Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform
Abstract
1. Introduction
2. Materials and Methods
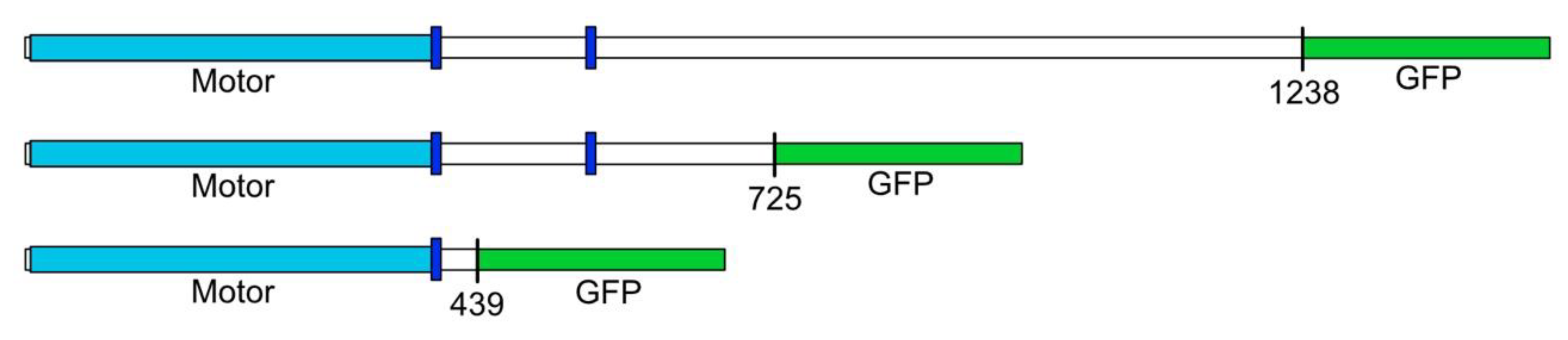
2.1. Molecular Constructs
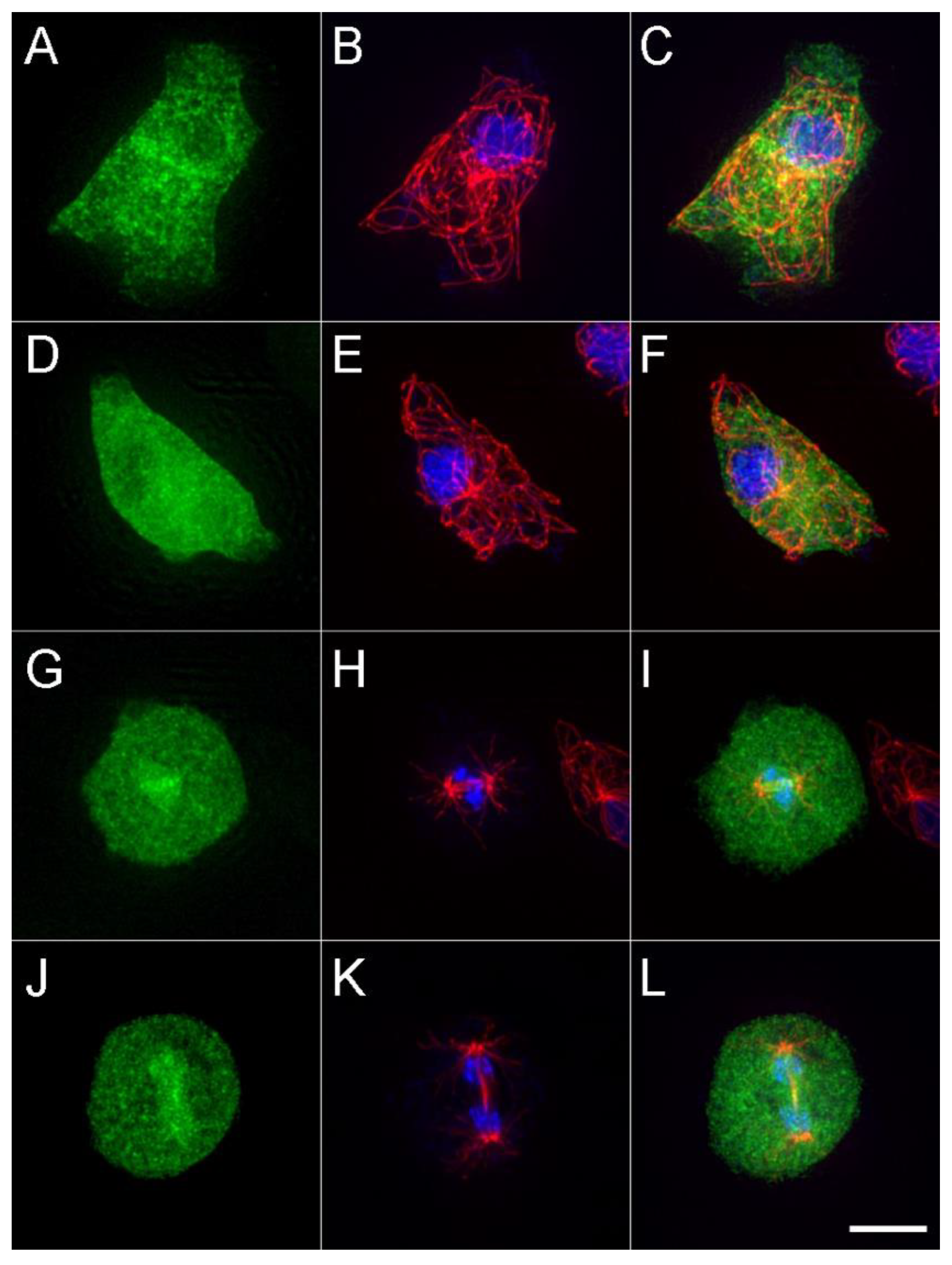
2.2. Biochemistry and Imaging
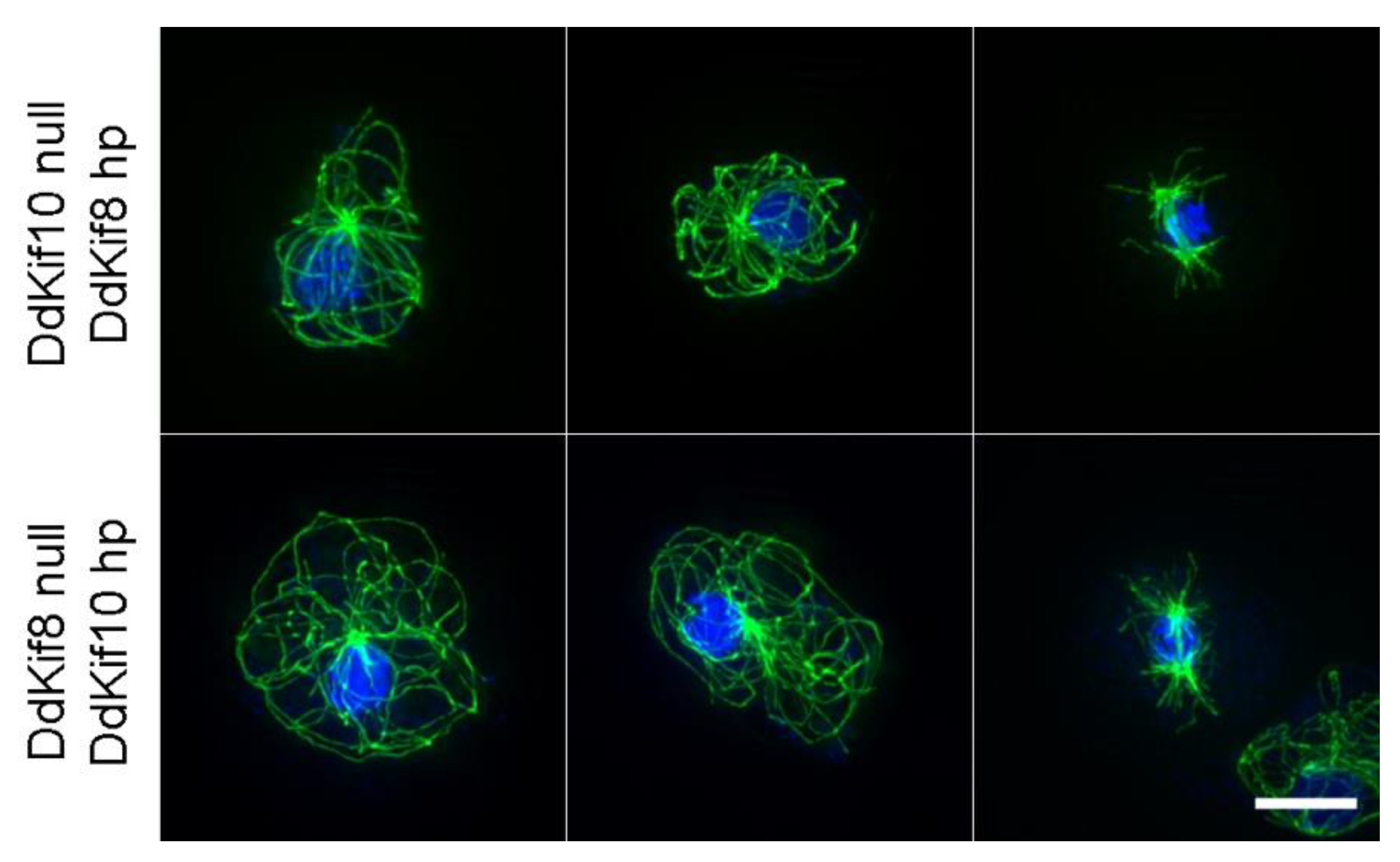
2.3. Hairpin Constructs
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miki, H.; Okada, Y.; Hirokawa, N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Hazelbaker, M.; Yount, L.A.; Walczak, E.C. Emerging insights into the function of Kinesin-8 proteins in microtubule length regulation. Biomolecules 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- DeZwaan, T.M.; Ellingson, E.; Pellman, D.; Roof, D.M. Kinesin-related KIP3 of Saccharomyces cerevisiae is required for a distinct step in nuclear migration. J. Cell Biol. 1997, 138, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Edzuka, T.; Goshima, G.; Yamada, M. Kinesin-13 and Kinesin-8 function during cell growth and division in the moss Physcomitrella patens. Plant Cell 2020, 32, 683. [Google Scholar] [CrossRef]
- Rischitor, P.E.; Konzack, S.; Fischer, R. The Kip3-like kinesin KipB moves along microtubules and determines spindle position during synchronized mitoses in Aspergillus nidulans hyphae. Euk. Cell 2004, 3, 632. [Google Scholar] [CrossRef]
- West, R.R.; Malmstrom, T.; Troxell, C.L.; McIntosh, J.R. Two related kinesins, klp5+ and klp6+, foster microtubule disassembly and are required for meiosis in fission yeast. Mol. Biol. Cell 2001, 12, 3919–3932. [Google Scholar] [CrossRef]
- Nag, D.K.; Tikhonenko, I.; Soga, I.; Koonce, M.P. Disruption of four kinesin genes in Dictyostelium. BMC Cell Biol. 2008, 9, 21. [Google Scholar] [CrossRef]
- Basu, S.; Fey, P.; Pandit, Y.; Dodson, R.; Kibbe, W.A.; Chisholm, R.L. Dictybase 2013: Integrating multiple Dictyostelid species. Nucleic Acids Res. 2013, 41, D676–D683. [Google Scholar] [CrossRef]
