cell Rev. 2017, 13, 567–574. [Google Scholar] [CrossRef]
- McClintick, J.N.; Edenberg, H.J. Effects of filtering by Present call on analysis of microarray experiments. BMC Bioinform. 2006, 7, 49. [Google Scholar] [CrossRef]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Pinheiro, A.; Nunes, M.J.; Milagre, I.; Rodrigues, E.; Silva, M.J.; de Almeida, I.T.; Rivera, I. Demethylation of the Coding Region Triggers the Activation of the Human Testis-Specific PDHA2 Gene in Somatic Tissues. PLoS ONE 2012, 7, e38076. [Google Scholar] [CrossRef]
- Danshina, P.V.; Geyer, C.B.; Dai, Q.; Goulding, E.H.; Willis, W.D.; Kitto, G.B.; McCarrey, J.R.; Eddy, E.M.; O’Brien, D.A. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol. Reprod. 2010, 82, 136–145. [Google Scholar] [CrossRef]
- Zinkham, W.H.; Blanco, A.; Clowry, L.J. An unusual isozyme of lactate dehydrogenase in mature testes: Localization, ontogeny, and kinetic properties. Ann. N. Y. Acad. Sci. 1964, 121, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhong, T.; Wang, M.; Xiang, X.; Ren, G.; Jia, Z.; Lin, Q.; Liu, Q.; Dong, J.; Li, L.; et al. Integrative Analysis Reveals Across-Cancer Expression Patterns and Clinical Relevance of Ribonucleotide Reductase in Human Cancers. Front. Oncol. 2019, 9, D805. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, B.; Zhang, H.; Song, J.; Zhang, Y.; Xing, J.; Yang, Z.; Wei, C.; Xu, T.; Yu, Z.; et al. RRM2 is a potential prognostic biomarker with functional significance in glioma. Int. J. Biol. Sci. 2019, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Hino, R.; Uozaki, H.; Maeda, D.; Ushiku, T.; Shinozaki, A.; Sakatani, T.; Fukayama, M. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Hum. Pathol. 2010, 41, 1742–1748. [Google Scholar] [CrossRef]
- Su, Y.-F.; Wu, T.-F.; Ko, J.-L.; Tsai, H.-T.; Tee, Y.-T.; Chien, M.-H.; Chou, C.-H.; Lin, W.-L.; Low, H.-Y.; Chou, M.-Y.; et al. The Expression of Ribonucleotide Reductase M2 in the Carcinogenesis of Uterine Cervix and Its Relationship with Clinicopathological Characteristics and Prognosis of Cancer Patients. PLoS ONE 2014, 9, e91644. [Google Scholar] [CrossRef]
- Hwang, T.L.; Liang, Y.; Chien, K.Y.; Yu, J.S. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics 2006, 6, 2259–2272. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Z.H.; Lu, X.; Liao, D.J.; Peng, G.L.; Xu, X.; Yin, W.Q.; He, J.X. Elevated expression of DTYMK is associated with poor prognosis in patients with Non-small cell lung cancer. Int. J. Clin. Exp. Med. 2016, 9. [Google Scholar]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Sun, Y.; Long, H.; Sun, L.; Sun, X.; Pang, L.; Chen, J.; Yi, Q.; Liang, T.; Shen, Y. PGM5 is a promising biomarker and may predict the prognosis of colorectal cancer patients. Cancer Cell Int. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Dieci, M.V.; Smutná, V.; Scott, V.; Yin, G.; Xu, R.; Vielh, P.; Mathieu, M.-C.; Vicier, C.; Laporte, M.; Drusch, F.; et al. Whole exome sequencing of rare aggressive breast cancer histologies. Breast Cancer Res. Treat. 2016, 156, 21–32. [Google Scholar] [CrossRef]
- Ward, S.T.; Weston, C.J.; Shepherd, E.L.; Hejmadi, R.; Ismail, T.; Adams, D.H. Evaluation of serum and tissue levels of VAP-1 in colorectal cancer. BMC Cancer 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Jin, B.; Gong, Z.; Yang, N.; Huang, Z.; Zeng, S.; Chen, H.; Hu, S.; Pan, G. Downregulation of betaine homocysteine methyltransferase (BHMT) in hepatocellular carcinoma associates with poor prognosis. Tumor Biol. 2015, 37, 5911–5917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tsoi, H.; Li, X.; Wang, H.; Gao, J.; Wang, K.; Go, M.Y.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.; et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP–WT1–TBL1 axis. Gut 2016, 65, 1482–1493. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.-L.; Xia, M.; Wu, C.; Song, J.; Wu, J.-J.; Laurence, A.; Xie, Q.-G.; Zhang, M.-Z.; Liang, H.-F.; et al. Loss of 11βHSD1 enhances glycolysis, facilitates intrahepatic metastasis, and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 2015, 7, 2038–2053. [Google Scholar]
- Sigoillot, F.D.; Sigoillot, S.M.; Guy, H.I. Breakdown of the regulatory control of pyrimidine biosynthesis in human breast cancer cells. Int. J. Cancer 2004, 109, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.L.; Guan, X.-J.; Ingram, R.; Tilghman, S.M. Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc. Natl. Acad. Sci. USA 2003, 100, 4622–4627. [Google Scholar] [CrossRef]
