Targeting the Class A Carbapenemase GES-5 via Virtual Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacophore Hypothesis
2.2. Virtual Screening
2.3. Cloning, Expression and Purification of Recombinant blages-5
2.4. Crystallization and Structure Determination
2.5. In Vitro Validation
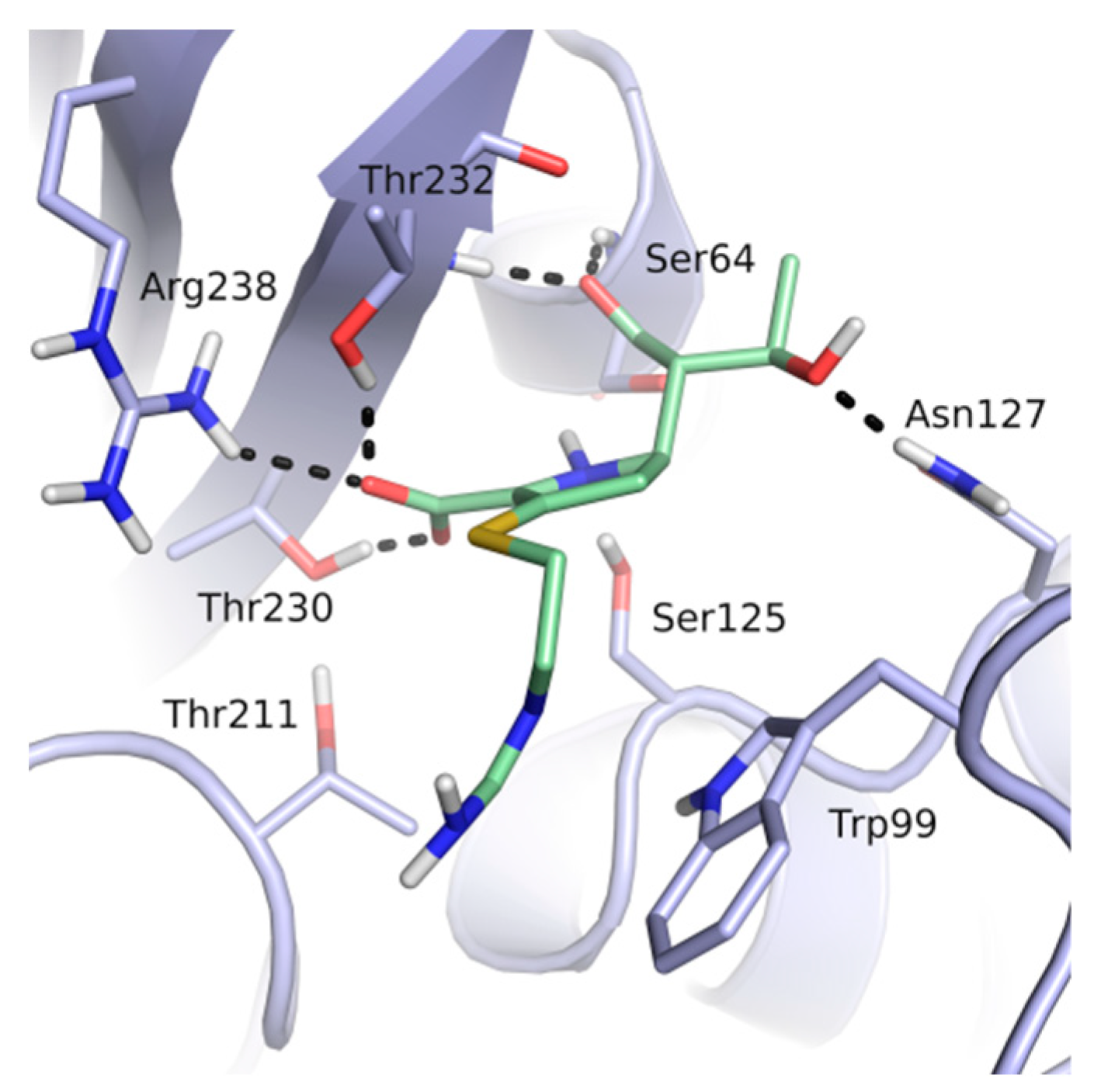
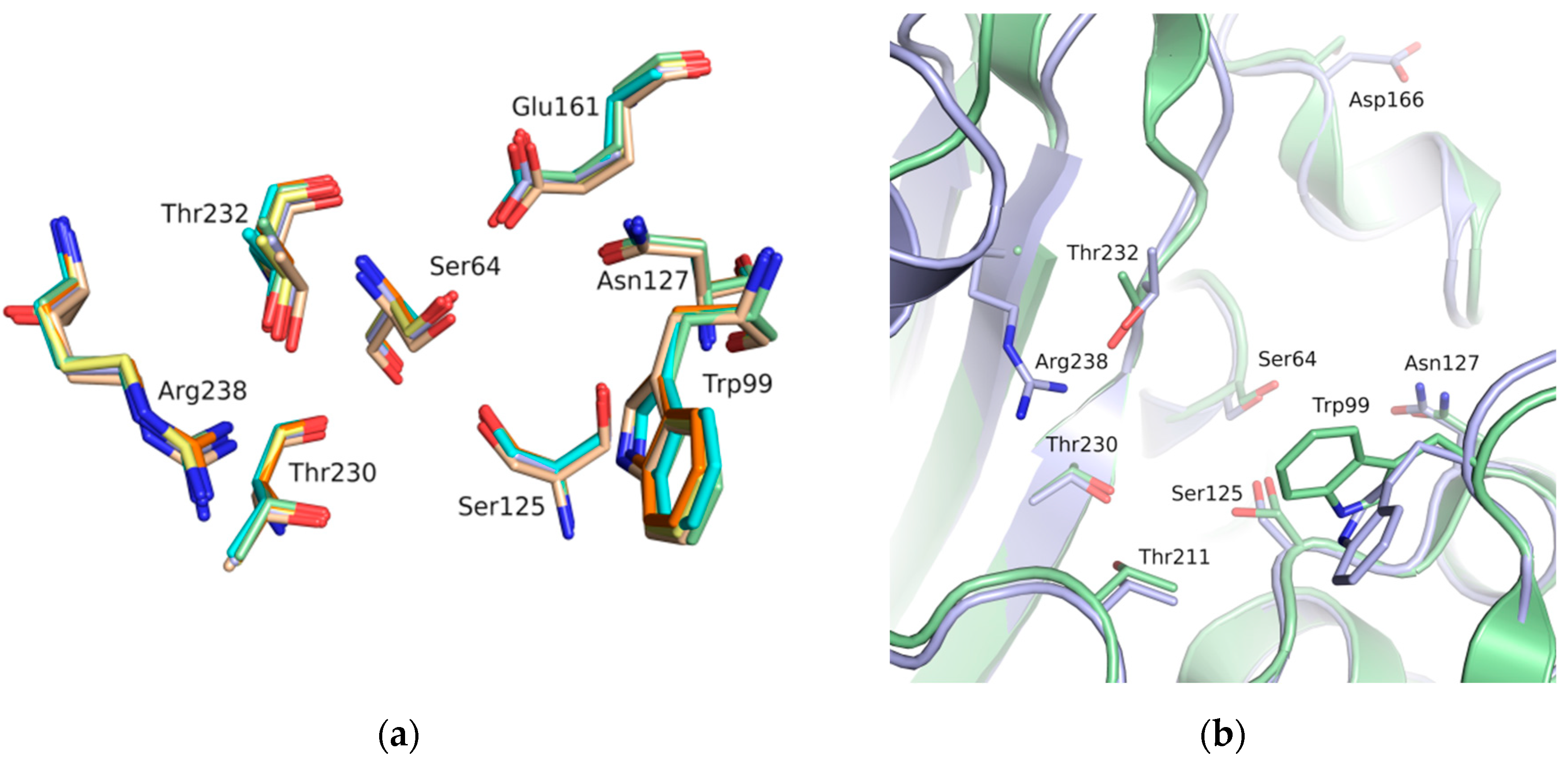
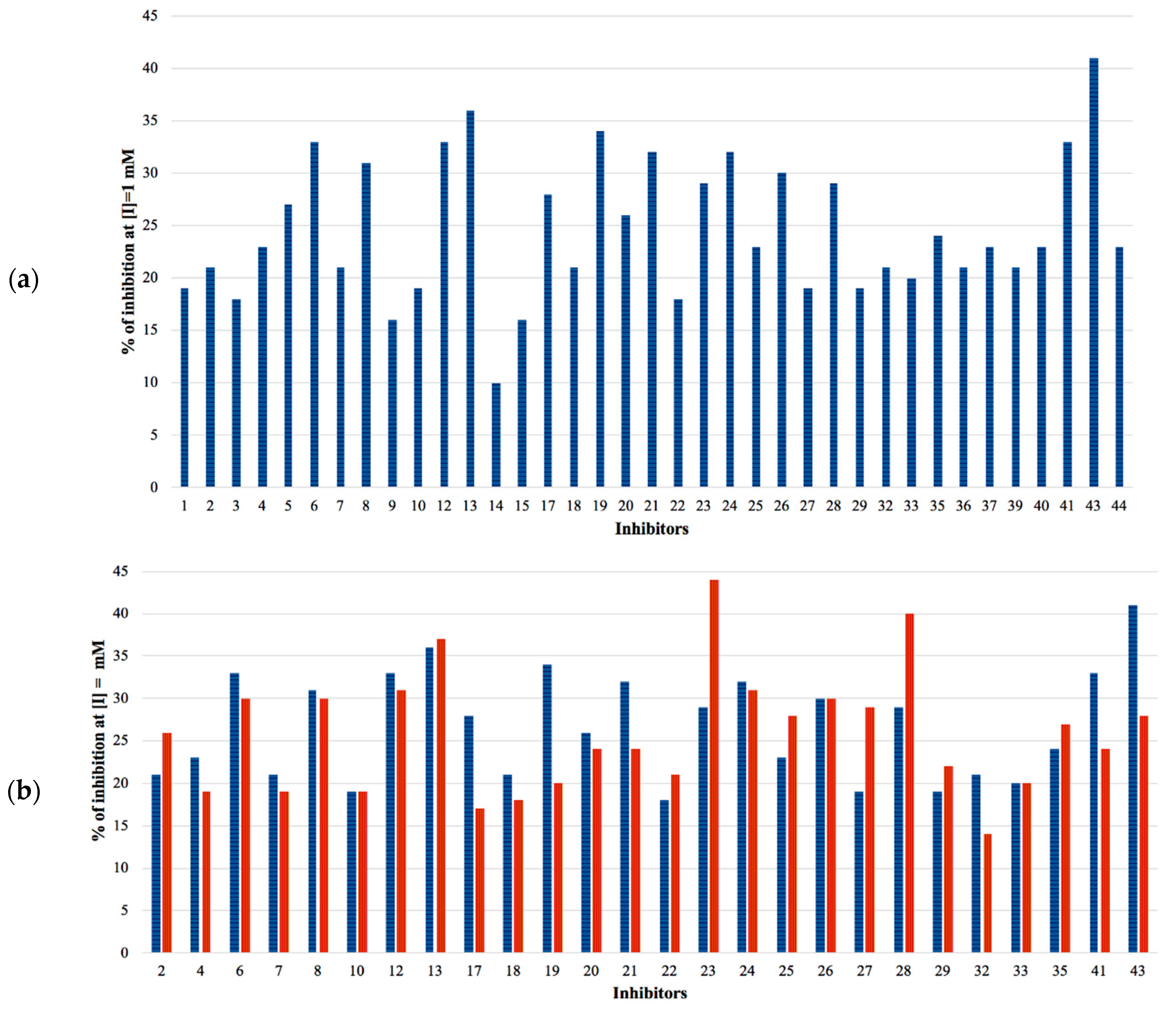
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Antimicrobial Resistance: Global Report on Surveillance; World Health Organisation: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 14 January 2020).
- Bellio, P.; Di Pietro, L.; Mancini, A.; Piovano, M.; Nicoletti, M.; Brisdelli, F.; Tondi, D.; Cendron, L.; Franceschini, N.; Amicosante, G.; et al. SOS response in bacteria: Inhibitory activity of lichen secondary metabolites against Escherichia coli RecA protein. Phytomedicine 2017, 29, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.Y.; Culyba, M.J.; Selwood, T.; Kubiak, J.M.; Hostetler, Z.M.; Jurewicz, A.J.; Keller, P.M.; Pope, A.J.; Quinn, A.; Schneck, J.; et al. Inhibitors of LexA Autoproteolysis and the Bacterial SOS Response Discovered by an Academic-Industry Partnership. ACS Infect. Dis. 2018, 4, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Thomas, I.L.E.; Naas, T.; Karim, A.; Nordmann, P. Biochemical