- Odell, J.; Sikirzhytski, V.; Tikhonenko, I.; Cobani, S.; Khodjakov, A.; Koonce, M. Force balances between interphase centrosomes as revealed by laser ablation. Mol. Biol. Cell 2019, 30, 1705–1715. [Google Scholar] [CrossRef]
- Egelhoff, T.T.; Titus, M.A.; Manstein, D.J.; Ruppel, K.M.; Spudich, J.A. Molecular genetic tools for study of the cytoskeleton in Dictyostelium. Methods Enzymol. 1991, 196, 319–334. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. FIJI: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.M.; Hisanaga, S.; Begg, D.A. An improved purification method for cytoplasmic dynein. J. Cell Biochem. 1984, 26, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Piperno, G.; Fuller, M.T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985, 101, 2085. [Google Scholar] [CrossRef] [PubMed]
- Girard, K.D.; Chaney, C.; Delannoy, M.; Kuo, S.C.; Robinson, D.N. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004, 23, 1536–1546. [Google Scholar] [CrossRef]
- Robinson, D.N.; Spudich, J.A. Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J. Cell Biol. 2000, 150, 823–838. [Google Scholar] [CrossRef]
- Knecht, D.A.; Jung, J.; Matthews, L. Quantification of transformation efficiency using a new method for clonal growth and selection of axenic Dictyostelium cells. Dev. Genet. 1990, 11, 403–409. [Google Scholar] [CrossRef]
- Kollmar, M.; Glöckner, G. Identification and phylogenetic analysis of Dictyostelium discoideum kinesin proteins. BMC Genom. 2003, 4, 47. [Google Scholar] [CrossRef]
- Koonce, M.P. 13 plus 1: A 30-year perspective on microtubule-based motility in Dictyostelium. Cells 2020, 9, 528. [Google Scholar] [CrossRef]
- Mayr, M.I.; Storch, M.; Howard, J.; Mayer, T.U. A non-motor microtubule binding site is essential for the high processivity and mitotic function of Kinesin-8 Kif18A. PLoS ONE 2011, 6, e27471. [Google Scholar] [CrossRef]
