Abstract
Nicotinamide (NAM) is a water-soluble form of Vitamin B3 (niacin) and a precursor of nicotinamide-adenine dinucleotide (NAD+) which regulates cellular energy metabolism. Except for its role in the production of adenosine triphosphate (ATP), NAD+ acts as a substrate for several enzymes including sirtuin 1 (SIRT1) and poly ADP-ribose polymerase 1 (PARP1). Notably, NAM is an inhibitor of both SIRT1 and PARP1. Accumulating evidence suggests that NAM plays a role in cancer prevention and therapy. Phase III clinical trials have confirmed its clinical efficacy for non-melanoma skin cancer chemoprevention or as an adjunct to radiotherapy against head and neck, laryngeal, and urinary bladder cancers. Evidence for other cancers has mostly been collected through preclinical research and, in its majority, is not yet evidence-based. NAM has potential as a safe, well-tolerated, and cost-effective agent to be used in cancer chemoprevention and therapy. However, more preclinical studies and clinical trials are needed to fully unravel its value.
1. Introduction
Cancer is a multistep process caused by an accumulation of molecular aberrations—activation of oncogenes, inhibition of tumor suppressor genes, epigenetic plasticity—that deregulate intracellular signaling pathways and drive cancer initiation and progression [1,2,3]. At the histologic level, normal tissues give rise to premalignant changes and, when the genetic alterations pile up to a higher number, the latter progress to malignancy (cancer) [1,2,3]. Through this process, cells gradually acquire traits that allow them to sustain uncontrolled proliferation, evade apoptosis, enhance angiogenesis, induce epithelial-mesenchymal transition (EMT), invade, and metastasize [4,5,6,7]. Of them, metastasis is the most common cause of morbidity and mortality [8]. Cancer cells’ ability to reprogram their metabolism is also critical for survival, growth, and metastasis [7]. Cancer metabolism could exhibit deregulated uptake of nutrients, intracellular metabolism, gene expression, and interactions with the microenvironment [9]. To suppress the genetic damage responsible for cancer initiation and progression, cells use diverse DNA repair systems which, if compromised, result in mutations; the latter characterize both premalignant and malignant changes [10]. The immune system is another defense mechanism our body uses against cancer; hence, immunosuppression is associated with higher cancer incidence [11,12,13]. Unfortunately, cancer cells might acquire the ability to evade immune system surveillance [7]. In contrast, chronic inflammation, a component of non-specific immunity, is tumor-promotive rather than suppressive [7,14].
Cancer chemoprevention involves the use of diverse natural, biological or synthetic agents to inhibit cancer initiation or progression [15,16]. Chemopreventive agents could include hormones, medications, vitamins, minerals, and vaccines [15]. Primary chemoprevention refers to the use of such agents in a healthy population, while secondary and tertiary chemoprevention aim to inhibit progression of a premalignant change to cancer or recurrence of an already treated cancer, respectively [16]. For instance, antiestrogens are prescribed after surgical treatment of hormone-positive breast cancer patients to reduce the chance of recurrence [17], and HPV (human papillomavirus) vaccination has been introduced to prevent cervical cancer [18]. Aspirin or other NSAIDs (nonsteroidal anti-inflammatory drugs) have been associated with lower incidence and mortality in colorectal cancer [19]. Metformin, an antidiabetic medication, has recently shown promising results for cancer chemoprevention and therapy [20]. Concerning vitamins, evidence shows that vitamin D could have a preventive role against colorectal cancer [21].
Cancer therapy is traditionally performed with surgery, chemotherapy, and radiotherapy [22]. To select the best possible management, it is important to grade and stage each malignant tumor. Tumor grading refers to its histologic level of aggressiveness [23,24], while staging to its extent into the body (local size and extent, regional lymph node involvement, and distant metastases) [25]. Notably, tumor heterogeneity is also taken into account when treating cancer patients and is the base of cancer precision therapy [26]. This heterogeneity can be found among distinct cancers of the same histologic type, between a primary cancer and its metastasis or among the cellular compartments that compose a tumor mass [27,28]. For instance, hormone-positive cancers are treated differently than HER2 (human epidermal growth factor receptor 2) positive breast cancers or TNBCs (triple-negative breast cancers) [29,30]. Tumor heterogeneity is studied with high throughput molecular tests and targeted therapies are presently standard of care [31,32]. Of interest, as cancer progresses, it accumulates genetic and epigenetic alterations thus becomes more heterogeneous. The latter could be associated with resistance to chemotherapy or targeted therapy [33,34]. Concerning radiotherapy, tumor hypoxia is one of the causes of resistance and agents that enhance tumor blood flow and oxygenation improve therapeutic response [35,36].
This review examines the role of nicotinamide (NAM) in cancer chemoprevention and therapy. It first starts with a brief description of NAM basic principles and metabolism, then continues with the existing evidence on its potential utility in chemoprevention, chemotherapy, and radiotherapy, and finishes with a discussion of the findings in addition to future perspectives.
2. NAM Basic Principles and Metabolism
NAM is a water-soluble form of Vitamin B3 (niacin) that can be found in animal and plant food, also as a cost-effective vitamin supplement [37,38,39]. It is absorbed in the stomach or intestine, metabolized in the liver, and excreted through the kidneys [40,41]. While its presence is vital for cellular energy metabolism, lack of NAM is associated with fatigue and anorexia. Pellagra, a disease caused by severe NAM deficiency, is identified by the presence of diarrhea, dementia, and dermatitis and can even lead to death if not treated [37].
NAM is a precursor of nicotinamide-adenine dinucleotide (NAD+), which takes part in several redox and non-redox reactions that regulate cellular energy metabolism (Figure 1) [38,39]. NAD+ acts as a co-enzyme in dehydrogenase reactions that result in the production of adenosine triphosphate (ATP). High NAD+ (or high NAD+/NADH ratio) suppresses the production of reactive oxygen species (ROS) and enhances mitochondrial quality. Therefore, it protects against oxidative stress and improves cell survival [42,43,44]. Except its vital part in redox homeostasis, NAD+ is also a substrate for enzymes in non-redox reactions, where it is cleaved back to NAM. The most well-studied enzymes in these reactions are the sirtuin 1 (SIRT1) and the poly ADP-ribose polymerase 1 (PARP1) [38,39]. SIRT1 and PARP1 carry out the protein posttranslational modifications called deacetylation and poly(ADP-ribosyl)ation (PARylation), respectively [38,39]. SIRT1 is a NAD+ dependent class III histone deacetylase (HDAC) of histones and various non-histone transcription factors (e.g., p53) involved in diverse intracellular processes including DNA repair, metabolism, apoptosis, proliferation, hormone response, aging, and carcinogenesis [39,45,46]. Whereas NAD+ stimulates SIRT1-mediated deacetylation of target proteins, low NAD+/NADH ratio diminishes SIRT1 activity [47]. PARP1, triggered by DNA damage (strand breaks), enhances DNA repair and maintains genome stability [40,48]. Of interest, mild and moderate genetic damage facilitate DNA repair or apoptosis, respectively; however, severe damage leads to PARP1 overactivation that depletes NAD+ and ATP leading to necrosis [40,47]. Notably, while SIRT1 and PARP1 are activated by NAD+ and DNA damage, respectively, NAM suppresses both through a negative feedback mechanism (Figure 1) [38,39].
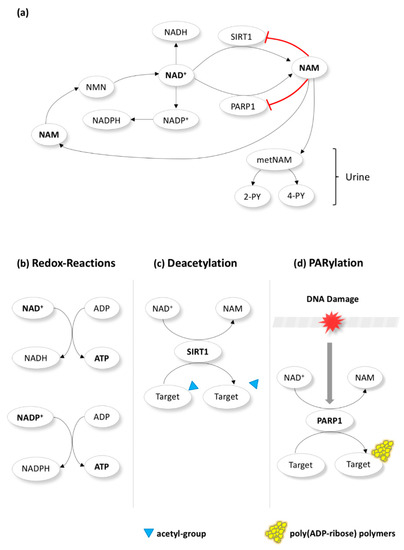
Figure 1.
Nicotinamide (NAM) metabolism. NAM is a precursor of nicotinamide-adenine dinucleotide (NAD+) and regulates cellular metabolism. NAM is first converted to NMN (nicotinamide mononucleotide) in the cytoplasm before becoming NAD+. The latter acts a co-enzyme in redox reactions (b) that produce adenosine triphosphate (ATP) or is phosphorylated towards NADP+ (which also takes part in redox reactions). Additionally, NAD+ is a substrate for enzymes in non-redox reactions, where it is cleaved back to NAM (a). The most well-studied enzymes in these reactions are the sirtuin 1 (SIRT1) and the poly ADP-ribose polymerase 1 (PARP1). SIRT1 and PARP1 carry out the protein posttranslational modifications called deacetylation (c) and poly(ADP-ribosyl)ation (PARylation)(d). Notably, while SIRT1 and PARP1 are activated by NAD+ (c) and DNA damage (d), respectively, NAM suppresses both through a negative feedback mechanism (a). NAM produced in non-redox reactions could “go back” to be converted once more to NAD+ via NMN. Alternatively, it could be metabolized to metNAM (methyl-NAM) and subsequently to 4-PY (N-methyl-4-pyridone-5-carboxamide) and 2-PY (N-methyl-2-pyridone-5-carboxamide) and excreted through the urine (a).
Several studies have shown that NAM could offer a benefit in several diverse diseases and pathologic conditions [39]. It is reported to have anti-inflammatory properties as it suppresses the expression of several pro-inflammatory mediators or the pro-inflammatory transcription factor NF-κΒ (nuclear factor kappa B) through the latter’s SIRT1-mediated deacetylation [37,39]. NAM is therapeutic for specific inflammatory skin lesions such as bullous pemphigoid and acne vulgaris [49]. In addition, it is regarded as neuroprotectant primarily due to increasing the levels of NAD+ [39]. Therefore, NAM could have a therapeutic role against trauma, ischemia, and neurodegeneration—Parkinson, Alzheimer, and Huntington diseases—[50] in addition to neuropsychological disorders including depression and schizophrenia [39,51]. Likewise, NAM could be beneficial against diabetes [52], HIV infection [53], and glaucoma [54]. Concerning cancer, NAM has already shown clinical efficacy against non-melanoma skin cancer (NMSC) [55] and radiotherapy resistance due to tumor hypoxia [35,36].
Oral administration of NAM is safe and well tolerated for doses below 3 g/day [39]. Several clinical trials have shown no significant differences regarding side effects between NAM-treated individuals and the placebo group [40,55,56,57]. In contrast, high oral doses of NAM, especially above 6 g/day, have been associated with gastrointestinal toxicity [40]. Adverse effects such as flushing, hypotension or headache due to vasodilation are believed to be caused by nicotinic acid (another form of vitamin B3, also a NAD+ precursor) rather than the NAM itself [39,41,47].
3. Nicotinamide and Cancer Chemoprevention
Most relevant studies, at both preclinical and clinical levels, have been performed in the field of skin cancer chemoprevention, especially for non-melanoma skin cancer (NMSC) (Table 1).

Table 1.
Summary of the evidence (preclinical/clinical) that supports the role of nicotinamide (NAM) in cancer chemoprevention.
NMSC is the most commonly diagnosed malignancy in Australia/New Zealand and North America, with a global estimated number of 1,042,056 new cases for 2018. Yet, it is characteristically absent from the top ten list of the estimated number of deaths in the same year [81]. Although most NMSCs follow an indolent course, their incidence and associated morbidity is on the rise [82]. NMSC is mainly divided into basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [83]. Actinic keratosis (AK), a common intra-epidermal lesion found in long-term photo-exposed skin areas, increases someone’s risk to develop NMSC [84,85,86]; around 10% of AKs are estimated to progress to invasive cancer [87]. Ultraviolet (UV) radiation from sunlight is associated with a higher risk of skin cancer [40,47]. The former depletes NAD+ thus ATP and cellular energy; hence, UV-induced DNA damage is followed by defective DNA repair and genetic instability [40,47]. Immunosuppression induced by UV radiation is another carcinogenesis factor [41,88,89,90]. This is obvious by the fact that cancer shows higher incidence in transplant recipients [91,92]. Similar to UV radiation, aging also depletes NAD+ levels, enhances the formation of ROS and hampers DNA repair; therefore, it promotes genomic instability [47]. As a result, skin cancer affects more commonly the elderly rather than younger patients [93].
NAM has exhibited skin cancer chemoprevention characteristics at the preclinical level (cell lines, animal models, human tissues). When administered together with butyric acid and calcium glucarate, it suppressed the DMBA (7,12-dimethylbenz (a) anthracene)-induced formation of skin tumors in animal models [58]. NAM suppressed ATP reduction in UV-irradiated keratinocytes in vitro [59] and enhanced the expression of enzymes involved in cellular energy metabolism [67]. In addition, it stimulated DNA repair when tested in UV-irradiated keratinocytes and ex vivo skin [60,61]; similarly, NAM showed a similar effect on genomic stability when tested in melanocytes [62]. As described before, chronic inflammation is a hallmark of cancer [7]. NAM displayed anti-inflammatory ability by suppressing the expression of diverse pro-inflammatory mediators including interleukins (IL) 1β, 6, 8, and 10, and tumor necrosis factor-alpha (TNF-α) in vitro [63] or by reducing the number of macrophages, which constitute the main regulator of chronic inflammation, in human NMSC tissues of patients treated with NAM [64].
NAM has displayed the ability to counteract UV-induced immunosuppression. Gessler et al. showed that topical NAM administration reduced immunosuppression and suppressed tumor formation in UV-irradiated animal models [65]. The same group reported similar results by administering oral niacin instead of topical NAM [94]. Several clinical studies have also confirmed the role of NAM against immunosuppression. Topical or oral NAM administration in human patients reduced immunosuppression in selected UV-irradiated sites, as measured with the Mantoux immunity reaction, compared to the patients that received placebo [66,67,68]. Oral NAM was also safe and well tolerated, while it was absorbed effectively by the tested patients, as shown with the increased NAD+ levels in their blood [68]. In addition, Thanos et al. showed that both topical and oral NAM reduced immunosuppression in skin areas undergoing photodynamic therapy [69].
Various clinical studies have exhibited NAM efficacy against skin pre-cancer and cancer. In earlier studies, topical or oral NAM administration reduced the incidence of AK in NAM-treated high-risk individuals compared to the ones that received placebo [70,71]. Notably, most of the information concerning NAM’s role in chemoprevention has derived from the ONTRAC (Oral Nicotinamide To Reduce Actinic Cancer) phase III clinical trial. This was carried out on 386 immunocompetent individuals diagnosed with at least two histologically confirmed NMSCs during the last five years [55]. Participants were divided into two groups, one that was treated with oral NAM and the other one with placebo for 12 months. The study first showed that oral NAM at a dose of 500 mg twice daily is safe and well tolerated. In addition, NAM reduced the incidence of AK and NMSC at significant levels; both BCC and SCC were reduced compared to the placebo group [55]. As part of the ONTRAC trial, oral NAM-treated patients showed no significant effect on their neurocognitive function or life outcome [95].
Two recent clinical studies evaluated the efficacy of oral NAM in immunocompromised patients. Although Chen et al. study did not reveal significant results due to its small sample size (n = 22), NAM was well tolerated and exhibited a 16% and 35% reduction of AKs and NMSCs, respectively [72]. Drago et al. included immunocompromised patients with kidney or liver transplants and reported that NAM suppressed preexisting AKs and even inhibited the development of new AKs or cancer in these patients [73].
Evidence for NAM chemopreventive role is sparse in cancers other than NMSC and only comes from preclinical studies (Table 1). NAM suppressed the formation of bladder tumors in BBN (N-butyl-N-(4-hydroxybutyl)-nitrosamine)-exposed animal models [74]. It also suppressed lung tumor formation in benzo(a)pyrene-exposed animal models, either when administered alone or synergistically with the glucocorticoid budesonide [75], in addition to urethane-exposed animal models [76]. Likewise, NAM suppressed liver tumor development in thioacetamide-exposed [78] or the formation of pre-neoplastic lesions in DEN (diethylnitrosamine)-exposed animal models, showing it plays a role in the early stages of cancer development [77]. In addition, oral NAM reduced the incidence of non-lymphocytic leukemia in alkylation-exposed animals [79]. Concerning kidney tumorigenesis, NAM treatment has shown both tumor-inhibiting and promoting capacity. Rakieten et al. reported that NAM inhibited the formation of renal tumors in streptozotocin-exposed animal models [80], whereas Rosenberg et al. reported that it enhanced kidney tubular tumor development [96]. Lastly, also in contrast to the previous studies, NAM induced the formation of pancreatic neuroendocrine tumors in animal models when administered together with streptozocin [97].
4. Nicotinamide and Cancer Therapy
4.1. Radiotherapy
Apart from its use in the chemoprevention of NMSCs, high level evidence in the form of randomized clinical trials suggests the clinical efficacy of NAM during radiotherapy of selected cancer types (Table 2). Cancers often show resistance to radiotherapy if they are poorly oxygenated [35]. ARCON (accelerated radiotherapy with carbogen and nicotinamide) could help overcome this issue. Carbogen (98% oxygen, 2% carbon dioxide) and nicotinamide enhance oxygenation and increase blood flow of hypoxic cancers improving the efficacy of radiotherapy [35,36].

Table 2.
Summary of evidence (preclinical/clinical) that supports the role of nicotinamide (NAM) in cancer radiotherapy.
Concerning head and neck cancers—a category composed of tumors growing in various sites such as the oral cavity, the oropharynx, and the larynx—ARCON was found to enhance locoregional control in a phase II clinical trial [98]. In addition, ARCON counteracted the negative prognostic impact of anemia in patients with head and neck cancer treated with radiotherapy in a phase III clinical trial. Notably, there was no significant association of hemoglobin levels with locoregional control, overall, and disease-free survival [100]. In contrast, a study by Bernier et al. concluded that ARCON showed no significant therapeutic benefit in terms of local tumor control and tumor response in patients treated with radiotherapy, while it was also accompanied by gastrointestinal toxicity connected with the high doses of NAM (6 g/day) used [99].
ARCON’s clinical efficacy seems more evident in laryngeal and urinary bladder cancers treated with radiotherapy. A phase III clinical trial showed that ARCON improved local tumor control in laryngeal cancer patients, especially in the presence of tumor hypoxia [104,105]. It also enhanced locoregional control and disease-free survival in anemic laryngeal cancer patients in addition to their quality of life after the treatment [106,107]. Likewise, ARCON was found to improve prognosis in patients with highly proliferative laryngeal cancers (detected with Ki-67 immunohistochemistry) [102]. In urinary bladder cancer, Hoskin et al. performed a phase II clinical trial where ARCON was found to be relatively safe and well tolerated; in addition, the latter enhanced local regional control and improved overall survival [108,109]. The same group reported some years later the results of their phase III clinical trial; NAM and carbogen improved overall and disease-free survival at a significant level in patients treated with radiotherapy [110].
In contrast to the favorable results in laryngeal and urinary bladder cancers, carbogen and nicotinamide showed no significant therapeutic benefit in terms of overall survival in glioblastoma patients treated with radiotherapy. Furthermore, gastrointestinal toxicity in the form of nausea and vomiting was noticed and linked with the high doses of NAM used in these clinical trials [112,113]. Lastly, ARCON also failed to show any significant therapeutic benefit in terms of tumor response in NSCLC (non-small cell lung cancer) patients in a phase I/II clinical trial [114].
4.2. Chemotherapy
In contrast to its role in chemoprevention or as an adjunct to radiotherapy in selected cancers, there is little evidence concerning the clinical efficacy of NAM as a chemotherapeutic regimen. To our knowledge, most of the published studies have been preclinical, whereas only a single clinical trial has been performed (Table 3).

Table 3.
Summary of evidence (preclinical, clinical) that supports the role of nicotinamide (NAM) in cancer chemotherapy.
Most studies were found to involve the breast. An earlier study showed that intraperitoneal NAM suppressed breast cancer volume in animal models [119]. More recently, two research groups reported that NAM suppressed proliferation and enhanced apoptosis when tested in the MC-7 hormone-positive breast cancer cell line; in one of these studies, SIRT1 inactivation by NAM was addressed as the main underlying mechanism [120,121]. Similarly, NAM also inhibited SIRT1 when tested in TNBC cell lines, suppressing cell cycle progression and DNA replication; at the same time, it enhanced apoptosis. Additionally, the same authors reported that NAM suppressed DNA repair by inhibiting PARP1 [122]. In another study, NAM suppressed metastasis to the lungs and brain and prolonged survival of TNBC xenografts through normalizing the NAD+/NADH ratio, enhancing autophagy and suppressing AKT/mTORC1 (protein kinase B/mammalian target of rapamycin complex 1) signaling [123].
In preclinical melanoma models, NAM was reported to suppress vasculogenic mimicry, a reported poor prognostic factor of melanoma [126]. It also inhibited migration in vitro, also invasion and metastasis in vivo through inhibiting SIRT1, while it improved animals’ survival [127]. Concerning liver-related primary cancers, NAM suppressed proliferation and enhanced apoptosis of HCC (hepatocellular carcinoma) in vitro mainly through stimulating the p53/p21 pathway [77]. Additionally, it reduced HCC growth, serum AFP (A-fetoprotein), and enhanced survival of thioacetamide-exposed animal models via suppressing IGF-1 (insulin-like growth factor 1), a well-known HCC promoter [78]. Similarly, NAM inhibited cell cycle progression, EMT (epithelial-mesenchymal transition), and invasion while it enhanced apoptosis of intrahepatic cholangiocarcinoma (bile duct carcinoma) in vitro [128]. In pancreatic cancer, NAM suppressed proliferation, cell cycle progression, invasion, and enhanced apoptosis in vitro by down-regulating SIRT1, KRAS (Kirsten Rat Sarcoma), and p-AKT (phosphated protein kinase B) [130], while it exhibited an analogous effect when administered in combination with valproate [129]. In urinary bladder cancer, NAM suppressed tumor proliferation, growth, and progression by modulating the expression of Myc—expression of the latter was reduced in cancerous compared to the normal urothelium—and its related genes [74]. When tested in the HeLa cell line, which is a model of cervical cancer, NAM suppressed proliferation and enhanced oxidative stress and apoptosis [132]. Lastly, NAM enhanced the delivery of chemotherapy to colon cancer metastases, when administered together with carbogen, in advanced cancer patients [131].
Notably, NAM seems to be of potential value in selected cancers resistant to chemotherapy. However, all studies have experimented on cell lines and evidence of high value is still absent. Concerning breast cancer, NAM reestablished sensitivity to chemotherapy in resistant hormone-positive [124,125] and TNBC cell lines [125]. NAM-induced suppression of SIRT1 and PARP1 was described as the main mechanism of action [124,125]. NAM also reestablished sensitivity to chemotherapy when tested in resistant pancreatic cancer cell lines [130].
Two studies have reported the potential role of NAM in the treatment of hematologic malignancies. Audrito et al. used cell lines derived from patient blood samples showing that NAM inhibited SIRT1, exhibiting an anti-proliferative and pro-apoptotic activity in CLL (chronic lymphocytic leukemia) [133]. Amengual et al. first experimented on preclinical models and found that NAM exhibited a synergistic cytotoxic action against DLBCL (diffuse large B-cell lymphoma) when administered together with a pan I/II deacetylase inhibitor (e.g., vorinostat, romidepsin, or belinostat), although it had a relatively weak effect by itself. The underlying mechanism was the acetylation of BCL6 (B-cell lymphoma 6) and p53, resulting in BCL6 suppression and p53 activation, respectively. The same group subsequently performed a phase I clinical trial, where patients with relapsed lymphoma treated with a combination of NAM and vorinostat. The overall response rate (ORR) was 24%, while about half of the patients demonstrated stable disease (SD) [134].
5. Discussion
Accumulating evidence suggests that NAM plays a role in cancer chemoprevention and therapy. Phase III clinical trials have demonstrated the efficacy of NAM in NMSC chemoprevention or as a component of ARCON in the treatment of head and neck, laryngeal and urinary bladder cancers, incorporating their results into clinical practice [55,100,104,105,110]. However, most of the evidence is not yet evidence-based, as it has mostly been acquired in preclinical studies. Nevertheless, it could give future directions with a goal to expand NAM applications in the fields of cancer prevention and therapy (Figure 2).
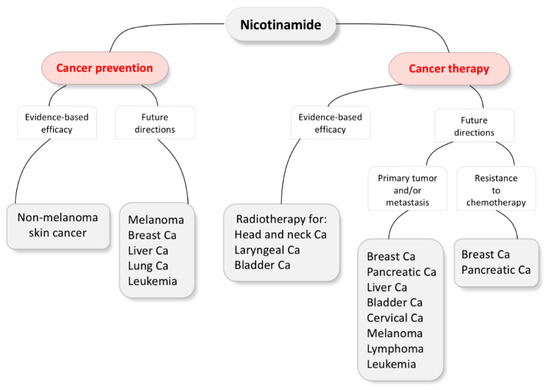
Figure 2.
Nicotinamide evidence-based efficacy and future directions in cancer prevention and therapy. Ca, carcinoma.
As shown in the ONTRAC trial, oral NAM is a safe, well-tolerated, and cost-effective chemopreventive agent against both basal and squamous cell skin cancers in high-risk immunocompetent individuals [55]. In addition, it also has value in NMSC prevention in immunocompromised patients with renal or liver transplants [72,73]. Besides the aforementioned studies, a Canadian pilot trial will also evaluate the chemopreventive role of NAM against NMSC in transplant recipients (NCT03769285).
Thomson et al. showed that NAM enhanced DNA repair in UV-irradiated melanocytes in vitro [62]. However, to our knowledge, no clinical trial has yet assessed the chemopreventive role of NAM against melanoma in high-risk patients. During the ONTRAC trial, a small number of the participants developed melanomas; nevertheless, these were distributed equally between the NAM-treated and placebo groups. In addition, the trial excluded patients with a history of melanoma the last 5 years, as it was designed to study NMSC and its precursor AK. For these reasons, the ONTRAC trial did not draw significant results concerning the role of NAM in melanoma chemoprevention [55,90].
NAM could potentially be of value to prevent breast cancer recurrence after primary treatment and a relevant clinical trial could be of value [135]. Likewise, as treated urinary bladder cancers are frequently followed with recurrences [136], a similar clinical trial on such high-risk patients could also end up in significant results.
NAM’s role in cancer therapy, except for ARCON, has still not been elucidated. A phase I clinical trial, where patients with relapsed lymphoma were treated with a combination of NAM and vorinostat, showed some promising results related to ORR and SD [134]. Of interest, a currently active phase II/III clinical trial is studying the clinical efficacy of NAM when prescribed synergistically with the EGFR (Epidermal growth factor receptor) inhibitors gefitinib or erlotinib in EGFR-mutated advanced NSCLC patients (NCT02416739).
Notably, NAM could have a role in reestablishing sensitivity to therapy in cases of chemoresistance, as shown in breast and pancreatic preclinical models [124,125,130]. It would also be interesting to investigate if NAM could reestablish treatment sensitivity in cases of resistance to distinct targeted therapies (e.g., tamoxifen or trastuzumab in hormone and HER2 positive breast cancer patients, respectively) or immunotherapy. Lastly, NAM could be of value in the therapy of aggressive cancers that still do not have effective targeted therapy such as TNBCs [122] and more studies in this direction would be desirable.
Preclinical studies have shown that NAM administration is tumor suppressive, being able to disrupt multiple key processes — e.g., proliferation, apoptosis, invasion, and metastasis — in a variety of cancers (Figure 3 and Table 3). Notably, NAM primarily seems to exhibit its chemotherapeutic capacity by suppressing SIRT1 and PARP1, as already shown in preclinical models of diverse cancers [120,122,124,125,127,130,133]. NAM-induced SIRT1 or PARP1 suppression also enhances sensitivity to chemotherapy in breast cancer cell lines [124,125]. While PARP1 inhibition suppresses DNA repair in cancer cells, SIRT1 inhibition results in p53 activation, suppressing proliferation and inducing cell cycle arrest and apoptosis [122,133,135]. In fact, the NAM-induced activation of the p53/p21 pathway has exhibited antitumor properties in breast cancer, lymphoma, leukemia, and HCC [77,122,133,134]. Besides inhibiting SIRT1 and PARP1, NAM has shown to inhibit the oncogenic KRAS/AKT pathway in skin and pancreatic cancers [58,130], modulate the expression of Myc oncogene in bladder cancer [74], and suppress IGF-1 in HCC [78].
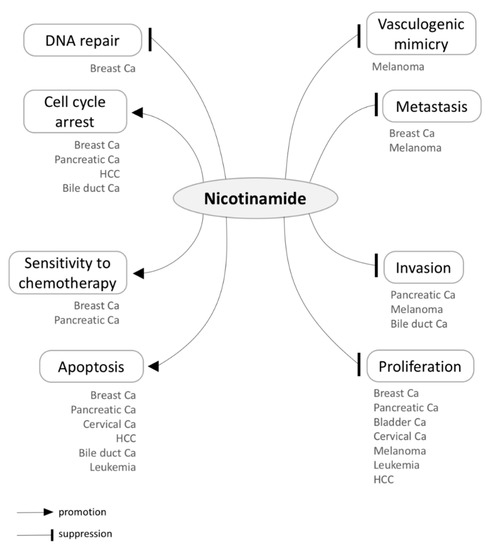
Figure 3.
Tumor suppressive effects of Nicotinamide (NAM) in a variety of cancers. Below each of the distinct processes influenced by NAM, the associated cancer types are listed according to the existing literature. HCC, hepatocellular carcinoma; Ca, carcinoma.
Despite the aforementioned evidence, more studies are needed to unravel NAM’s entire molecular mechanism in diverse cancer scenarios, before its widespread implementation into clinical practice. As previously mentioned, NAM increases the levels of NAD+, boosts energy metabolism and protects cells from oxidative stress [39]. NAM also inhibits SIRT1 and PARP1, both of which have been shown to be up-regulated in multiple cancers [39,48,137,138,139]. However, while it suppresses SIRT1, NAM is quickly metabolized into NAD+ which activates SIRT1. Therefore, it is not easy to say if NAM inhibits or in fact stimulates SIRT1 in the long term and more preclinical research in this direction would be vital [38]. To make things more complicated, SIRT1 exhibits a dichotomous behavior and has been described as both tumor promoter and suppressor, while both SIRT1 up-regulation and down-regulation have been associated with cancer progression [45,139,140,141,142]. Some studies have suggested that SIRT1 behavior is dependent on the oncogenic context [142]. For instance, SIRT1 overexpression has been reported to be a poor prognostic factor in TNBCs [143,144] but a favorable one in the luminal A hormone-positive breast cancers [145,146]. In contrast, Tan et al. connected SIRT1 overexpression with shorter survival in luminal cancers [147], while Wu et al. linked it with cancer progression and dismal prognosis in both TNBCs and non-TNBCs [148]. In fact, evidence that supports both a tumor promoter and suppressor function of SIRT1 has been reported for TNBC and luminal breast cancer subtypes [142]. Concerning PARP1, its up-regulation has been reported in multiple cancers, while its inhibition has the capacity to suppress angiogenesis, metastasis, and tumor-induced inflammation [48].
Notably, any potential carcinogenic effects in normal tissues induced by NAM administration should be carefully studied. SIRT1 inhibition by NAM could suppress metabolism, a deregulation of which is potentially oncogenic [7,9], while suppression of PARP1 could result in accumulated genetic damage in the long term [39,46].
NAM has shown promise as a well-tolerated and cost-effective agent against cancer prevention and therapy. However, more preclinical studies and clinical trials are needed to unravel its clinical value as well as long-term safety.
Author Contributions
Conceptualization: I.P.N.; Writing—Original Draft Preparation: I.P.N.; Writing—Review & Editing: I.P.N., S.A.P., and H.S.R.; Visualization: I.P.N.; Supervision: I.P.N. and H.S.R.; Project Administration: I.P.N. and H.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0048) and Basic Science Research Program through Seoul National University Hospital Research Fund (03-2016-0390 & 03-2019-0400).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Braakhuis, B.J.M.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar] [PubMed]
- Yokota, J. Tumor progression and metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Bernstein, C.; R, A.; Nfonsam, V.; Bernstei, H. DNA Damage, DNA Repair and Cancer. In New Research Directions in DNA Repair; Chen, C., Ed.; InTech: Vienna, Austria, 2013; ISBN 9789535111146. [Google Scholar]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Janssen, L.M.E.; Ramsay, E.E.; Logsdon, C.D.; Overwijk, W.W. The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer 2017, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Penn, I.; Starzl, T.E. Immunosuppression and cancer. Transplant. Proc. 1973, 5, 943–947. [Google Scholar] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Res. 2015, 4, 916. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Garcia-Albeniz, X.; Chan, A.T. Aspirin for the prevention of colorectal cancer. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 461–472. [Google Scholar] [CrossRef]
- Zi, F.; Zi, H.; Li, Y.; He, J.; Shi, Q.; Cai, Z. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol. Lett. 2018, 15, 683–690. [Google Scholar] [CrossRef]
- Guraya, S.Y. Chemopreventive role of vitamin D in colorectal carcinoma. Journal of Microscopy and Ultrastructure 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutiérrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Aubry, M.-C.; Deschamps, C.; Marks, R.S.; Okuno, S.H.; Williams, B.A.; Sugimura, H.; Pankratz, V.S.; Yang, P. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: An analysis of 5018 hospital- and 712 population-based cases. J. Thorac. Cardiovasc. Surg. 2006, 131, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, E.I.; Meric-Bernstam, F. Personalized cancer therapy-leveraging a knowledge base for clinical decision-making. Cold Spring Harb Mol Case Stud 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Sounni, N.E.; Noel, A. Targeting the tumor microenvironment for cancer therapy. Clin. Chem. 2013, 59, 85–93. [Google Scholar] [CrossRef]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef]
- Schnitt, S.J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. 2010, 23 Suppl 2, S60–S64. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Wang, Y.; Schmid-Bindert, G.; Zhou, C. Erlotinib in the treatment of advanced non-small cell lung cancer: An update for clinicians. Ther. Adv. Med. Oncol. 2012, 4, 19–29. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Beijnen, J.H.; Schellens, J.H.M. Trastuzumab. Oncologist 2011, 16, 800–810. [Google Scholar] [CrossRef]
- Luqmani, Y.A. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14 Suppl 1, 35–48. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Kaanders, J.H.A.M.; Bussink, J.; van der Kogel, A.J. ARCON: A novel biology-based approach in radiotherapy. Lancet Oncol. 2002, 3, 728–737. [Google Scholar] [CrossRef]
- Tharmalingam, H.; Hoskin, P. Clinical trials targeting hypoxia. Br. J. Radiol. 2018, 20170966. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell. Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef]
- Song, S.B.; Park, J.S.; Chung, G.J.; Lee, I.H.; Hwang, E.S. Diverse therapeutic efficacies and more diverse mechanisms of nicotinamide. Metabolomics 2019, 15, 137. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28 Suppl 1, 15–22. [Google Scholar] [CrossRef]
- Choi, H.J.; Jang, S.-Y.; Hwang, E.S. High-Dose Nicotinamide Suppresses ROS Generation and Augments Population Expansion during CD8(+) T Cell Activation. Mol. Cells 2015, 38, 918–924. [Google Scholar]
- Kwak, J.Y.; Ham, H.J.; Kim, C.M.; Hwang, E.S. Nicotinamide exerts antioxidative effects on senescent cells. Mol. Cells 2015, 38, 229–235. [Google Scholar] [CrossRef]
- Song, S.B.; Jang, S.-Y.; Kang, H.T.; Wei, B.; Jeoun, U.-W.; Yoon, G.S.; Hwang, E.S. Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy. Mol. Cells 2017, 40, 503–514. [Google Scholar]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W. Emerging Roles of SIRT1 in Cancer Drug Resistance. Genes Cancer 2013, 4, 82–90. [Google Scholar] [CrossRef]
- Fania, L.; Mazzanti, C.; Campione, E.; Candi, E.; Abeni, D.; Dellambra, E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20, 5946. [Google Scholar] [CrossRef]
- Wang, L.; Liang, C.; Li, F.; Guan, D.; Wu, X.; Fu, X.; Lu, A.; Zhang, G. PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs. Int. J. Mol. Sci. 2017, 18, 2111. [Google Scholar] [CrossRef]
- Niren, N.M. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: A review. Cutis 2006, 77, 11–16. [Google Scholar]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef]
- Rennie, G.; Chen, A.C.; Dhillon, H.; Vardy, J.; Damian, D.L. Nicotinamide and neurocognitive function. Nutr. Neurosci. 2015, 18, 193–200. [Google Scholar] [CrossRef]
- Crinó, A.; Schiaffini, R.; Ciampalini, P.; Suraci, M.C.; Manfrini, S.; Visalli, N.; Matteoli, M.C.; Patera, P.; Buzzetti, R.; Guglielmi, C.; et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2005, 18, 749–754. [Google Scholar] [CrossRef]
- Murray, M.F. Nicotinamide: An oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin. Infect. Dis. 2003, 36, 453–460. [Google Scholar] [CrossRef]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W.M. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Knip, M.; Douek, I.F.; Moore, W.P.; Gillmor, H.A.; McLean, A.E.; Bingley, P.J.; Gale, E.A.; European Nicotinamide Diabetes Intervention Trial Group. Safety of high-dose nicotinamide: A review. Diabetologia 2000, 43, 1337–1345. [Google Scholar] [CrossRef]
- Gale, E.A.M.; Bingley, P.J.; Emmett, C.L.; Collier, T.; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004, 363, 925–931. [Google Scholar] [CrossRef]
- Tiwari, P.; Sahay, S.; Pandey, M.; Qadri, S.S.Y.H.; Gupta, K.P. Preventive effects of butyric acid, nicotinamide, calcium glucarate alone or in combination during the 7, 12-dimethylbenz (a) anthracene induced mouse skin tumorigenesis via modulation of K-Ras-PI3K-AKTpathway and associated microRNAs. Biochimie 2016, 121, 112–122. [Google Scholar] [CrossRef]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis 2013, 34, 1144–1149. [Google Scholar] [CrossRef]
- Thompson, B.C.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS One 2015, 10, e0117491. [Google Scholar] [CrossRef]
- Thompson, B.C.; Surjana, D.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Exp. Dermatol. 2014, 23, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Monfrecola, G.; Gaudiello, F.; Cirillo, T.; Fabbrocini, G.; Balato, A.; Lembo, S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-α gene expression in HaCaT keratinocytes after ultraviolet B irradiation. Clin. Exp. Dermatol. 2013, 38, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Martin, A.J.; Chen, A.C.; Scolyer, R.A.; Lyons, J.G.; McKenzie, C.A.; Madore, J.; Halliday, G.M.; Damian, D.L. A Reduction in Inflammatory Macrophages May Contribute to Skin Cancer Chemoprevention by Nicotinamide. J. Invest. Dermatol. 2019, 139, 467–469. [Google Scholar] [CrossRef]
- Gensler, H.L. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr. Cancer 1997, 29, 157–162. [Google Scholar] [CrossRef]
- Damian, D.L.; Patterson, C.R.S.; Stapelberg, M.; Park, J.; Barnetson, R.S.C.; Halliday, G.M. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Invest. Dermatol. 2008, 128, 447–454. [Google Scholar] [CrossRef]
- Sivapirabu, G.; Yiasemides, E.; Halliday, G.M.; Park, J.; Damian, D.L. Topical nicotinamide modulates cellular energy metabolism and provides broad-spectrum protection against ultraviolet radiation-induced immunosuppression in humans. Br. J. Dermatol. 2009, 161, 1357–1364. [Google Scholar] [CrossRef]
- Yiasemides, E.; Sivapirabu, G.; Halliday, G.M.; Park, J.; Damian, D.L. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis 2009, 30, 101–105. [Google Scholar] [CrossRef]
- Thanos, S.M.; Halliday, G.M.; Damian, D.L. Nicotinamide reduces photodynamic therapy-induced immunosuppression in humans. Br. J. Dermatol. 2012, 167, 631–636. [Google Scholar] [CrossRef]
- Moloney, F.; Vestergaard, M.; Radojkovic, B.; Damian, D. Randomized, double-blinded, placebo controlled study to assess the effect of topical 1% nicotinamide on actinic keratoses. Br. J. Dermatol. 2010, 162, 1138–1139. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Martin, A.J.; Moloney, F.J.; Damian, D.L. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J. Invest. Dermatol. 2012, 132, 1497–1500. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Dalziell, R.A.; McKenzie, C.A.; Lowe, P.M.; Eris, J.M.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Bielski, V.A.; et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br. J. Dermatol. 2016, 175, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Cogorno, L.; Calvi, C.; Marsano, L.A.; Parodi, A. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: A case-control study. Eur. J. Dermatol. 2017, 27, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Yun, S.-J.; Kim, J.; Lee, O.-J.; Bae, S.-C.; Kim, W.-J. Identification of gene expression signature modulated by nicotinamide in a mouse bladder cancer model. PLoS One 2011, 6, e26131. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, A.R.; Seabloom, D.E.; Wuertz, B.R.; Antonides, J.D.; Steele, V.E.; Wattenberg, L.W.; Ondrey, F.G. Chemoprevention of Lung Carcinogenesis by Dietary Nicotinamide and Inhaled Budesonide. Cancer Prev. Res. 2019, 12, 69–78. [Google Scholar] [CrossRef]
- Gotoh, H.; Nomura, T.; Nakajima, H.; Hasegawa, C.; Sakamoto, Y. Inhibiting effects of nicotinamide on urethane-induced malformations and tumors in mice. Mutat. Res. 1988, 199, 55–63. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, K.B.; Lee, M.-J.; Bae, S.-C.; Jang, J.-J. Nicotinamide inhibits the early stage of carcinogen-induced hepatocarcinogenesis in mice and suppresses human hepatocellular carcinoma cell growth. J. Cell. Physiol. 2012, 227, 899–908. [Google Scholar] [CrossRef]
- Al-Gayyar, M.M.H.; Bagalagel, A.; Noor, A.O.; Almasri, D.M.; Diri, R. The therapeutic effects of nicotinamide in hepatocellular carcinoma through blocking IGF-1 and effecting the balance between Nrf2 and PKB. Biomed. Pharmacother. 2019, 112, 108653. [Google Scholar] [CrossRef]
- Bartleman, A.-P.; Jacobs, R.; Kirkland, J.B. Niacin supplementation decreases the incidence of alkylation-induced nonlymphocytic leukemia in Long-Evans rats. Nutr. Cancer 2008, 60, 251–258. [Google Scholar] [CrossRef]
- Rakieten, N.; Gordon, B.S.; Beaty, A.; Cooney, D.A.; Schein, P.S.; Dixon, R.L. Modification of renal tumorigenic effect of streptozotocin by nicotinamide: Spontaneous reversibility of streptozotocin diabetes. Proc. Soc. Exp. Biol. Med. 1976, 151, 356–361. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Leiter, U.; Garbe, C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Adv. Exp. Med. Biol. 2008, 624, 89–103. [Google Scholar] [PubMed]
- Roewert-Huber, J.; Stockfleth, E.; Kerl, H. Pathology and pathobiology of actinic (solar) keratosis - an update. Br. J. Dermatol. 2007, 157 Suppl 2, 18–20. [Google Scholar] [CrossRef]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F.; Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Dodds, A.; Chia, A.; Shumack, S. Actinic keratosis: Rationale and management. Dermatol. Ther. 2014, 4, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42, 23–24. [Google Scholar] [CrossRef]
- Ullrich, S.E. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005, 571, 185–205. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Minocha, R.; Damian, D.L.; Halliday, G.M. Melanoma and nonmelanoma skin cancer chemoprevention: A role for nicotinamide? Photodermatol. Photoimmunol. Photomed. 2018, 34, 5–12. [Google Scholar] [CrossRef]
- Bordea, C.; Wojnarowska, F.; Millard, P.R.; Doll, H.; Welsh, K.; Morris, P.J. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation 2004, 77, 574–579. [Google Scholar] [CrossRef]
- Moloney, F.J.; Comber, H.; O’Lorcain, P.; O’Kelly, P.; Conlon, P.J.; Murphy, G.M. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br. J. Dermatol. 2006, 154, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Colloca, G.; Sollena, P.; Andrea, B.; Balducci, L.; Cho, W.C.; Bernabei, R.; Peris, K. Skin Cancer Epidemics in the Elderly as An Emerging Issue in Geriatric Oncology. Aging Dis. 2017, 8, 643–661. [Google Scholar] [CrossRef]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Dhillon, H.M.; Vardy, J.L.; Dalziell, R.A.; Choy, B.; Fernández-Peñas, P.; Dixon, A.; Renton, C.; St George, G.; Chinniah, N.; et al. Neurocognitive Function and Quality of Life Outcomes in the ONTRAC Study for Skin Cancer Chemoprevention by Nicotinamide. Geriatrics 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.R.; Novicki, D.L.; Jirtle, R.L.; Novotny, A.; Michalopoulos, G. Promoting effect of nicotinamide on the development of renal tubular cell tumors in rats initiated with diethylnitrosamine. Cancer Res. 1985, 45, 809–814. [Google Scholar]
- Rakieten, N.; Gordon, B.S.; Beaty, A.; Cooney, D.A.; Davis, R.D.; Schein, P.S. Pancreatic islet cell tumors produced by the combined action of streptozotocin and nicotinamide. Proc. Soc. Exp. Biol. Med. 1971, 137, 280–283. [Google Scholar] [CrossRef]
- Kaanders, J.H.A.M.; Pop, L.A.M.; Marres, H.A.M.; Bruaset, I.; van den Hoogen, F.J.A.; Merkx, M.A.W.; van der Kogel, A.J. ARCON: Experience in 215 patients with advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 769–778. [Google Scholar] [CrossRef]
- Bernier, J.; Denekamp, J.; Rojas, A.; Minatel, E.; Horiot, J.; Hamers, H.; Antognoni, P.; Dahl, O.; Richaud, P.; van Glabbeke, M.; et al. ARCON: Accelerated radiotherapy with carbogen and nicotinamide in head and neck squamous cell carcinomas. The experience of the Co-operative group of radiotherapy of the european organization for research and treatment of cancer (EORTC). Radiother. Oncol. 2000, 55, 111–119. [Google Scholar] [CrossRef]
- Hoogsteen, I.J.; Pop, L.A.M.; Marres, H.A.M.; Merkx, M.A.W.; van den Hoogen, F.J.A.; van der Kogel, A.J.; Kaanders, J.H.A.M. Oxygen-modifying treatment with ARCON reduces the prognostic significance of hemoglobin in squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 83–89. [Google Scholar] [CrossRef]
- Bussink, J.; Kaanders, J.H.; Rijken, P.F.; Peters, J.P.; Hodgkiss, R.J.; Marres, H.A.; van der Kogel, A.J. Vascular architecture and microenvironmental parameters in human squamous cell carcinoma xenografts: Effects of carbogen and nicotinamide. Radiother. Oncol. 1999, 50, 173–184. [Google Scholar] [CrossRef]
- Rademakers, S.E.; Hoogsteen, I.J.; Rijken, P.F.; Terhaard, C.H.; Doornaert, P.A.; Langendijk, J.A.; van den Ende, P.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H. Prognostic value of the proliferation marker Ki-67 in laryngeal carcinoma: Results of the accelerated radiotherapy with carbogen breathing and nicotinamide phase III randomized trial. Head Neck 2015, 37, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Kaanders, J.H.; Pop, L.A.; Marres, H.A.; Liefers, J.; van den Hoogen, F.J.; van Daal, W.A.; van der Kogel, A.J. Accelerated radiotherapy with carbogen and nicotinamide (ARCON) for laryngeal cancer. Radiother. Oncol. 1998, 48, 115–122. [Google Scholar] [CrossRef]
- Janssens, G.O.; Rademakers, S.E.; Terhaard, C.H.; Doornaert, P.A.; Bijl, H.P.; van den Ende, P.; Chin, A.; Marres, H.A.; de Bree, R.; van der Kogel, A.J.; et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: Results of a phase III randomized trial. J. Clin. Oncol. 2012, 30, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.O.; Terhaard, C.H.; Doornaert, P.A.; Bijl, H.P.; van den Ende, P.; Chin, A.; Pop, L.A.; Kaanders, J.H. Acute toxicity profile and compliance to accelerated radiotherapy plus carbogen and nicotinamide for clinical stage T2-4 laryngeal cancer: Results of a phase III randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 532–538. [Google Scholar] [CrossRef]
- Janssens, G.O.; Rademakers, S.E.; Terhaard, C.H.; Doornaert, P.A.; Bijl, H.P.; van den Ende, P.; Chin, A.; Takes, R.P.; de Bree, R.; Hoogsteen, I.J.; et al. Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer. Clin. Cancer Res. 2014, 20, 1345–1354. [Google Scholar] [CrossRef]
- Janssens, G.O.; Langendijk, J.A.; Terhaard, C.H.; Doornaert, P.A.; van den Ende, P.; de Jong, M.A.; Takes, R.P.; Span, P.N.; Kaanders, J.H. Quality-of-life after radiotherapy for advanced laryngeal cancer: Results of a phase III trial of the Dutch Head and Neck Society. Radiother. Oncol. 2016, 119, 213–220. [Google Scholar] [CrossRef]
- Hoskin, P.; Rojas, A.; Saunders, M. Accelerated radiotherapy, carbogen, and nicotinamide (ARCON) in the treatment of advanced bladder cancer: Mature results of a Phase II nonrandomized study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1425–1431. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Phillips, H.; Saunders, M.I. Acute and late morbidity in the treatment of advanced bladder carcinoma with accelerated radiotherapy, carbogen, and nicotinamide. Cancer 2005, 103, 2287–2297. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bentzen, S.M.; Saunders, M.I. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J. Clin. Oncol. 2010, 28, 4912–4918. [Google Scholar] [CrossRef]
- Hulshof, M.C.; Rehmann, C.J.; Booij, J.; van Royen, E.A.; Bosch, D.A.; González González, D. Lack of perfusion enhancement after administration of nicotinamide and carbogen in patients with glioblastoma: A 99mTc-HMPAO SPECT study. Radiother. Oncol. 1998, 48, 135–142. [Google Scholar] [CrossRef]
- Miralbell, R.; Mornex, F.; Greiner, R.; Bolla, M.; Storme, G.; Hulshof, M.; Bernier, J.; Denekamp, J.; Rojas, A.M.; Pierart, M.; et al. Accelerated radiotherapy, carbogen, and nicotinamide in glioblastoma multiforme: Report of European Organization for Research and Treatment of Cancer trial 22933. J. Clin. Oncol. 1999, 17, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.M.; Noël, G.; Chiras, J.; Hoang-Xuan, K.; Delattre, J.Y.; Baillet, F.; Mazeron, J.J. Radiotherapy and chemotherapy with or without carbogen and nicotinamide in inoperable biopsy-proven glioblastoma multiforme. Radiother. Oncol. 2003, 67, 45–51. [Google Scholar] [CrossRef]
- Bernier, J.; Denekamp, J.; Rojas, A.; Trovò, M.; Horiot, J.C.; Hamers, H.; Antognoni, P.; Dahl, O.; Richaud, P.; Kaanders, J.; et al. ARCON: Accelerated radiotherapy with carbogen and nicotinamide in non small cell lung cancer: A phase I/II study by the EORTC. Radiother. Oncol. 1999, 52, 149–156. [Google Scholar] [CrossRef]
- van Laarhoven, H.W.M.; Bussink, J.; Lok, J.; Punt, C.J.A.; Heerschap, A.; van Der Kogel, A.J. Effects of nicotinamide and carbogen in different murine colon carcinomas: Immunohistochemical analysis of vascular architecture and microenvironmental parameters. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 310–321. [Google Scholar] [CrossRef]
- van Laarhoven, H.W.M.; Bussink, J.; Lok, J.; Verhagen, I.; Punt, C.J.A.; Heerschap, A.; Kaanders, J.H.A.M.; van der Kogel, A.J. Modulation of hypoxia in murine liver metastases of colon carcinoma by nicotinamide and carbogen. Radiat. Res. 2005, 164, 245–249. [Google Scholar] [CrossRef]
- Young, A.; Berry, R.; Holloway, A.F.; Blackburn, N.B.; Dickinson, J.L.; Skala, M.; Phillips, J.L.; Brettingham-Moore, K.H. RNA-seq profiling of a radiation resistant and radiation sensitive prostate cancer cell line highlights opposing regulation of DNA repair and targets for radiosensitization. BMC Cancer 2014, 14, 808. [Google Scholar] [CrossRef]
- Fenton, B.M.; Lord, E.M.; Paoni, S.F. Enhancement of tumor perfusion and oxygenation by carbogen and nicotinamide during single- and multifraction irradiation. Radiat. Res. 2000, 153, 75–83. [Google Scholar] [CrossRef]
- Horsman, M.R.; Khalil, A.A.; Chaplin, D.J.; Overgaard, J. The ability of nicotinamide to inhibit the growth of a C3H mouse mammary carcinoma. Acta Oncol. 1995, 34, 443–446. [Google Scholar] [CrossRef]
- Wang, T.; Cui, H.; Ma, N.; Jiang, Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol. Lett. 2013, 6, 600–604. [Google Scholar] [CrossRef]
- Jafary, H.; Ahmadian, S.; Soleimani, M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2014, 35, 2701–2710. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.; Woo, J.; Yue, W.; Kim, K.; Choi, S.; Jang, J.-J.; Kim, Y.; Park, I.A.; Han, D.; et al. Reconstruction of pathway modification induced by nicotinamide using multi-omic network analyses in triple negative breast cancer. Sci. Rep. 2017, 7, 3466. [Google Scholar] [CrossRef] [PubMed]
- Santidrian, A.F.; Matsuno-Yagi, A.; Ritland, M.; Seo, B.B.; LeBoeuf, S.E.; Gay, L.J.; Yagi, T.; Felding-Habermann, B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J. Clin. Invest. 2013, 123, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Guo, Y.; Zhou, J.; Dai, K.; Xu, Q.; Jin, X. Nicotinamide Overcomes Doxorubicin Resistance of Breast Cancer Cells through Deregulating SIRT1/Akt Pathway. Anticancer Agents Med. Chem. 2019, 19, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Gómez, G.; Díaz-Chávez, J.; Chávez-Blanco, A.; Gonzalez-Fierro, A.; Jiménez-Salazar, J.E.; Damián-Matsumura, P.; Gómez-Quiroz, L.E.; Dueñas-González, A. Nicotinamide sensitizes human breast cancer cells to the cytotoxic effects of radiation and cisplatin. Oncol. Rep. 2015, 33, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, O.; Greenberg, E.; Shalmon, B.; Kubi, A.; Treves, A.J.; Shapira-Frommer, R.; Avivi, C.; Ortenberg, R.; Ben-Ami, E.; Schachter, J.; et al. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS One 2013, 8, e57160. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, R.; Jimbow, K.; Tanimura, A.; Sato, M.; Horimoto, K.; Hayashi, T.; Hisahara, S.; Sugino, T.; Hirobe, T.; Yamashita, T.; et al. SIRT1 regulates lamellipodium extension and migration of melanoma cells. J. Invest. Dermatol. 2014, 134, 1693–1700. [Google Scholar] [CrossRef]
- Wang, Y.; Ryu, H.S.; Jang, J.J. Nicotinamide suppresses cell growth by G1-phase arrest and induces apoptosis in intrahepatic cholangiocarcinoma. Molecular & Cellular Toxicology 2018, 14, 43–51. [Google Scholar]
- Jafary, H.; Ahmadian, S.; Soleimani, M. Synergistic anticancer activity of valproate combined with nicotinamide enhances anti-proliferation response and apoptosis in MIAPaca2 cells. Mol. Biol. Rep. 2014, 41, 3801–3812. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Zhao, G.; Qin, Q.; Wang, B.; Liu, L.; Liu, Y.; Deng, S.-C.; Tian, K.; Wang, C.-Y. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology 2013, 13, 140–146. [Google Scholar] [CrossRef]
- Gupta, N.; Saleem, A.; Kötz, B.; Osman, S.; Aboagye, E.O.; Phillips, R.; Vernon, C.; Wasan, H.; Jones, T.; Hoskin, P.J.; et al. Carbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patients. Clin. Cancer Res. 2006, 12, 3115–3123. [Google Scholar] [CrossRef][Green Version]
- Feng, Y.; Wang, Y.; Jiang, C.; Fang, Z.; Zhang, Z.; Lin, X.; Sun, L.; Jiang, W. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci. 2017, 181, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Vaisitti, T.; Rossi, D.; Gottardi, D.; D’Arena, G.; Laurenti, L.; Gaidano, G.; Malavasi, F.; Deaglio, S. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011, 71, 4473–4483. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.E.; Clark-Garvey, S.; Kalac, M.; Scotto, L.; Marchi, E.; Neylon, E.; Johannet, P.; Wei, Y.; Zain, J.; O’Connor, O.A. Sirtuin and pan-class I/II deacetylase (DAC) inhibition is synergistic in preclinical models and clinical studies of lymphoma. Blood 2013, 122, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Dell’Omo, G.; Ciana, P. Nicotinamide in the prevention of breast cancer recurrences? Oncotarget 2019, 10, 5495–5496. [Google Scholar] [CrossRef]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S.; Urologic Diseases in America Project. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef]
- Choupani, J.; Mansoori Derakhshan, S.; Bayat, S.; Alivand, M.R.; Shekari Khaniani, M. Narrower insight to SIRT1 role in cancer: A potential therapeutic target to control epithelial--mesenchymal transition in cancer cells. J. Cell. Physiol. 2018, 233, 4443–4457. [Google Scholar] [CrossRef]
- Frazzi, R. SIRT1 in Secretory Organ Cancer. Front. Endocrinol. 2018, 9, 569. [Google Scholar] [CrossRef]
- Sun, M.; Du, M.; Zhang, W.; Xiong, S.; Gong, X.; Lei, P.; Zha, J.; Zhu, H.; Li, H.; Huang, D.; et al. Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis. Front. Endocrinol. 2019, 10, 121. [Google Scholar] [CrossRef]
- Fang, Y.; Nicholl, M.B. Sirtuin 1 in malignant transformation: Friend or foe? Cancer Lett. 2011, 306, 10–14. [Google Scholar] [CrossRef]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.-G.; Huang, J.-D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef]
- Rifaï, K.; Idrissou, M.; Penault-Llorca, F.; Bignon, Y.-J.; Bernard-Gallon, D. Breaking down the Contradictory Roles of Histone Deacetylase SIRT1 in Human Breast Cancer. Cancers 2018, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-S.; Hyun, C.L.; Park, I.A.; Kim, J.Y.; Chung, Y.R.; Im, S.-A.; Lee, K.-H.; Moon, H.-G.; Ryu, H.S. SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer. Tumour Biol. 2016, 37, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Jung, Y.Y.; Park, I.A.; Kim, H.; Chung, Y.R.; Kim, J.Y.; Park, S.Y.; Im, S.-A.; Lee, K.-H.; Moon, H.-G.; et al. Oncogenic role of SIRT1 associated with tumor invasion, lymph node metastasis, and poor disease-free survival in triple negative breast cancer. Clin. Exp. Metastasis 2016, 33, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Kim, H.; Park, S.Y.; Park, I.A.; Jang, J.J.; Choe, J.-Y.; Jung, Y.Y.; Im, S.-A.; Moon, H.-G.; Lee, K.-H.; et al. Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype. Hum. Pathol. 2015, 46, 1027–1035. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.-H.; Park, I.A.; Chung, Y.R.; Im, S.-A.; Noh, D.-Y.; Han, W.; Moon, H.-G.; Jung, Y.Y.; Ryu, H.S. Expression of SIRT1 and apoptosis-related proteins is predictive for lymph node metastasis and disease-free survival in luminal A breast cancer. Virchows Arch. 2015, 467, 563–570. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Y.; Maimaiti, Y.; Wang, C.; Yan, Y.; Zhou, J.; Ruan, S.; Huang, T. Combination of SIRT1 and Src overexpression suggests poor prognosis in luminal breast cancer. Onco. Targets. Ther. 2018, 11, 2051–2061. [Google Scholar] [CrossRef]
- Wu, M.; Wei, W.; Xiao, X.; Guo, J.; Xie, X.; Li, L.; Kong, Y.; Lv, N.; Jia, W.; Zhang, Y.; et al. Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer. Med. Oncol. 2012, 29, 3240–3249. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).