- Thompson, A.J.; Williams, R.J.; Hakki, Z.; Alonzi, D.S.; Wennekes, T.; Gloster, T.M.; Songsrirote, K.; Thomas-Oates, J.E.; Wrodnigg, T.M.; Spreitz, J.; et al. Structural and mechanistic insight into N-glycan processing by endo-α-mannosidase. Proc. Natl. Acad. Sci. USA 2012, 109, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X.; Gao, G.; Tao, L.; Wei, L. RLEdb: A database of rate-limiting enzymes and their regulation in human, rat, mouse, yeast and E. coli. Cell Res. 2009, 19, 793–795. [Google Scholar] [CrossRef]
- Dempster, J.M.; Rossen, J.; Kazachkova, M.; Pan, J.; Kugener, G.; Root, D.E.; Tsherniak, A. Extracting Biological Insights from the Project Achilles Genome-Scale CRISPR Screens in Cancer Cell Lines. bioRxiv 2019, 20, 720243. [Google Scholar]
- Meyers, R.M.; Bryan, J.G.; McFarland, J.M.; Weir, B.A.; Sizemore, A.E.; Xu, H.; Dharia, N.V.; Montgomery, P.G.; Cowley, G.S.; Pantel, S.; et al. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat. Genet. 2017, 49, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Li, G.; Liu, P.; Wang, Z.; Li, N. Glycolysis-Based Genes Associated with the Clinical Outcome of Pancreatic Ductal Adenocarcinoma Identified by The Cancer Genome Atlas Data Analysis. DNA Cell Biol. 2020, 39, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Romero, P.; Wagg, J.; Green, M.L.; Kaiser, D.; Krummenacker, M.; Karp, P.D. Computational prediction of human metabolic pathways from the complete human genome. Genome Biol. 2005, 6, R2. [Google Scholar] [CrossRef]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef]
- Peng, X.; Chen, Z.; Farshidfar, F.; Xu, X.; Lorenzi, P.L.; Wang, Y.; Cheng, F.; Tan, L.; Mojumdar, K.; Du, D.; et al. Molecular Characterization and Clinical Relevance of Metabolic Expression Subtypes in Human Cancers. Cell Rep. 2018, 23, 255–269.e4. [Google Scholar] [CrossRef]
- Satoh, K.; Yachida, S.; Sugimoto, M.; Oshima, M.; Nakagawa, T.; Akamoto, S.; Tabata, S.; Saitoh, K.; Kato, K.; Sato, S.; et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc. Natl. Acad. Sci. USA 2017, 114, E7697–E7706. [Google Scholar] [CrossRef]
- Gaude, E.; Gaude, E.; Frezza, C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat. Commun. 2016, 7, 13041. [Google Scholar] [CrossRef] [PubMed]


| Enzyme Name | PMS Gene Set | Cancer Type | Reference |
|---|---|---|---|
| RRM1 | Upregulated | Lung cancer | [33] |
| RRM2 | Upregulated | Lung, gastric, uterine cervix, glioma | [33,34,35,36] |
| PGK1 | Upregulated | Pancreatic ductal adenocarcinoma (PDAC) | [37] |
| DTYMK | Upregulated | Non-small cell lung cancer | [38] |
| PKM | Upregulated | Colorectal cancer | [39] |
| PGM5 | Downregulated | Colorectal cancer | [40] |
| PYGM | Downregulated | Breast cancer | [41] |
| AOC3 | Downregulated | Colorectal cancer | [42] |
| BHMT2 | Downregulated | Hepatocellular carcinoma | [43] |
| CA4 | Downregulated | Colorectal cancer | [44] |
| HSD11B1 | Downregulated | Hepatocellular carcinoma | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Rmaileh, A.; Solaimuthu, B.; Tanna, M.; Khatib, A.; Ben Yosef, M.; Hayashi, A.; Lichtenstein, M.; Shaul, Y.D. Large-Scale Differential Gene Expression Transcriptomic Analysis Identifies a Metabolic Signature Shared by All Cancer Cells. Biomolecules 2020, 10, 701. https://doi.org/10.3390/biom10050701
Abu Rmaileh A, Solaimuthu B, Tanna M, Khatib A, Ben Yosef M, Hayashi A, Lichtenstein M, Shaul YD. Large-Scale Differential Gene Expression Transcriptomic Analysis Identifies a Metabolic Signature Shared by All Cancer Cells. Biomolecules. 2020; 10(5):701. https://doi.org/10.3390/biom10050701
Chicago/Turabian StyleAbu Rmaileh, Areej, Balakrishnan Solaimuthu, Mayur Tanna, Anees Khatib, Michal Ben Yosef, Arata Hayashi, Michal Lichtenstein, and Yoav D. Shaul. 2020. "Large-Scale Differential Gene Expression Transcriptomic Analysis Identifies a Metabolic Signature Shared by All Cancer Cells" Biomolecules 10, no. 5: 701. https://doi.org/10.3390/biom10050701
APA StyleAbu Rmaileh, A., Solaimuthu, B., Tanna, M., Khatib, A., Ben Yosef, M., Hayashi, A., Lichtenstein, M., & Shaul, Y. D. (2020). Large-Scale Differential Gene Expression Transcriptomic Analysis Identifies a Metabolic Signature Shared by All Cancer Cells. Biomolecules, 10(5), 701. https://doi.org/10.3390/biom10050701