- Su, X.; Qiu, W.; Gupta, M.L., Jr.; Pereira-Leal, J.B.; Reck-Peterson, S.L.; Pellman, D. Mechanisms underlying the dual-mode regulation of microtubule dynamics by Kip3/Kinesin-8. Mol. Cell 2011, 43, 751–763. [Google Scholar] [CrossRef]
- Koonce, M.P.; Samsó, M. Overexpression of cytoplasmic dynein’s globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol. Biol. Cell 1996, 7, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Tikhonenko, I.; Magidson, V.; Gräf, R.; Khodjakov, A.; Koonce, M.P. A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell Mol. Life Sci. 2013, 70, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Niekamp, S.; Coudray, N.; Zhang, N.; Vale, R.D.; Bhabha, G. Coupling of ATPase activity, microtubule binding, and mechanics in the dynein motor domain. EMBO J. 2019, 38, e101414. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.A.; Strauss, J.; Magidson, V.; Tikhonenko, I.; Khodjakov, A.; Koonce, M.P. Pushing forces drive the comet-like motility of microtubule arrays in Dictyostelium. Mol. Biol. Cell 2005, 16, 3334–3340. [Google Scholar] [CrossRef]
- Tikhonenko, I.; Nag, D.K.; Robinson, D.N.; Koonce, M.P. Microtubule-nucleus interactions in Dictyostelium discoideum mediated by central motor kinesins. Euk. Cell 2009, 8, 723–731. [Google Scholar] [CrossRef]
- Martens, H.; Novotny, J.; Oberstrass, J.; Steck, T.L.; Postlethwait, P.; Nellen, W. RNAi in Dictyostelium: The role of RNA-directed RNA polymerases and double-stranded RNAse. Mol. Biol. Cell 2002, 13, 445–453. [Google Scholar] [CrossRef]
- Rosel, D.; Kimmel, A.R. The COP9 signalosome regulates cell proliferation of Dictyostelium discoideum. Eur. J. Cell Biol. 2006, 85, 1023–1034. [Google Scholar] [CrossRef]
- Stumpff, J.; Du, Y.; English, C.A.; Maliga, Z.; Wagenbach, M.; Asbury, C.L.; Wordeman, L.; Ohi, R. A tethering mechanism controls the processivity and kinetochore-microtubule plus-end enrichment of the Kinesin-8 Kif18A. Mol. Cell 2011, 43, 764–775. [Google Scholar] [CrossRef]
- Edzuka, T.; Goshima, G. Drosophila Kinesin-8 stabilizes the kinetochore–microtubule interaction. J. Cell Biol. 2018, 218, 474–488. [Google Scholar] [CrossRef]
- Erent, M.; Drummond, D.R.; Cross, R.A. S. Pombe Kinesins-8 promote both nucleation and catastrophe of microtubules. PLoS ONE 2012, 7, e30738. [Google Scholar] [CrossRef]
- Grissom, P.M.; Fiedler, T.; Grishchuk, E.L.; Nicastro, D.; West, R.R.; McIntosh, J.R. Kinesin-8 from fission yeast: A heterodimeric, plus-end–directed motor that can couple microtubule depolymerization to cargo movement. Mol. Biol. Cell 2008, 20, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.L.; Carvalho, P.; Roof, D.M.; Pellman, D. Plus end-specific depolymerase activity of Kip3, a Kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat. Cell Biol. 2006, 8, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Helenius, J.; Tanaka, K.; Hyman, A.A.; Tanaka, T.U.; Howard, J. Yeast Kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat. Cell Biol. 2006, 8, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.; Joseph, A.P.; Peña, A.; Möckel, M.M.; Mayer, T.U.; Topf, M.; Moores, C.A. Structural basis of human Kinesin-8 function and inhibition. Proc. Natl. Acad. Sci. USA 2017, 114, 9539–9584. [Google Scholar] [CrossRef]
- Goshima, G.; Vale, R.D. Cell cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells. Mol. Biol. Cell 2005, 16, 3896–3907. [Google Scholar] [CrossRef]
- Mayr, M.I.; Hümmer, S.; Bormann, J.; Grüner, T.; Adio, S.; Woehlke, G.; Mayer, T.U. The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr. Biol. 2007, 17, 488–498. [Google Scholar] [CrossRef]
- Savoian, M.S.; Glover, D.M. Drosophila Klp67A binds prophase kinetochores to subsequently regulate congression and spindle length. J. Cell Sci. 2010, 123, 767–776. [Google Scholar] [CrossRef]
- West, R.R.; McIntosh, J.R. Novel interactions of fission yeast Kinesin 8 revealed through in vivo expression of truncation alleles. Cell Motil. Cytoskelet. 2008, 65, 626–640. [Google Scholar] [CrossRef]
- Kim, H.; Fonseca, C.; Stumpff, J. A unique Kinesin-8 surface loop provides specificity for chromosome alignment. Mol. Biol. Cell 2014, 25, 3319–3329. [Google Scholar] [CrossRef]
- Ogawa, T.; Nitta, R.; Okada, Y.; Hirokawa, N. A common mechanism for microtubule destabilizers—M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell 2004, 116, 591–602. [Google Scholar] [CrossRef]
- Shipley, K.; Hekmat-Nejad, M.; Turner, J.; Moores, C.; Anderson, R.; Milligan, R.; Sakowicz, R.; Fletterick, R. Structure of a kinesin microtubule depolymerization machine. EMBO J. 2004, 23, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Samereier, M.; Baumann, O.; Meyer, I.; Gräf, R. Analysis of Dictyostelium TACC reveals differential interactions with CP224 and unusual dynamics of Dictyostelium microtubules. Cell Mol. Life Sci. 2010, 68, 275–287. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koonce, M.P.; Tikhonenko, I. Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform. Biomolecules 2020, 10, 563. https://doi.org/10.3390/biom10040563
Koonce MP, Tikhonenko I. Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform. Biomolecules. 2020; 10(4):563. https://doi.org/10.3390/biom10040563
Chicago/Turabian StyleKoonce, Michael P., and Irina Tikhonenko. 2020. "Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform" Biomolecules 10, no. 4: 563. https://doi.org/10.3390/biom10040563
APA StyleKoonce, M. P., & Tikhonenko, I. (2020). Bioenergetics of the Dictyostelium Kinesin-8 Motor Isoform. Biomolecules, 10(4), 563. https://doi.org/10.3390/biom10040563



