Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action
Abstract
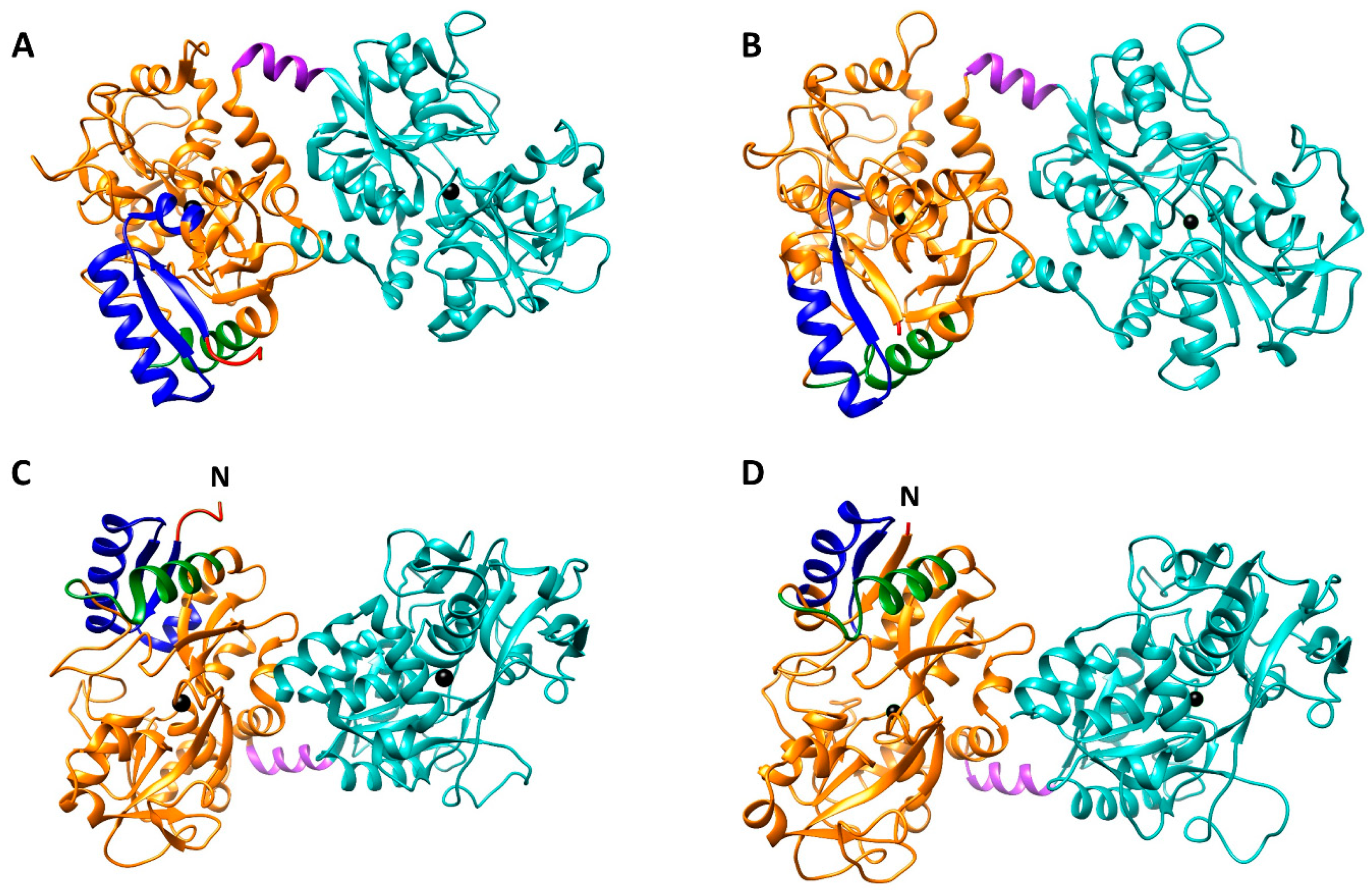
1. Lactoferrin: A Brief Overview
2. Lactoferrin Bioavailability: Absorption and Body Delivery
3. Lactoferrin and Cancer
3.1. Lactoferrin Anti-Cancer Activity: Modulation of Cell Cycle
3.2. Lactoferrin Anti-Cancer Activity: Induction of Apoptosis
3.3. Lactoferrin Anti-Cancer Activity: Inhibition of Cell Migration, Invasion, and Metastasis
3.4. Lactoferrin Anti-Cancer Activity: Immunomodulation
3.5. Lactoferrin as a Cargo for Cancer Targeting
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorensen, M.; Sorensen, S. The Proteins in Whey. Compte rendu des Travaux du Laboratoire de Carlsberg Ser. Chim. 1939, 23, 55–99. [Google Scholar]
- Johanson, B. Isolation of an iron-containing red protein from human milk. Acta. Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B. 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients. 2019, 11, 2350. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef]
- Baker, E.N.; Lindley, P.F. New perspectives on the structure and function of transferrins. J. Inorg. Biochem. 1992, 47, 147–160. [Google Scholar] [CrossRef]
- Metz-Boutigue, M.H.; Jolles, J.; Mazurier, J.; FranGoise Schoentgen, F.G.; Legrand, D.; Spik, G.; Montreuil, J.; Jolles, P. Human lactotransferrin: Amino acid sequence and structural comparisons with other transferrins. FEBS 1984, 145, 659–676. [Google Scholar] [CrossRef]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Day, C.L.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A.; Thomas, D.H. Three-dimensional structure of lactoferrin in various functional states. Adv. Exp. Med. Biol. 1994, 357, 1–12. [Google Scholar] [CrossRef]
- Baker, E.P.; Rumball, S.V.; Anderson, B.F. Transferrins: Insights into structure and function from studies on lactoferrin. Trends Biochem Sci. 1987, 12, 350–353. [Google Scholar] [CrossRef]
- Bruns, C.M.; Nowalk, A.J.; Arvai, A.S.; McTigue, M.A.; Vaughan, K.G.; Mietzner, T.A.; McRee, D.E. Structure of Haemophilus influenzae Fe (+3)-binding protein reveals convergent evolution within a superfamily. Nat. Struct. Biol. 1997, 4, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Luck, A.N.; Mason, A.B. Transferrin-mediated cellular iron delivery. Curr Top. Membr. 2012, 69, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today. 2005, 10, 267–273. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G.; Von Hunolstein, C.; Visca, P.; Orsi, N.; Antonini, E. Studies of the antimicrobial activity of ovotransferrin. Int. J. Tissue React. 1983, 5, 97–105. [Google Scholar] [PubMed]
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta 2012, 1820, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; RosA, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules. 2019, 24, 1323. [Google Scholar] [CrossRef]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: Regulatory pathways and the role of lactoferrin. Biometals 2018, 31, 399–414. [Google Scholar] [CrossRef]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional Structure of Diferric Bovine Lactoferrin at 2.8A Resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Paramasivam, M.; Srinivasan, A.; Yadav, M.P.; Singh, T.P. Three-dimensional structure of mare diferric lactoferrin at 2.6 A resolution. J. Mol. Biol. 1999, 289, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Paramasivam, M.; Yadav, S.; Srinivasan, A.; Singh, T.P. Structure of buffalo lactoferrin at 2.5 Å resolution using crystals grown at 303 K shows different orientations of the N and C lobes. Acta Crystallogr. D. Biol. Crystallogr. 1999, 55, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rumball, S.V.; Baker, E.N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990, 344, 784–787. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef]
- Van der Kraan, M.I.; Groenink, J.; Nazmi, K.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 18, 8:2. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin--a natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell. Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Scotti, M.J.; Conte, M.P.; Paesano, R.; Valenti, P. Physico-chemical properties influence the functions and efficacy of commercial bovine lactoferrins. Biometals 2018, 31, 301–312. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Apo- and holo-lactoferrin stimulate proliferation of mouse crypt cells but through different cellular signaling pathways. Int. J. Biochem. Cell. Biol. 2012, 44, 91–100. [Google Scholar] [CrossRef]