Sequence Analyses of GES-1, a Novel Class A Extended-Spectrum beta-Lactamase and the Class 1 Integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 622–632. [Google Scholar] [CrossRef]
- Tondi, D.; Cross, S.; Venturelli, A.; Costi, M.; Cruciani, G.; Spyrakis, F. Decoding the structural basis for carbapenem hydrolysis by class A-lactamases: Fishing for a pharmacophore. Curr. Drug Targets 2016, 17, 983–1005. [Google Scholar] [CrossRef]
- Naas, T.; Dortet, L.I.; Iorga, B. Structural and Functional Aspects of Class A Carbapenemases. Curr. Drug Targets 2016, 17, 1006–1028. [Google Scholar] [CrossRef]
- Chihi, H.; Bonnin, R.A.; Bourouis, A.; Mahrouki, S.; Besbes, S.; Ben Moussa, M.; Belhadj, O.; Naas, T. GES-11-producing Acinetobacter baumannii clinical isolates from Tunisian hospitals: Long-term dissemination of GES-type carbapenemases in North Africa. J. Glob. Antimicrob. Resist. 2016, 5, 47–50. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Jousset, A.B.; Urvoy, N.; Gauthier, L.; Tlili, L.; Creton, E.; Cotellon, G.; Arthur, F.; Dortet, L.; Naas, T. Detection of GES-5 Carbapenemase in Klebsiella pneumoniae, a Newcomer in France. Antimicrob. Agents Chemother. 2017, 61, e02263-16. [Google Scholar] [CrossRef]
- Poirel, L.; Weldhagen, G.F.; Naas, T.; De Champs, C.; Dove, M.G.; Nordmann, P. GES-2, a class A β-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 2001, 45, 2598–2603. [Google Scholar] [CrossRef]
- Smith, C.A.; Frase, H.; Toth, M.; Kumarasiri, M.; Wiafe, K.; Munoz, J.; Mobashery, S.; Vakulenko, S.B. Structural basis for progression toward the carbapenemase activity in the GES family of β-lactamases. J. Am. Chem. Soc. 2012, 134, 19512–19515. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Nossoni, Z.; Toth, M.; Stewart, N.K.; Frase, H.; Vakulenko, S.B. Role of the Conserved Disulfide Bridge in Class A Carbapenemases. J. Biol. Chem. 2016, 291, 22196–22206. [Google Scholar] [CrossRef] [PubMed]
- Cendron, L.; Quotadamo, A.; Maso, L.; Bellio, P.; Montanari, M.; Celenza, G.; Venturelli, A.; Costi, M.P.; Tondi, D. X-ray Crystallography Deciphers the Activity of Broad-Spectrum Boronic Acid β-Lactamase Inhibitors. ACS Med. Chem. Lett. 2019, 10, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Caccamo, M.; Kantardjieff, K.A.; Vakulenko, S. Structure of GES-1 at atomic resolution: Insights into the evolution of carbapenamase activity in the class A extended-spectrum β-lactamases. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 982–992. [Google Scholar] [CrossRef]
- Bebrone, C.; Bogaerts, P.; Delbrück, H.; Bennink, S.; Kupper, M.B.; De Castro, R.R.; Glupczynski, Y.; Hoffmann, K.M. GES-18, a new carbapenem-hydrolyzing GES-type β-lactamase from Pseudomonas aeruginosa that contains Ile80 and Ser170 residues. Antimicrob. Agents Chemother. 2013, 57, 396–401. [Google Scholar] [CrossRef]
- Delbrück, H.; Bogaerts, P.; Kupper, M.B.; De Castro, R.R.; Bennink, S.; Glupczynski, Y.; Galleni, M.; Hoffmann, K.M.; Bebrone, C. Kinetic and crystallographic studies of extended-spectrum GES-11, GES-12, and GES-14 β-lactamases. Antimicrob. Agents Chemother. 2012, 56, 5618–5625. [Google Scholar] [CrossRef]
- Stewart, N.K.; Smith, C.A.; Frase, H.; Black, D.J.; Vakulenko, S.B. Kinetic and structural requirements for carbapenemase activity in GES-type beta-lactamases. Biochemistry 2015, 54, 588–597. [Google Scholar] [CrossRef]
- Linciano, P.; Vicario, M.; Kekez, I.; Bellio, P.; Celenza, G.; Martín-Blecua, I.; Blázquez, J.; Cendron, L.; Tondi, D. Phenylboronic acids probing molecular recognition against class A and class C β-lactamases. Antibiotics 2019, 8, 171. [Google Scholar] [CrossRef]
- Spyrakis, F.; Bellio, P.; Quotadamo, A.; Linciano, P.; Benedetti, P.; D’Arrigo, G.; Baroni, M.; Cendron, L.; Celenza, G.; Tondi, D. First virtual screening and experimental validation of inhibitors targeting GES-5 carbapenemase. J. Comput. Aided. Mol. Des. 2019, 33, 295–305. [Google Scholar] [CrossRef]
- Klein, R.; Linciano, P.; Celenza, G.; Bellio, P.; