Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools
Abstract
1. Introduction
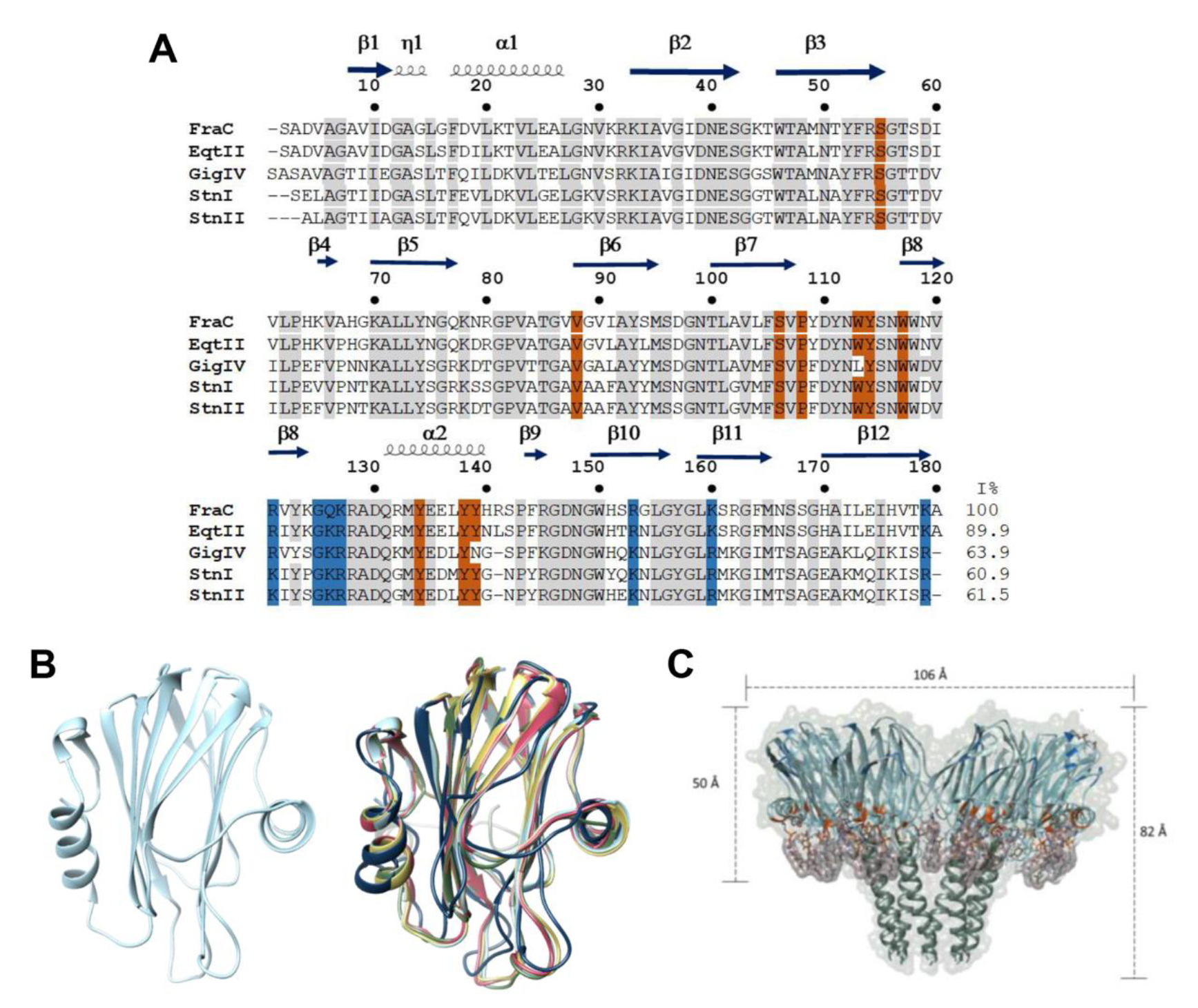
2. Structure and Function of Actinoporins
3. Therapeutic and Biotechnological Tools Based on Actinoporins
3.1. Actinoporin-Based Immunotoxin For Cancer Therapy
3.2. Biosensors Based On Actinoporins Nanopores
3.3. Actinoporins Used to Identify SM in the Cell Membranes
3.4. Actinoporins As an Adjuvant for Vaccine Design
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.-S.; Liu, X.-L.; Hui, Q.; Lu, S.-Y.; Qu, L.-L.; Li, Y.-S.; Zhou, Y.; Ren, H.-L.; Hu, P. Clinical targeting recombinant immunotoxins for cancer therapy. Onco Targets Ther. 2017, 10, 3645–3665. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.; Pastan, I. Pseudomonas Exotoxin Immunotoxins and Anti-Tumor Immunity: From Observations at the Patient’s Bedside to Evaluation in Preclinical Models. Toxins 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Mutter, N.L.; Soskine, M.; Huang, G.; Albuquerque, I.S.; Bernardes, G.J.L.; Maglia, G. Modular Pore-Forming Immunotoxins with Caged Cytotoxicity Tailored by Directed Evolution. ACS Chem. Biol. 2018, 13, 3153–3160. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, S.-O.; Yoon, A.-R.; Yoo, J.Y.; Kim, M.K.; Yun, C.-O.; Kim, C.-W. Suicide cancer gene therapy using pore-forming toxin, streptolysin O. Mol. Cancer Ther. 2006, 5, 1610–1619. [Google Scholar] [CrossRef]
- Vitetta, E.S.; Krolick, K.A.; Miyama-Inaba, M.; Cushley, W.; Uhr, J.W. Immunotoxins: A new approach to cancer therapy. Science 1983, 219, 644–650. [Google Scholar] [CrossRef]
- Blythman, H.E.; Casellas, P.; Gros, O.; Gros, P.; Jansen, F.K.; Paolucci, F.; Pau, B.; Vidal, H. Immunotoxins: Hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 1981, 290, 145–146. [Google Scholar] [CrossRef]
- Vitetta, E.S.; Uhr, J.W. Immunotoxins: Redirecting nature’s poisons. Cell 1985, 41, 653–654. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Karlin, L.; Robak, T.; Gladstone, D.E.; le Coutre, P.; Dietrich, S.; Gotic, M.; et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia 2018, 32, 1768–1777. [Google Scholar] [CrossRef]
- Hairy Cell Leukemia Treatment Approved. Cancer Discov. 2018, 8, OF1. [CrossRef]
- Tejuca, M.; Anderluh, G.; Dalla Serra, M. Sea anemone cytolysins as toxic components of immunotoxins. Toxicon 2009, 54, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed]
- Rojko, N.; Dalla Serra, M.; Maček, P.; Anderluh, G. Pore formation by actinoporins, cytolysins from sea anemones. Biochim. Biophys. Acta 2016, 1858, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Anderluh, G. Pore-forming toxins in Cnidaria. Semin. Cell Dev. Biol. 2017, 72, 133–141. [Google Scholar] [CrossRef]
- Morante, K.; Caaveiro, J.M.M.; Tanaka, K.; González-Mañas, J.M.; Tsumoto, K. A pore-forming toxin requires a specific residue for its activity in membranes with particular physicochemical properties. J. Biol. Chem. 2015, 290, 10850–10861. [Google Scholar] [CrossRef]
- Monastyrnaya, M.; Leychenko, E.; Isaeva, M.; Likhatskaya, G.; Zelepuga, E.; Kostina, E.; Trifonov, E.; Nurminski, E.; Kozlovskaya, E. Actinoporins from the sea anemones, tropical Radianthus macrodactylus and northern Oulactis orientalis: Comparative analysis of structure-function relationships. Toxicon 2010, 56, 1299–1314. [Google Scholar] [CrossRef]
- Leichenko, E.V.; Monastirnaya, M.M.; Zelepuga, E.A.; Tkacheva, E.S.; Isaeva, M.P.; Likhatskaya, G.N.; Anastyuk, S.D.; Kozlovskaya, E.P. Hct-a is a new actinoporin family from the heteractis crispa sea anemone. Acta Naturae 2014, 6, 89–98. [Google Scholar] [CrossRef]
- Rivera-de-Torre, E.; Palacios-Ortega, J.; García-Linares, S.; Gavilanes, J.G.; Martínez-Del-Pozo, Á. One single salt bridge explains the different cytolytic activities shown by actinoporins sticholysin I and II from the venom of Stichodactyla helianthus. Arch. Biochem. Biophys. 2017, 636, 79–89. [Google Scholar] [CrossRef]
- Palacios-Ortega, J.; García-Linares, S.; Rivera-de-Torre, E.; Gavilanes, J.G.; Martínez-Del-Pozo, Á.; Slotte, J.P. Sticholysin, Sphingomyelin, and Cholesterol: A Closer Look at a Tripartite Interaction. Biophys. J. 2019, 116, 2253–2265. [Google Scholar] [CrossRef]
- Palacios-Ortega, J.; García-Linares, S.; Åstrand, M.; Al Sazzad, M.A.; Gavilanes, J.G.; Martínez-del-Pozo, Á.; Slotte, J.P. Regulation of Sticholysin II-Induced Pore Formation by Lipid Bilayer Composition, Phase State, and Interfacial Properties. Langmuir 2016, 32, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- García-Linares, S.; Palacios-Ortega, J.; Yasuda, T.; Åstrand, M.; Gavilanes, J.G.; Martínez-del-Pozo, Á.; Slotte, J.P. Toxin-induced pore formation is hindered by intermolecular hydrogen bonding in sphingomyelin bilayers. Biochim. Biophys. Acta 2016, 1858, 1189–1195. [Google Scholar] [CrossRef]
- Valcarcel, C.A.; Dalla Serra, M.; Potrich, C.; Bernhart, I.; Tejuca, M.; Martinez, D.; Pazos, F.; Lanio, M.E.; Menestrina, G. Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus. Biophys. J. 2001, 80, 2761–2774. [Google Scholar] [CrossRef]
- Garcia, P.S.; Chieppa, G.; Desideri, A.; Cannata, S.; Romano, E.; Luly, P.; Rufini, S. Sticholysin II: A pore-forming toxin as a probe to recognize sphingomyelin in artificial and cellular membranes. Toxicon 2012, 60, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Abe, M.; Murate, M.; Inaba, T.; Yilmaz, N.; Hullin-Matsuda, F.; Kishimoto, T.; Schieber, N.L.; Taguchi, T.; Arai, H.; et al. Visualization of the heterogeneous membrane distribution of sphingomyelin associated with cytokinesis, cell polarity, and sphingolipidosis. FASEB J. 2015, 29, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Hendrich, A.B.; Michalak, K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug Targets 2003, 4, 23–30. [Google Scholar] [CrossRef]
- Soletti, R.C.; de Faria, G.P.; Vernal, J.; Terenzi, H.; Anderluh, G.; Borges, H.L.; Moura-Neto, V.; Gabilan, N.H. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-forming proteins in human glioblastoma cells. Anticancer Drugs 2008, 19, 517–525. [Google Scholar] [CrossRef]
- Ramírez-Carreto, S.; Pérez-García, E.I.; Salazar-García, S.I.; Bernáldez-Sarabia, J.; Licea-Navarro, A.; Rudiño-Piñera, E.; Pérez-Martínez, L.; Pedraza-Alva, G.; Rodríguez-Almazán, C. Identification of a pore-forming protein from sea anemone Anthopleura dowii Verrill (1869) venom by mass spectrometry. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e147418. [Google Scholar] [CrossRef]
- Tejuca, M.; Díaz, I.; Figueredo, R.; Roque, L.; Pazos, F.; Martínez, D.; Iznaga-Escobar, N.; Pérez, R.; Alvarez, C.; Lanio, M.E. Construction of an immunotoxin with the pore forming protein StI and ior C5, a monoclonal antibody against a colon cancer cell line. Int. Immunopharmacol. 2004, 4, 731–744. [Google Scholar] [CrossRef]
- Potrich, C.; Tomazzolli, R.; Dalla Serra, M.; Anderluh, G.; Malovrh, P.; Macek, P.; Menestrina, G.; Tejuca, M. Cytotoxic activity of a tumor protease-activated pore-forming toxin. Bioconjug. Chem. 2005, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, J.; Xu, R.; Dong, Y.; Sun, A.; Shen, Y.; Wei, D. Gigantoxin-4-4D5 scFv is a novel recombinant immunotoxin with specific toxicity against HER2/neu-positive ovarian carcinoma cells. Appl. Microbiol. Biotechnol. 2016, 100, 6403–6413. [Google Scholar] [CrossRef] [PubMed]
- Boersma, A.J.; Bayley, H. Continuous stochastic detection of amino acid enantiomers with a protein nanopore. Angew. Chem. Int. Ed. Engl. 2012, 51, 9606–9609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; de Zoysa, R.S.S.; Wang, D.; Jayawardhana, D.A.; Guan, X. Real-time monitoring of peptide cleavage using a nanopore probe. J. Am. Chem. Soc. 2009, 131, 6324–6325. [Google Scholar] [CrossRef] [PubMed]
- Manrao, E.A.; Derrington, I.M.; Laszlo, A.H.; Langford, K.W.; Hopper, M.K.; Gillgren, N.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat. Biotechnol. 2012, 30, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.C.; Long, Y.-T.; Stefureac, R.-I.; Bediako-Amoa, I.; Kraatz, H.-B.; Lee, J.S. Structure of Peptides Investigated by Nanopore Analysis. Nano Lett. 2004, 4, 1273–1277. [Google Scholar] [CrossRef]
- Robertson, J.W.F.; Reiner, J.E. The Utility of Nanopore Technology for Protein and Peptide Sensing. Proteomics 2018, 18, e1800026. [Google Scholar] [CrossRef]
- Meller, A.; Nivon, L.; Branton, D. Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 2001, 86, 3435–3438. [Google Scholar] [CrossRef]
- Huang, G.; Willems, K.; Soskine, M.; Wloka, C.; Maglia, G. Electro-osmotic capture and ionic discrimination of peptide and protein biomarkers with FraC nanopores. Nat. Commun. 2017, 8, 935. [Google Scholar] [CrossRef]
- Watanabe, H.; Gubbiotti, A.; Chinappi, M.; Takai, N.; Tanaka, K.; Tsumoto, K.; Kawano, R. Analysis of Pore Formation and Protein Translocation Using Large Biological Nanopores. Anal. Chem. 2017, 89, 11269–11277. [Google Scholar] [CrossRef]
- Zhao, S.; Restrepo-Pérez, L.; Soskine, M.; Maglia, G.; Joo, C.; Dekker, C.; Aksimentiev, A. Electro-Mechanical Conductance Modulation of a Nanopore Using a Removable Gate. ACS Nano 2019, 13, 2398–2409. [Google Scholar] [CrossRef]
- Huang, G.; Voet, A.; Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 2019, 10, 835. [Google Scholar] [CrossRef]
- Laborde, R.J.; Sanchez-Ferras, O.; Luzardo, M.C.; Cruz-Leal, Y.; Fernández, A.; Mesa, C.; Oliver, L.; Canet, L.; Abreu-Butin, L.; Nogueira, C.V.; et al. Novel Adjuvant Based on the Pore-Forming Protein Sticholysin II Encapsulated into Liposomes Effectively Enhances the Antigen-Specific CTL-Mediated Immune Response. J. Immunol. 2017, 198, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yap, L.L.; Chua, K.L.; Khoo, H.E. A multigene family of Heteractis magnificalysins (HMgs). Toxicon 2008, 51, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Leychenko, E.; Isaeva, M.; Tkacheva, E.; Zelepuga, E.; Kvetkina, A.; Guzev, K.; Monastyrnaya, M.; Kozlovskaya, E. Multigene Family of Pore-Forming Toxins from Sea Anemone Heteractis crispa. Mar. Drugs 2018, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Kristan, K.C.; Viero, G.; Dalla Serra, M.; Macek, P.; Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 2009, 54, 1125–1134. [Google Scholar] [CrossRef]
- Anderluh, G.; Barlic, A.; Podlesek, Z.; Macek, P.; Pungercar, J.; Gubensek, F.; Zecchini, M.L.; Serra, M.D.; Menestrina, G. Cysteine-scanning mutagenesis of an eukaryotic pore-forming toxin from sea anemone: Topology in lipid membranes. Eur. J. Biochem. 1999, 263, 128–136. [Google Scholar] [CrossRef]
- Kohno, Y.; Satoh, H.; Iguchi, A.; Nagai, H. Characterization of a new hemolytic protein toxin from the sea anemone Anthopleura asiatica. Fish. Sci. 2009, 75, 1049–1054. [Google Scholar] [CrossRef]
- Tkacheva, E.S.; Leychenko, E.V.; Monastyrnaya, M.M.; Issaeva, M.P.; Zelepuga, E.A.; Anastuk, S.D.; Dmitrenok, P.S.; Kozlovskaya, E.P. New Actinoporins from sea anemone Heteractis crispa: Cloning and functional expression. Biochem. Mosc. 2011, 76, 1131–1139. [Google Scholar] [CrossRef]
- Bellomio, A.; Morante, K.; Barlic, A.; Gutiérrez-Aguirre, I.; Viguera, A.R.; González-Mañas, J.M. Purification, cloning and characterization of fragaceatoxin C, a novel actinoporin from the sea anemone Actinia fragacea. Toxicon 2009, 54, 869–880. [Google Scholar] [CrossRef]
- Álvarez, C.; Mancheño, J.M.; Martínez, D.; Tejuca, M.; Pazos, F.; Lanio, M.E. Sticholysins, two pore-forming toxins produced by the Caribbean sea anemone Stichodactyla helianthus: Their interaction with membranes. Toxicon 2009, 54, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, W.; Wang, L.-H.; Wang, J.-G.; Liu, X.-Y.; Jiao, B.-H. Purification and characterization of gigantoxin-4, a new actinoporin from the sea anemone Stichodactyla gigantea. Int. J. Biol. Sci. 2011, 7, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.E.; Bellomoio, A.; Morante, K.; Agirre, J.; Gil-Cartón, D.; González-Mañas, J.M.; Guérin, D.M.A. Pores of the toxin fraC assemble into 2D hexagonal clusters in both crystal structure and model membranes. J. Struct. Biol. 2012, 180, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Pungercar, J.; Strukelj, B.; Macek, P.; Gubensek, F. Cloning, sequencing, and expression of equinatoxin II. Biochem. Biophys. Res. Commun. 1996, 220, 437–442. [Google Scholar] [CrossRef]
- Lanio, M.E.; Morera, V.; Alvarez, C.; Tejuca, M.; Gómez, T.; Pazos, F.; Besada, V.; Martínez, D.; Huerta, V.; Padrón, G.; et al. Purification and characterization of two hemolysins from Stichodactyla helianthus. Toxicon 2001, 39, 187–194. [Google Scholar] [CrossRef]
- Mancheño, J.M.; Martín-Benito, J.; Martínez-Ripoll, M.; Gavilanes, J.G.; Hermoso, J.A. Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure 2003, 11, 1319–1328. [Google Scholar] [CrossRef]
- García-Linares, S.; Castrillo, I.; Bruix, M.; Menéndez, M.; Alegre-Cebollada, J.; Martínez-del-Pozo, Á.; Gavilanes, J.G. Three-dimensional structure of the actinoporin sticholysin I. Influence of long-distance effects on protein function. Arch. Biochem. Biophys. 2013, 532, 39–45. [Google Scholar] [CrossRef]
- Mechaly, A.E.; Bellomio, A.; Gil-Cartón, D.; Morante, K.; Valle, M.; González-Mañas, J.M.; Guérin, D.M.A. Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure 2011, 19, 181–191. [Google Scholar] [CrossRef]
- Hinds, M.G.; Zhang, W.; Anderluh, G.; Hansen, P.E.; Norton, R.S. Solution structure of the eukaryotic pore-forming cytolysin equinatoxin II: Implications for pore formation. J. Mol. Biol. 2002, 315, 1219–1229. [Google Scholar] [CrossRef]
- García-Linares, S.; Rivera-de-Torre, E.; Morante, K.; Tsumoto, K.; Caaveiro, J.M.M.; Gavilanes, J.G.; Slotte, J.P.; Martínez-Del-Pozo, Á. Differential Effect of Membrane Composition on the Pore-Forming Ability of Four Different Sea Anemone Actinoporins. Biochemistry 2016, 55, 6630–6641. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Avigad, L.S. Properties of a toxin from the sea anemone Stoichacis helianthus, including specific binding to sphingomyelin. Proc. Natl. Acad. Sci. USA 1976, 73, 467–471. [Google Scholar] [CrossRef] [PubMed]
- García-Sáez, A.J.; Buschhorn, S.B.; Keller, H.; Anderluh, G.; Simons, K.; Schwille, P. Oligomerization and pore formation by equinatoxin II inhibit endocytosis and lead to plasma membrane reorganization. J. Biol. Chem. 2011, 286, 37768–37777. [Google Scholar] [CrossRef]
- Ros, U.; Edwards, M.A.; Epand, R.F.; Lanio, M.E.; Schreier, S.; Yip, C.M.; Alvarez, C.; Epand, R.M. The sticholysin family of pore-forming toxins induces the mixing of lipids in membrane domains. Biochim. Biophys. Acta 2013, 1828, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Barlic, A.; Gutiérrez-Aguirre, I.; Caaveiro, J.M.M.; Cruz, A.; Ruiz-Argüello, M.-B.; Pérez-Gil, J.; González-Mañas, J.M. Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J. Biol. Chem. 2004, 279, 34209–34216. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Pungercar, J.; Krizaj, I.; Strukelj, B.; Gubensek, F.; Macek, P. N-terminal truncation mutagenesis of equinatoxin II, a pore-forming protein from the sea anemone Actinia equina. Protein Eng. 1997, 10, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Barlic, A.; Krizaj, I.; Menestrina, G.; Gubensĕk, F.; Macek, P. Avidin-FITC topological studies with three cysteine mutants of equinatoxin II, a sea anemone pore-forming protein. Biochem. Biophys. Res. Commun. 1998, 242, 187–190. [Google Scholar] [CrossRef]
- Athanasiadis, A.; Anderluh, G.; Macek, P.; Turk, D. Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure 2001, 9, 341–346. [Google Scholar] [CrossRef]
- Mechaly, A.E.; Bellomio, A.; Morante, K.; González-Mañas, J.M.; Guérin, D.M.A. Crystallization and preliminary crystallographic analysis of fragaceatoxin C, a pore-forming toxin from the sea anemone Actinia fragacea. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 357–360. [Google Scholar] [CrossRef]
- Kristan, K.; Podlesek, Z.; Hojnik, V.; Gutiérrez-Aguirre, I.; Guncar, G.; Turk, D.; González-Mañas, J.M.; Lakey, J.H.; Macek, P.; Anderluh, G. Pore formation by equinatoxin, a eukaryotic pore-forming toxin, requires a flexible N-terminal region and a stable beta-sandwich. J. Biol. Chem. 2004, 279, 46509–46517. [Google Scholar] [CrossRef]
- Tejuca, M.; Serra, M.D.; Ferreras, M.; Lanio, M.E.; Menestrina, G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 1996, 35, 14947–14957. [Google Scholar] [CrossRef]
- Macek, P.; Belmonte, G.; Pederzolli, C.; Menestrina, G. Mechanism of action of equinatoxin II, a cytolysin from the sea anemone Actinia equina L. belonging to the family of actinoporins. Toxicology 1994, 87, 205–227. [Google Scholar] [CrossRef]
- de los Rios, V.; Mancheño, J.M.; Lanio, M.E.; Oñaderra, M.; Gavilanes, J.G. Mechanism of the leakage induced on lipid model membranes by the hemolytic protein sticholysin II from the sea anemone Stichodactyla helianthus. Eur. J. Biochem. 1998, 252, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Hervis, Y.P.; Valle, A.; Dunkel, S.; Klare, J.P.; Canet, L.; Lanio, M.E.; Alvarez, C.; Pazos, I.F.; Steinhoff, H.-J. Architecture of the pore forming toxin sticholysin I in membranes. J. Struct. Biol. 2019, 208, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Caaveiro, J.M.M.; Morante, K.; González-Mañas, J.M.; Tsumoto, K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 2015, 6, 6337. [Google Scholar] [CrossRef]
- Morante, K.; Bellomio, A.; Gil-Cartón, D.; Redondo-Morata, L.; Sot, J.; Scheuring, S.; Valle, M.; González-Mañas, J.M.; Tsumoto, K.; Caaveiro, J.M.M. Identification of a Membrane-bound Prepore Species Clarifies the Lytic Mechanism of Actinoporins. J. Biol. Chem. 2016, 291, 19210–19219. [Google Scholar] [CrossRef]
- Pentón, D.; Pérez-Barzaga, V.; Díaz, I.; Reytor, M.L.; Campos, J.; Fando, R.; Calvo, L.; Cilli, E.M.; Morera, V.; Castellanos-Serra, L.R.; et al. Validation of a mutant of the pore-forming toxin sticholysin-I for the construction of proteinase-activated immunotoxins. Protein Eng. Des. Sel. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Wloka, C.; Mutter, N.L.; Soskine, M.; Maglia, G. Alpha-Helical Fragaceatoxin C Nanopore Engineered for Double-Stranded and Single-Stranded Nucleic Acid Analysis. Angew. Chem. Int. Ed. Engl. 2016, 55, 12494–12498. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Panchal, R.G. Novel therapeutic strategies to selectively kill cancer cells. Biochem. Pharmacol. 1998, 55, 247–252. [Google Scholar] [CrossRef]
- Lai, D.; Visser-Grieve, S.; Yang, X. Tumour suppressor genes in chemotherapeutic drug response. Biosci. Rep. 2012, 32, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2012, 64, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Ward, R.; Barton, M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. (R. Coll. Radiol.) 2004, 16, 549–560. [Google Scholar] [CrossRef]
- Sapra, P.; Shor, B. Monoclonal antibody-based therapies in cancer: Advances and challenges. Pharmacol. Ther. 2013, 138, 452–469. [Google Scholar] [CrossRef]
- Banerji, U.; Workman, P. Critical parameters in targeted drug development: The pharmacological audit trail. Semin. Oncol. 2016, 43, 436–445. [Google Scholar] [CrossRef]
- Mack, F.; Ritchie, M.; Sapra, P. The next generation of antibody drug conjugates. Semin. Oncol. 2014, 41, 637–652. [Google Scholar] [CrossRef]
- Potrich, C.; Anderluh, G.; Maček, P. Construction of new immunotoxins by linking equinatoxin II to monoclonal antibodies via the biotin-avidin interaction. Cytotoxic effects on human tumor cells = Citotoksični efekti imunotoxinov sestavljenih iz ekvinatoksina II in monoklonskih protiteles na človeške tumorske celice. Acta Biol. Slov. 2000, 43, 47–51. [Google Scholar]
- Pederzolli, C.; Belmonte, G.; Dalla Serra, M.; Macek, P.; Menestrina, G. Biochemical and cytotoxic properties of conjugates of transferrin with equinatoxin II, a cytolysin from a sea anemone. Bioconjug. Chem. 1995, 6, 166–173. [Google Scholar] [CrossRef]
- Choi, K.Y.; Swierczewska, M.; Lee, S.; Chen, X. Protease-activated drug development. Theranostics 2012, 2, 156–178. [Google Scholar] [CrossRef]
- Reunanen, N.; Kähäri, V. Matrix Metalloproteinases in Cancer Cell Invasion; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Casallanovo, F.; de Oliveira, F.J.F.; de Souza, F.C.; Ros, U.; Martínez, Y.; Pentón, D.; Tejuca, M.; Martínez, D.; Pazos, F.; Pertinhez, T.A.; et al. Model peptides mimic the structure and function of the N-terminus of the pore-forming toxin sticholysin II. Biopolymers 2006, 84, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ros, U.; Rodríguez-Vera, W.; Pedrera, L.; Valiente, P.A.; Cabezas, S.; Lanio, M.E.; García-Sáez, A.J.; Alvarez, C. Differences in activity of actinoporins are related with the hydrophobicity of their N-terminus. Biochimie 2015, 116, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mutter, N.L.; Volarić, J.; Szymanski, W.; Feringa, B.L.; Maglia, G. Reversible Photocontrolled Nanopore Assembly. J. Am. Chem. Soc. 2019, 141, 14356–14363. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Beers, R.; Xiang, L.; Lee, B.; Weldon, J.E.; Kreitman, R.J.; Pastan, I. Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc. Natl. Acad. Sci. USA 2011, 108, 5742–5747. [Google Scholar] [CrossRef]
- Onda, M.; Beers, R.; Xiang, L.; Nagata, S.; Wang, Q.; Pastan, I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc. Natl. Acad. Sci. USA 2008, 105, 11311–11316. [Google Scholar] [CrossRef]
- Oukhaled, A.; Bacri, L.; Pastoriza-Gallego, M.; Betton, J.-M.; Pelta, J. Sensing Proteins through Nanopores: Fundamental to Applications. ACS Chem. Biol. 2012, 7, 1935–1949. [Google Scholar] [CrossRef]
- Movileanu, L. Interrogating single proteins through nanopores: Challenges and opportunities. Trends Biotechnol. 2009, 27, 333–341. [Google Scholar] [CrossRef]
- Merzlyak, P.G.; Capistrano, M.-F.P.; Valeva, A.; Kasianowicz, J.J.; Krasilnikov, O.V. Conductance and ion selectivity of a mesoscopic protein nanopore probed with cysteine scanning mutagenesis. Biophys. J. 2005, 89, 3059–3070. [Google Scholar] [CrossRef]
- Maglia, G.; Heron, A.J.; Stoddart, D.; Japrung, D.; Bayley, H. Analysis of single nucleic acid molecules with protein nanopores. Meth. Enzymol. 2010, 475, 591–623. [Google Scholar] [PubMed]
- Dzubiella, J.; Allen, R.J.; Hansen, J.-P. Electric field-controlled water permeation coupled to ion transport through a nanopore. J. Chem. Phys. 2004, 120, 5001–5004. [Google Scholar] [CrossRef] [PubMed]
- Ivica, J.; Williamson, P.T.F.; de Planque, M.R.R. Salt Gradient Modulation of MicroRNA Translocation through a Biological Nanopore. Anal. Chem. 2017, 89, 8822–8829. [Google Scholar] [CrossRef] [PubMed]
- Kececi, K.; Sexton, L.T.; Buyukserin, F.; Martin, C.R. Resistive-pulse detection of short dsDNAs using a chemically functionalized conical nanopore sensor. Nanomedicine (Lond.) 2008, 3, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.C.; Choi, Y.; Horne, L.P.; Baker, L.A.; Siwy, Z.S.; Martin, C.R. Resistive-Pulse DNA Detection with a Conical Nanopore Sensor. Langmuir 2006, 22, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Noakes, M.T.; Brinkerhoff, H.; Laszlo, A.H.; Derrington, I.M.; Langford, K.W.; Mount, J.W.; Bowman, J.L.; Baker, K.S.; Doering, K.M.; Tickman, B.I.; et al. Increasing the accuracy of nanopore DNA sequencing using a time-varying cross membrane voltage. Nat. Biotechnol. 2019, 37, 651–656. [Google Scholar] [CrossRef]
- Cao, C.; Li, M.-Y.; Cirauqui, N.; Wang, Y.-Q.; Dal Peraro, M.; Tian, H.; Long, Y.-T. Mapping the sensing spots of aerolysin for single oligonucleotides analysis. Nat. Commun. 2018, 9, 2823. [Google Scholar] [CrossRef]
- Akeson, M.; Branton, D.; Kasianowicz, J.J.; Brandin, E.; Deamer, D.W. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 1999, 77, 3227–3233. [Google Scholar] [CrossRef]
- Carter, J.-M.; Hussain, S. Robust long-read native DNA sequencing using the ONT CsgG Nanopore system. Wellcome Open Res. 2017, 2, 23. [Google Scholar] [CrossRef]
- Jain, M.; Tyson, J.R.; Loose, M.; Ip, C.L.C.; Eccles, D.A.; O’Grady, J.; Malla, S.; Leggett, R.M.; Wallerman, O.; Jansen, H.J.; et al. MinION Analysis and Reference Consortium: Phase 2 data release and analysis of R9.0 chemistry. F1000Res 2017, 6, 760. [Google Scholar] [CrossRef]
- Goldstein, S.; Beka, L.; Graf, J.; Klassen, J.L. Evaluation of strategies for the assembly of diverse bacterial genomes using MinION long-read sequencing. BMC Genomics 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Minei, R.; Hoshina, R.; Ogura, A. De novo assembly of middle-sized genome using MinION and Illumina sequencers. BMC Genomics 2018, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Wongsurawat, T.; Jenjaroenpun, P.; Taylor, M.K.; Lee, J.; Tolardo, A.L.; Parvathareddy, J.; Kandel, S.; Wadley, T.D.; Kaewnapan, B.; Athipanyasilp, N.; et al. Rapid Sequencing of Multiple RNA Viruses in Their Native Form. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Razaghi, R.; Gilpatrick, T.; Molnar, M.; Sadowski, N.; Simpson, J.T.; Sedlazeck, F.J.; Timp, W. Simultaneous profiling of chromatin accessibility and methylation on human cell lines with nanopore sequencing. bioRxiv 2019, 504993. [Google Scholar]
- Tan, S.; Dvorak, C.M.T.; Estrada, A.; Gebhart, C.; Marthaler, D.G.; Murtaugh, M.P. MinION sequencing of Streptococcus suis allows for functional characterization of bacteria by multilocus sequence typing and antimicrobial resistance profiling. J. Microbiol. Methods 2019, 105817. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Kang, Y.; Chen, W.; Fang, W.; Wang, K.; Zhang, Q.; Fu, A.; Zhou, S.; Cheng, C.; et al. Identification of pathogens in culture-negative infective endocarditis cases by metagenomic analysis. Ann. Clin. Microbiol. Antimicrob. 2018, 17. [Google Scholar] [CrossRef]
- Ho, C.-W.; Van Meervelt, V.; Tsai, K.-C.; De Temmerman, P.-J.; Mast, J.; Maglia, G. Engineering a nanopore with co-chaperonin function. Sci. Adv. 2015, 1, e1500905. [Google Scholar] [CrossRef]
- Galenkamp, N.S.; Soskine, M.; Hermans, J.; Wloka, C.; Maglia, G. Direct electrical quantification of glucose and asparagine from bodily fluids using nanopores. Nat. Commun. 2018, 9, 4085. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Ying, Y.-L.; Li, Y.; Kraatz, H.-B.; Long, Y.-T. Nanopore analysis of β-amyloid peptide aggregation transition induced by small molecules. Anal. Chem. 2011, 83, 1746–1752. [Google Scholar] [CrossRef]
- Gu, L.-Q.; Cheley, S.; Bayley, H. Electroosmotic enhancement of the binding of a neutral molecule to a transmembrane pore. Proc. Natl. Acad. Sci. USA 2003, 100, 15498–15503. [Google Scholar] [CrossRef]
- Boukhet, M.; Piguet, F.; Ouldali, H.; Pastoriza-Gallego, M.; Pelta, J.; Oukhaled, A. Probing driving forces in aerolysin and α-hemolysin biological nanopores: Electrophoresis versus electroosmosis. Nanoscale 2016, 8, 18352–18359. [Google Scholar] [CrossRef] [PubMed]
- Asandei, A.; Schiopu, I.; Chinappi, M.; Seo, C.H.; Park, Y.; Luchian, T. Electroosmotic Trap Against the Electrophoretic Force Near a Protein Nanopore Reveals Peptide Dynamics During Capture and Translocation. ACS Appl. Mater Interfaces 2016, 8, 13166–13179. [Google Scholar] [CrossRef] [PubMed]
- Soskine, M.; Biesemans, A.; De Maeyer, M.; Maglia, G. Tuning the size and properties of ClyA nanopores assisted by directed evolution. J. Am. Chem. Soc. 2013, 135, 13456–13463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Z.; Haque, F.; Guo, P. Engineering of protein nanopores for sequencing, chemical or protein sensing and disease diagnosis. Curr. Opin. Biotechnol. 2018, 51, 80–89. [Google Scholar] [CrossRef]
- Zhang, X.; Price, N.E.; Fang, X.; Yang, Z.; Gu, L.-Q.; Gates, K.S. Characterization of Interstrand DNA-DNA Cross-Links Using the α-Hemolysin Protein Nanopore. ACS Nano 2015, 9, 11812–11819. [Google Scholar] [CrossRef]
- Ouldali, H.; Sarthak, K.; Ensslen, T.; Piguet, F.; Manivet, P.; Pelta, J.; Behrends, J.C.; Aksimentiev, A.; Oukhaled, A. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.-Q.; Li, M.-Y.; Ying, Y.-L.; Long, Y.-T. Direct Sensing of Single Native RNA with a Single-Biomolecule Interface of Aerolysin Nanopore. Langmuir 2018, 34, 14940–14945. [Google Scholar] [CrossRef]
- Laszlo, A.H.; Derrington, I.M.; Gundlach, J.H. MspA nanopore as a single-molecule tool: From sequencing to SPRNT. Methods 2016, 105, 75–89. [Google Scholar] [CrossRef]
- Cao, J.; Jia, W.; Zhang, J.; Xu, X.; Yan, S.; Wang, Y.; Zhang, P.; Chen, H.-Y.; Huang, S. Giant single molecule chemistry events observed from a tetrachloroaurate(III) embedded Mycobacterium smegmatis porin A nanopore. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Brown, C.G.; Clarke, J. Nanopore development at Oxford Nanopore. Nat. Biotechnol. 2016, 34, 810–811. [Google Scholar] [CrossRef]
- Restrepo-Pérez, L.; Huang, G.; Bohländer, P.R.; Worp, N.; Eelkema, R.; Maglia, G.; Joo, C.; Dekker, C. Resolving Chemical Modifications to a Single Amino Acid within a Peptide Using a Biological Nanopore. ACS Nano 2019, 13, 13668–13676. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kobayashi, T. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochim. Biophys. Acta 2014, 1841, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. [Google Scholar] [CrossRef]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Bakrac, B.; Kladnik, A.; Macek, P.; McHaffie, G.; Werner, A.; Lakey, J.H.; Anderluh, G. A toxin-based probe reveals cytoplasmic exposure of Golgi sphingomyelin. J. Biol. Chem. 2010, 285, 22186–22195. [Google Scholar] [CrossRef]
- Bakrac, B.; Gutiérrez-Aguirre, I.; Podlesek, Z.; Sonnen, A.F.-P.; Gilbert, R.J.C.; Macek, P.; Lakey, J.H.; Anderluh, G. Molecular determinants of sphingomyelin specificity of a eukaryotic pore-forming toxin. J. Biol. Chem. 2008, 283, 18665–18677. [Google Scholar] [CrossRef]
- Hong, Q.; Gutierrez-Aguirre, I.; Barlic, A.; Malovrh, P.; Kristan, K.; Podlesek, Z.; Macek, P.; Turk, D.; Gonzalez-Manas, J.M.; Lakey, J.H.; et al. Two-step membrane binding by Equinatoxin II, a pore-forming toxin from the sea anemone, involves an exposed aromatic cluster and a flexible helix. J. Biol. Chem. 2002, 277, 41916–41924. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, A.; Sekizawa, Y.; Emoto, K.; Sakuraba, H.; Inoue, K.; Kobayashi, H.; Umeda, M. Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 1998, 273, 5300–5306. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Murate, M.; Kobayashi, T. Protein probes to visualize sphingomyelin and ceramide phosphoethanolamine. Chem. Phys. Lipids 2018, 216, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Robinson, B.W.S.; Knolle, P.A. Cross-priming in health and disease. Nat. Rev. Immunol. 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Platzer, B.; Stout, M.; Fiebiger, E. Antigen cross-presentation of immune complexes. Front. Immunol. 2014, 5, 140. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Korsholm, K.S.; Rosenkrands, I.; Lindenstrøm, T.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2007, 6, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Zhou, F.; Reddy, R.; Huang, L.; Rouse, B.T. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J. Exp. Med. 1992, 175, 609–612. [Google Scholar] [CrossRef]
- Korsholm, K.S.; Hansen, J.; Karlsen, K.; Filskov, J.; Mikkelsen, M.; Lindenstrøm, T.; Schmidt, S.T.; Andersen, P.; Christensen, D. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine 2014, 32, 3927–3935. [Google Scholar] [CrossRef]
- Bakrač, B.; Anderluh, G. Molecular Mechanism of Sphingomyelin-Specific Membrane Binding and Pore Formation by Actinoporins. In Proteins Membrane Binding and Pore Formation; Anderluh, G., Lakey, J., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; pp. 106–115. ISBN 978-1-4419-6327-7. [Google Scholar]
- Antonini, V.; Pérez-Barzaga, V.P.; Bampi, S.; Pentón, D.; Martínez, D.; Dalla Serra, M.; Tejuca, M. Functional characterization of sticholysin I and W111C mutant reveals the sequence of the actinoporin’s pore assembly. PLoS ONE 2014, 9, e110824. [Google Scholar] [CrossRef]
- Morante, K.; Caaveiro, J.M.M.; Viguera, A.R.; Tsumoto, K.; González-Mañas, J.M. Functional characterization of Val60, a key residue involved in the membrane-oligomerization of fragaceatoxin C, an actinoporin from Actinia fragacea. FEBS Lett. 2015, 589, 1840–1846. [Google Scholar] [CrossRef]
- Rivera-de-Torre, E.; García-Linares, S.; Alegre-Cebollada, J.; Lacadena, J.; Gavilanes, J.G.; Martínez-Del-Pozo, Á. Synergistic Action of Actinoporin Isoforms from the Same Sea Anemone Species Assembled into Functionally Active Heteropores. J. Biol. Chem. 2016, 291, 14109–14119. [Google Scholar] [CrossRef]
- Fedorov, S.; Dyshlovoy, S.; Monastyrnaya, M.; Shubina, L.; Leychenko, E.; Kozlovskaya, E.; Jin, J.-O.; Kwak, J.-Y.; Bode, A.M.; Dong, Z.; et al. The anticancer effects of actinoporin RTX-A from the sea anemone Heteractis crispa (=Radianthus macrodactylus). Toxicon 2010, 55, 811–817. [Google Scholar] [CrossRef]
- Gupta, V.R.; Patel, H.K.; Kostolansky, S.S.; Ballivian, R.A.; Eichberg, J.; Blanke, S.R. Sphingomyelin Functions as a Novel Receptor for Helicobacter pylori VacA. PLoS Pathogens 2008, 4, e1000073. [Google Scholar] [CrossRef]
- Miller, M.E.; Adhikary, S.; Kolokoltsov, A.A.; Davey, R.A. Ebolavirus Requires Acid Sphingomyelinase Activity and Plasma Membrane Sphingomyelin for Infection. J. Virol. 2012, 86, 7473–7483. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, J.; Chen, J.; Durrant, D.; McIntyre, T.M.; Lee, R.M. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene 2004, 23, 3650–3658. [Google Scholar] [CrossRef][Green Version]
- Kummerow, F.A. Interaction between sphingomyelin and oxysterols contributes to atherosclerosis and sudden death. Am. J. Cardiovasc. Dis. 2013, 3, 17–26. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Carreto, S.; Miranda-Zaragoza, B.; Rodríguez-Almazán, C. Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools. Biomolecules 2020, 10, 539. https://doi.org/10.3390/biom10040539
Ramírez-Carreto S, Miranda-Zaragoza B, Rodríguez-Almazán C. Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools. Biomolecules. 2020; 10(4):539. https://doi.org/10.3390/biom10040539
Chicago/Turabian StyleRamírez-Carreto, Santos, Beatriz Miranda-Zaragoza, and Claudia Rodríguez-Almazán. 2020. "Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools" Biomolecules 10, no. 4: 539. https://doi.org/10.3390/biom10040539
APA StyleRamírez-Carreto, S., Miranda-Zaragoza, B., & Rodríguez-Almazán, C. (2020). Actinoporins: From the Structure and Function to the Generation of Biotechnological and Therapeutic Tools. Biomolecules, 10(4), 539. https://doi.org/10.3390/biom10040539