- Cutone, A.; Colella, B.; Pagliaro, A.; Rosa, L.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Di Bartolomeo, S.; Musci, G. Native and iron-saturated bovine lactoferrin differently hinder migration in a model of human glioblastoma by reverting epithelial-to-mesenchymal transition-like process and inhibiting interleukin-6/STAT3 axis. Cell Signal. 2020, 65, 109461. [Google Scholar] [CrossRef]
- Haridas, M.; Anderson, B.F.; Baker, E.N. Structure of human diferric lactoferrin refined at 2.2 A resolution. Acta Crystallogr. D. Biol. Crystallogr. 1995, 51, 629–646. [Google Scholar] [CrossRef]
- Thomassen, E.A.; van Veen, H.A.; van Berkel, P.H.; Nuijens, J.H.; Abrahams, J.P. The protein structure of recombinant human lactoferrin produced in the milk of transgenic cows closely matches the structure of human milk-derived lactoferrin. Transgenic Res. 2005, 14, 397–405. [Google Scholar] [CrossRef]
- Le Parc, A.; Dallas, D.C.; Duaut, S.; Leonil, J.; Martin, P.; Barile, D. Characterization of goat milk lactoferrin N-glycans and comparison with the N-glycomes of human and bovine milk. Electrophoresis 2014, 35, 1560–1570. [Google Scholar] [CrossRef]
- Yu, T.; Guo, C.; Wang, J.; Hao, P.; Sui, S.; Chen, X.; Zhang, R.; Wang, P.; Yu, G.; Zhang, L.; et al. Comprehensive characterization of the site-specific N-glycosylation of wild-type and recombinant human lactoferrin expressed in the milk of transgenic cloned cattle. Glycobiology 2011, 21, 206–224. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Nishimura, T.; Yoshida, S. Presence of a glycan at a potential N-glycosylation site, Asn-281, of bovine lactoferrin. J. Dairy Sci. 2000, 83, 683–689. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Scotti, M.J.; Conte, A.L.; Marazzato, M.; Zagaglia, C.; Longhi, C.; Berlutti, F.; Musci, G.; et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. Int. J. Mol. Sci. 2019, 20, 5666. [Google Scholar] [CrossRef]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Rosa, L.; Bragonzi, A.; Berlutti, F. Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of Pseudomonas aeruginosa lung infections. Biochem. Cell. Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar]
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Hayes, T.G.; Falchook, G.F.; Varadhachary, G.R.; Smith, D.P.; Davis, L.D.; Dhingra, H.M.; Hayes, B.P.; Varadhachary, A. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest. New Drugs. 2006, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomedicine. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; To, M.; Saruta, J.; Sato, C.; Yamamoto, Y.; Kondo, Y.; Shimizu, T.; Kamata, Y.; Tsukinoki, K. Salivary lactoferrin is transferred into the brain via the sublingual route. Biosci. Biotechnol. Biochem. 2017, 81, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.Y.; Koh, P.Y.; Somani, S.; Al Robaian, M.; Karim, R.; Yean, Y.L.; Mitchell, J.; Tate, R.J.; Edrada-Ebel, R.; Blatchford, D.R.; et al. Tumor regression following intravenous administration of lactoferrin- and lactoferricin-bearing dendriplexes. Nanomedicine 2015, 11, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, E.T.; Sokolov, A.V.; Pavlichenko, N.N.; Kostevich, V.A.; Abdurasulova, I.N.; Chechushkov, A.V.; Voynova, I.V.; Elizarova, A.Y.; Kolmakov, N.N.; Bass, M.G.; et al. Erythropoietin and Nrf2: Key factors in the neuroprotection provided by apo-lactoferrin. Biometals 2018, 31, 425–443. [Google Scholar] [CrossRef]
- Paesano, R.; Pietropaoli, M.; Berlutti, F.; Valenti, P. Bovine lactoferrin in preventing preterm delivery associated with sterile inflammation. Biochem Cell Biol. 2012, 90, 468–475. [Google Scholar] [CrossRef]
- Saarinen, U.M.; Siimes, M.A.; Dallman, P.R. Iron absorption in infants: High bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J. Pediatr. 1977, 91, 36–39. [Google Scholar] [CrossRef]
- Davidsson, L.; Kastenmayer, P.; Yuen, M.; Lönnerdal, B.; Hurrell, R.F. Influence of lactoferrin on iron absorption from human milk in infants. Pediatr. Res. 1994, 35, 117–124. [Google Scholar] [CrossRef]
- Baintner, K. Intestinal Absorption of Macromolecules and Immune Transmission from Mother to Young; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar] [CrossRef]
- Payne, L.C.; Marsh, C.L. Gamma Globulin Absorption in the Baby Pig: The Nonselective Absorption of Heterologous Globulins and Factors Influencing Absorption Time. J. Nutr. 1962, 76, 151–158. [Google Scholar] [CrossRef]
- Weström, B.; Svendsen, J.; Tagesson, C. Intestinal permeability to polyethyleneglycol 600 in relation to macromolecular ‘closure’ in the neonatal pig. Gut. 1984, 25, 520–525. [Google Scholar] [CrossRef]
- Talukder, M.J.; Takeuchi, T.; Harada, E. Transport of colostral macromolecules into the cerebrospinal fluid via plasma in newborn calves. J. Dairy Sci. 2002, 85, 514–524. [Google Scholar] [CrossRef]
- Talukder, M.J.; Takeuchi, T.; Harada, E. Receptor-mediated transport of lactoferrin into the cerebrospinal fluid via plasma in young calves. J. Vet. Med. Sci. 2003, 65, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Harada, E.; Itoh, Y.; Sitizyo, K.; Takeuchi, T.; Araki, Y.; Kitagawa, H. Characteristic transport of lactoferrin from the intestinal lumen into the bile via the blood in piglets. Comp. Biochem Physiol A Mol Integr Physiol. 1999, 124, 321–327. [Google Scholar] [CrossRef]
- Knapp, R.D.; Hutchens, T.W. Maternal lactoferrin in the urine of preterm infants. Evidence for retention of structure and function. Adv. Exp. Med. Biol. 1994, 357, 177–181. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar]
- Troost, F.J.; Saris, W.H.; Brummer, R.J. Orally ingested human lactoferrin is digested and secreted in the upper gastrointestinal tract in vivo in women with ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.; Brummer, R.J. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Cox, T.M.; Mazurier, J.; Spik, G.; Montreuil, J.; Peters, T.J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim. Biophys. Acta. 1979, 588, 120–128. [Google Scholar] [CrossRef]
- Ezekiel, E. The iron-binding proteins in milk and the secretion of iron by the mammary gland in the rat 1965. Biochim. Biophys. Acta. 1965, 107, 511–518. [Google Scholar] [CrossRef]
- Huebers, H.A.; Huebers, E.; Csiba, E.; Rummel, W.; Finch, C.A. The significance of transferrin for intestinal iron absorption. Blood. 1983, 61, 283–290. [Google Scholar] [CrossRef]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lönnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef]
- Conesa, C.; Pocoví, C.; Pérez, M.D.; Calvo, M.; Sánchez, L. Recombinant human lactoferrin and iron transport across Caco-2 monolayers: Effect of heat treatment on the binding to cells. J. Agric. Food Chem. 2008, 56, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Pocoví, C.; Pérez, M.D.; Calvo, M.; Sánchez, L. Transport of iron bound to recombinant human lactoferrin from rice and iron citrate across Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2009, 73, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Oshima, K.; Kuhara, T.; Shin, K.; Abe, F.; Iwatsuki, K.; Nadano, D.; Matsuda, T. A lactoferrin-receptor, intelectin, affects uptake, sub-cellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells. J. Biochem. 2013, 154, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Mikogami, T.; Heyman, M.; Spik, G.; Desjeux, J.F. Apical-to-basolateral transepithelial transport of human lactoferrin in the intestinal cell line HT-29cl.19A. Am. J. Physiol. 1994, 267, G308–G315. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Nakamura, M.; Nogita, T.; Sato, A. Cellular Uptake and Release of Intact Lactoferrin and Its Derivatives in an Intestinal Enterocyte Model of Caco-2 Cells. Biol. Pharm. Bull. 2019, 42, 989–995. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell. Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Kitagawa, H.; Yoshizawa, Y.; Yokoyama, T.; Takeuchi, T.; Talukder, M.J.; Shimizu, H.; Ando, K.; Harada, E. Persorption of bovine lactoferrin from the intestinal lumen into the systemic circulation via the portal vein and the mesenteric lymphatics in growing pigs. J. Vet. Med. Sci. 2003, 65, 567–572. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Blais, A.; Dubarry, M.; Rautureau, M.; Boyaka, P.N.; Tome, D. Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues. Int. Immunopharmacol. 2007, 7, 1387–1393. [Google Scholar] [CrossRef]
- Prieels, J.P.; Pizzo, S.V.; Glasgow, L.R.; Paulson, J.C.; Hill, R.L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc. Natl. Acad. Sci. USA 1978, 75, 2215–2219. [Google Scholar] [CrossRef]
- Peen, E.; Johansson, A.; Engquist, M.; Skogh, T. Hepatic and extrahepatic clearance of circulating human lactoferrin: An experimental study in rat. Eur. J. Haematol. 1998, 61, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Lönnerdal, B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am. J. Physiol. 1991, 261, 841–846. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Kuhara, T.; Ushida, Y.; Sekine, K.; Moore, M.A.; Tsuda, H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin. Exp. Metastasis. 1999, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Iigo, M.; Itoh, T.; Ushida, Y.; Sekine, K.; Terada, N.; Okamura, H.; Tsuda, H. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer 2000, 38, 192–199. [Google Scholar] [CrossRef]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 8. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lönnerdal, B. Baculovirus expression of mouse lactoferrin receptor and tissue distribution in the mouse. Biometals 2004, 17, 301–309. [Google Scholar] [CrossRef]
- Mancinelli, R.; Olivero, F.; Carpino, G.; Overi, D.; Rosa, L.; Lepanto, M.S.; Cutone, A.; Franchitto, A.; Alpini, G.; Onori, P.; et al. Role of lactoferrin and its receptors on biliary epithelium. Biometals 2018, 31, 369–379. [Google Scholar] [CrossRef]
- Lin, L.; Hu, K. LRP-1: Functions, signaling and implications in kidney and other diseases. Int. J. Mol. Sci. 2014, 15, 22887–22901. [Google Scholar] [CrossRef] [PubMed]
- Meilinger, M.; Haumer, M.; Szakmary, K.A.; Steinböck, F.; Scheiber, B.; Goldenberg, H.; Huettinger, M. Removal of lactoferrin from plasma is mediated by binding to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor and transport to endosomes. FEBS Lett. 1995, 360, 70–74. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Vigié, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.C.; Carpentier, M.; Briand, J.P.; Mazurier, J.; Hovanessian, A.G. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Wong, H.; Ashida, K.Y.; Schryvers, A.B.; Lönnerdal, B. The N1 domain of human lactoferrin is required for internalization by caco-2 cells and targeting to the nucleus. Biochemistry. 2008, 47, 10915–10920. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, R.; Lönnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol. 2012, 90, 476–484. [Google Scholar] [CrossRef]
- Losfeld, M.E.; Khoury, D.E.; Mariot, P.; Carpentier, M.; Krust, B.; Briand, J.P.; Mazurier, J.; Hovanessian, A.G.; Legrand, D. The cell surface expressed nucleolin is a glycoprotein that triggers calcium entry into mammalian cells. Exp. Cell. Res. 2009, 315, 357–369. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol. Pharm. Bull. 2008, 31, 1605–1608. [Google Scholar] [CrossRef]
- Paesano, R.; Natalizi, T.; Berlutti, F.; Valenti, P. Body iron delocalization: The serious drawback in iron disorders in both developing and developed countries. Pathog. Glob. Health. 2012, 106, 200–216. [Google Scholar] [CrossRef]
- Bezault, J.; Bhimani, R.; Wiprovnick, J.; Furmanski, P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994, 54, 2310–2312. [Google Scholar]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.; Das, M.R. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Siebert, P.D.; Huang, B.C. Identification of an alternative form of human lactoferrin mRNA that is expressed differentially in normal tissues and tumor-derived cell lines. Proc. Natl. Acad. Sci. USA 1997, 94, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Hoedt, E.; Hardivillé, S.; Mariller, C.; Elass, E.; Perraudin, J.P.; Pierce, A. Discrimination and evaluation of lactoferrin and delta-lactoferrin gene expression levels in cancer cells and under inflammatory stimuli using TaqMan real-time PCR. Biometals 2010, 23, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Panella, T.J.; Liu, Y.H.; Huang, A.T.; Teng, C.T. Polymorphism and altered methylation of the lactoferrin gene in normal leukocytes, leukemic cells, and breast cancer. Cancer Res. 1991, 51, 3037–3043. [Google Scholar] [PubMed]
- Teng, C.; Gladwell, W.; Raphiou, I.; Liu, E. Methylation and expression of the lactoferrin gene in human tissues and cancer cells. Biometals 2004, 17, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ling, T.; Wu, H.; Wang, K. Re-expression of Lactotransferrin, a candidate tumor suppressor inactivated by promoter hypermethylation, impairs the malignance of oral squamous cell carcinoma cells. J. Oral Pathol. Med. 2014, 44, 578–584. [Google Scholar] [CrossRef]
- Tsuda, H.; Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Saito, D.; Akasu, T.; Alexander, D.B.; Futakuchi, M.; Fukamachi, K.; et al. Cancer prevention by bovine lactoferrin: From animal studies to human trial. Biometals 2010, 23, 399–409. [Google Scholar] [CrossRef]
- Ushida, Y.; Sekine, K.; Kuhara, T.; Takasuka, N.; Iigo, M.; Maeda, M.; Tsuda, H. Possible chemopreventive effects of bovine lactoferrin on esophagus and lung carcinogenesis in the rat. Jpn. J. Cancer Res. 1999, 90, 262–267. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabata, K.; Kohno, H.; Honjo, S.; Murakami, M.; Ota, T.; Tsuda, H. Chemopreventive effect of bovine lactoferrin on 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats. Jpn. J. Cancer Res. 2000, 91, 25–33. [Google Scholar] [CrossRef]
- Sugihara, Y.; Zuo, X.; Takata, T.; Jin, S.; Miyauti, M.; Isikado, A.; Imanaka, H.; Tatsuka, M.; Qi, G.; Shimamoto, F1. Inhibition of DMH-DSS-induced colorectal cancer by liposomal bovine lactoferrin in rats. Oncol. Lett. 2017, 14, 5688–5694. [Google Scholar] [CrossRef]
- Hegazy, R.R.; Mansour, D.F.; Salama, A.A.; Abdel-Rahman, R.F.; Hassan, A.M. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacol. Rep. 2019, 71, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Yamamoto, Y.; Ashino, H.; Oikawa, T.; Hazato, T.; Tsuda, H.; Iigo, M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer 2004, 111, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. (Schol Ed.). 2011, 3, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer. 2004, 111, 398–403. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, R.; Przepiorski, A.; Reddy, S.; Palmano, K.P.; Krissansen, G.W. “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice. BMC Cancer 2012, 12, 591. [Google Scholar] [CrossRef]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of Orally Administered Bovine Lactoferrin on the Growth of Adenomatous Colorectal Polyps in a Randomized, Placebo-Controlled Clinical Trial. Cancer Prev. Res. (Phila) 2009, 2, 975–983. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Elass-Rochard, E.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Role of heparan sulphate proteoglycans in the regulation of human lactoferrin binding and activity in the MDA-MB-231 breast cancer cell line. Eur. J. Cell. Biol. 1998, 77, 344–351. [Google Scholar] [CrossRef]
- Pereira, C.S.; Guedes, J.P.; Gonçalves, M.; Loureiro, L.; Castro, L.; Gerós, H.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 2016, 7, 62144–62158. [Google Scholar] [CrossRef]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Côrte-Real, M. Bovine Milk Lactoferrin Selectively Kills Highly Metastatic Prostate Cancer PC-3 and Osteosarcoma MG-63 Cells In Vitro. Front. Oncol. 2018, 8, 200. [Google Scholar] [CrossRef]
- Legrand, D.; van Berkel, P.H.; Salmon, V.; van Veen, H.A.; Slomianny, M.C.; Nuijens, J.H.; Spik, G. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem. J. 1997, 327, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Kühnle, A.; Veelken, R.; Galuska, C.E.; Saftenberger, M.; Verleih, M.; Schuppe, H.C.; Rudloff, S.; Kunz, C.; Galuska, S.P. Polysialic acid interacts with lactoferrin and supports its activity to inhibit the release of neutrophil extracellular traps. Carbohydr. Polym. 2019, 208, 32–41. [Google Scholar] [CrossRef] [PubMed]
- El Yazidi-Belkoura, I.; Legrand, D.; Nuijens, J.; Slomianny, M.C.; van Berkel, P.; Spik, G. The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT29-18-C1 cells is mediated through basic residues located in the N-terminus. Biochim. Biophys. Acta 2001, 1568, 197–204. [Google Scholar] [CrossRef]
- Riedl, S.; Leber, R.; Rinner, B.; Schaider, H.; Lohner, K.; Zweytick, D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim. Biophys. Acta 2015, 1848, 2918–2931. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell. Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef]
- Vargas Casanova, Y.; Rodríguez Guerra, J.A.; Umaña Pérez, Y.A.; Leal Castro, A.L.; Almanzar Reina, G.; García Castañeda, J.E.; Rivera Monroy, Z.J. Antibacterial Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Cytotoxic Effect against MDA-MB-468 and MDA-MB-231 Breast Cancer Cell Lines. Molecules 2017, 22, 1641. [Google Scholar] [CrossRef]
- Sharma, A.; Shandilya, U.K.; Sodhi, M.; Mohanty, A.K.; Jain, P.; Mukesh, M. Evaluation of Milk Colostrum Derived Lactoferrin of Sahiwal (Bos indicus) and Karan Fries (Cross-Bred) Cows for Its Anti-Cancerous Potential. Int. J. Mol. Sci. 2019, 20, 6318. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.F.; Jie, M.M.; Li, B.S.; Hu, C.J.; Xie, R.; Tang, B.; Yang, S.M. Peptide-Based Treatment: A Promising Cancer Therapy. J. Immunol. Res. 2015, 2015, 761820. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Han, X.; Shan, Y.J.; Zhang, L.W.; Du, M.; Liu, M.; Yi, H.X.; Ma, Y. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J. Dairy Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fan, F.; Shi, P.; Tu, M.; Yu, C.; Yu, C.; Du, M. Lactoferrin promotes MC3T3-E1 osteoblast cells proliferation via MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 107, 137–143. [Google Scholar] [CrossRef]
- Kohno, Y.; Shiraki, K.; Mura, T.; Ikawa, S. Iron-saturated lactoferrin as a co-mitogenic substance for neonatal rat hepatocytes in primary culture. Acta Paediatr. 1993, 82, 650–655. [Google Scholar] [CrossRef]
- Yanaihara, A.; Toma, Y.; Saito, H.; Yanaihara, T. Cell proliferation effect of lactoferrin in human endometrial stroma cells. Mol. Hum. Reprod. 2000, 6, 469–473. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef]
- Huang, N.; Bethell, D.; Card, C.; Cornish, J.; Marchbank, T.; Wyatt, D.; Mabery, K.; Playford, R. Bioactive recombinant human lactoferrin, derived from rice, stimulates mammalian cell growth. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 464–471. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell Biochem. 1999, 74, 486–498. [Google Scholar] [CrossRef]
- Xiao, Y.; Monitto, C.L.; Minhas, K.M.; Sidransky, D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004, 10, 8683–8686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nicolau, A.; Lima, C.F.; Rodrigues, L.R. Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr. Cancer. 2014, 66, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Febriyanti Ayuningtyas, N.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Freiburghaus, C.; Janicke, B.; Lindmark-Månsson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484. [Google Scholar] [CrossRef]
- Hengartner, M.O. Apoptosis: Corralling the corpses. Cell 2001, 104, 325–328. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care. 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell. 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2 family proteins: Regulators of apoptosis and chemoresistance in hematologic malignancies. Semin. Hematol. 1997, 34, 9–19. [Google Scholar]
- Kanwar, R.K.; Kanwar, J.R. Immunomodulatory lactoferrin in the regulation of apoptosis modulatory proteins in cancer. Protein Pept. Lett. 2013, 20, 450–458. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.W.; Pyo, C.W.; Yoo, N.K.; Kim, J.; Choi, S.Y. Requirement of the JNK-associated Bcl-2 pathway for human lactoferrin-induced apoptosis in the Jurkat leukemia T cell line. Biochimie 2009, 91, 102–108. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.M.; Pyo, C.W.; Hahm, D.H.; Choi, S.Y. E2F1-directed activation of Bcl-2 is correlated with lactoferrin-induced apoptosis in Jurkat leukemia T lymphocytes. Biometals 2010, 23, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Ou, Y.; Han, Z.; Li, K.; Wang, P.; Zhou, S. Inhibition of Tumor Growth by Recombinant Adenovirus Containing Human Lactoferrin through Inducing Tumor Cell Apoptosis in Mice Bearing EMT6 Breast Cancer. Arch. Pharm. Res. 2011, 34, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendón, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis. 2019, 24, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jiang, H.R.; Li, H.B.; Zhang, T.N.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis. 2004, 25, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin modifies apoptosis-related gene expression in the colon of the azoxymethane-treated rat. Cancer Lett. 2004, 213, 21–29. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer. 2015, 15. [Google Scholar] [CrossRef]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008, 14, 5000–5005. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Roy, K.; Sasidharan, S.; Krishnakumar, S.; Prasad, N.; Sehgal, R.; Kanwar, R.K. Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine (Lond). 2015, 10, 35–55. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Watanabe, R.; Koike, Y.; Mitobe, M.; Shimazaki, K.; Watanabe, S.; Azuma, I. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: Involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 1997, 237, 624–628. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Zhao, Y.; Zhang, A.; Wang, L.; Zhang, G. The effect of LfcinB9 on human ovarian cancer cell SK-OV-3 is mediated by inducing apoptosis. J. Pept. Sci. 2014, 20, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Banno, Y.; Kato, Y.; Nozawa, Y.; Kawaguchi, M. Pepsin-digested bovine lactoferrin induces apoptotic cell death with JNK/SAPK activation in oral cancer cells. J. Pharmacol. Sci. 2005, 98, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tu, J.; Zhou, C.; Li, J.; Huang, L.; Tao, L.; Zhao, L. The effect of Lfcin-B on non-small cell lung cancer H460 cells is mediated by inhibiting VEGF expression and inducing apoptosis. Arch. Pharm. Res. 2015, 3, 261–271. [Google Scholar] [CrossRef]
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell. Biol. 2012, 90, 497–503. [Google Scholar] [CrossRef]
- Raja, S.K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Uchida, R.; Aoki, R.; Aoki-Yoshida, A.; Tajima, A.; Takayama, Y. Promoting effect of lactoferrin on barrier function and epithelial differentiation of human keratinocytes. Biochem. Cell. Biol. 2017, 95, 64–68. [Google Scholar] [CrossRef]
- Saito, S.; Takayama, Y.; Mizumachi, K.; Suzuki, C. Lactoferrin promotes hyaluronan synthesis in human dermal fibroblasts. Biotechnol. Lett. 2011, 33, 33–39. [Google Scholar] [CrossRef]
- Pattamatta, U.; Willcox, M.; Stapleton, F.; Cole, N.; Garrett, Q. Bovine lactoferrin stimulates human corneal epithelial alkali wound healing in vitro. Invest. Ophthalmol. Vis. Sci. 2009, 50, 1636–1643. [Google Scholar] [CrossRef]
- Tang, L.; Wu, J.J.; Ma, Q.; Cui, T.; Andreopoulos, F.; Gil, J.; Valdes, J.; Davis, S.C.; Li, J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. Br. J. Dermatol. 2010, 163, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Pattamatta, U.; Willcox, M.; Stapleton, F.; Garrett, Q. Bovine lactoferrin promotes corneal wound healing and suppresses IL-1 expression in alkali wounded mouse cornea. Curr. Eye Res. 2013, 38, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Calvani, F.; Cutone, A.; Lepanto, M.S.; Rosa, L.; Valentini, V.; Valenti, P. Efficacy of bovine lactoferrin in the post-surgical treatment of patients suffering from bisphosphonate-related osteonecrosis of the jaws: An open-label study. Biometals 2018, 31, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shinoda, I.; Samejima, Y.; Miyauchi, H.; Fukuwatari, Y.; Hayasawa, H. Lactoferrin as a suppressor of cell migration of gastrointestinal cell lines. J. Cell. Physiol. 1997, 170, 101–105. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Nguyen, P.T.; Imanaka, H.; Takata, T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018, 507, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Colella, B.; Faienza, F.; Di Bartolomeo, S. EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology. Cancers (Basel). 2019, 11, 312. [Google Scholar] [CrossRef]
- Lau, J.; Ilkhanizadeh, S.; Wang, S.; Miroshnikova, Y.A.; Salvatierra, N.A.; Wong, R.A.; Schmidt, C.; Weaver, V.M.; Weiss, W.A.; Persson, A.I. STAT3 blockade inhibits radiation- induced malignant progression in glioma. Cancer Res. 2015, 75, 4302–4311. [Google Scholar] [CrossRef]
- Hemavathy, K.; Ashraf, S.I.; Ip, Y.T. Snail/slug family of repressors: Slowly going into the fast lane of development and cancer. Gene 2000, 257, 1–12. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Watanabe, S.; Watanabe, R.; Hata, K.; Shimazaki, K.; Azuma, I. Bovine lactoferrin and Lactoferricin inhibit tumor metastasis in mice. Adv. Exp. Med. Biol. 1998, 443, 285–291. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, X.; Wang, J.; Ye, Q.; Zheng, X.; Peng, Q.; Zheng, Y.; Liu, P.; Zhang, X.; Li, Z.; et al. Lactoferrin deficiency induces a pro-metastatic tumor microenvironment through recruiting myeloid-derived suppressor cells in mice. Oncogene 2020, 39, 122–135. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature. 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Shimamura, M.; Matsuda, E.; Fujita, K.; Nomoto, H.; Satoh, J.; Kojima, S.; Alexander, D.B.; Moore, M.A.; Tsuda, H. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: A possible explanation for inhibition of carcinogenesis and metastasis. Cytokine 2004, 25, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, W. Inhibitory effects of human lactoferrin on U14 cervical carcinoma through upregulation of the immune response. Oncol Lett. 2014, 7, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.S.; Li, G.; Varadhachary, A.; Petrak, K.; Schneyer, M.; Li, D.; Ongkasuwan, J.; Zhang, X.; Taylor, R.J.; Strome, S.E.; et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin. Cancer Res. 2007, 13, 1601–1610. [Google Scholar] [CrossRef]
- Iigo, M.; Alexander, D.B.; Long, N.; Xu, J.; Fukamachi, K.; Futakuchi, M.; Takase, M.; Tsuda, H. Anticarcinogenesis pathways activated by bovine lactoferrin in the murine small intestine. Biochimie 2009, 91, 86–101. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef]
- Chea, C.; Haing, S.; Miyauchi, M.; Shrestha, M.; Imanaka, H.; Takata, T. Molecular mechanisms underlying the inhibitory effects of bovine lactoferrin on osteosarcoma. Biochem. Biophys. Res. Commun. 2019, 508, 946–952. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Ramadan, G.; Zoheiry, M.K.; El-Beih, N.M. Antihepatocarcinogenic activity of whey protein concentrate and lactoferrin in diethylnitrosamine-treated male albino mice. Environ. Toxicol. 2019, 34, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Frioni, A.; Berlutti, F.; Valenti, P.; Musci, G.; Bonaccorsi di Patti, M.C. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 2014, 27, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jeong, Y.Y.; Moon, W.K.; Jon, S. Integrin-targeting thermally cross-linked superparamagnetic iron oxide nanoparticles for combined cancer imaging and drug delivery. Nanotechnology 2010, 21, 415102. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.; Kendall, J.; Denny, W.; Garg, S. Targeting of nanoparticles in cancer: Drug delivery and diagnostics. Anticancer Drugs. 2011, 22, 949–962. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin Oncol. 2014, 141, 769–784. [Google Scholar] [CrossRef]
- Shankaranarayanan, J.S.; Kanwar, J.R.; Al-Juhaishi, A.J.; Kanwar, R.K. Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice. Sci Rep. 2016, 6, 32062. [Google Scholar] [CrossRef]
- Wei, M.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Xu, Y.; Wu, C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomedicine. 2015, 10, 5123–5137. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Min, Q.; Ling, C.; Maiti, D.; Xu, J.; Qin, L.; Yang, K. Holo-Lactoferrin Modified Liposome for Relieving Tumor Hypoxia and Enhancing Radiochemotherapy of Cancer. Small. 2019, 15, 1803703. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kondapi, A.K. Lactoferrin nanoparticle mediated targeted delivery of 5-fluorouracil for enhanced therapeutic efficacy. Int. J. Biol. Macromol. 2017, 95, 232–237. [Google Scholar] [CrossRef]
- Altwaijry, N.; Somani, S.; Parkinson, J.A.; Tate, R.J.; Keating, P.; Warzecha, M.; Mackenzie, G.R.; Leung, H.Y.; Dufès, C. Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-α, TRAIL, and interleukin-12. Drug Deliv. 2018, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zheng, Z.; He, Y.; Zhong, H.; Kang, X.; Shi, M.; Liu, T.; Jiao, Z.; Huang, Y. Antiglioma via regulating oxidative stress and remodeling tumor-associated macrophage using lactoferrin-mediated biomimetic codelivery of simvastatin/fenretinide. J. Control. Release. 2018, 287, 12–23. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chen, Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef]
- Singh, I.; Swami, R.; Pooja, D.; Jeengar, M.K.; Khan, W.; Sistla, R. Lactoferrin bioconjugated solid lipid nanoparticles: A new drug delivery system for potential brain targeting. J. Drug Target. 2016, 24, 212–223. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. https://doi.org/10.3390/biom10030456
Cutone A, Rosa L, Ianiro G, Lepanto MS, Bonaccorsi di Patti MC, Valenti P, Musci G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules. 2020; 10(3):456. https://doi.org/10.3390/biom10030456
Chicago/Turabian StyleCutone, Antimo, Luigi Rosa, Giusi Ianiro, Maria Stefania Lepanto, Maria Carmela Bonaccorsi di Patti, Piera Valenti, and Giovanni Musci. 2020. "Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action" Biomolecules 10, no. 3: 456. https://doi.org/10.3390/biom10030456
APA StyleCutone, A., Rosa, L., Ianiro, G., Lepanto, M. S., Bonaccorsi di Patti, M. C., Valenti, P., & Musci, G. (2020). Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules, 10(3), 456. https://doi.org/10.3390/biom10030456










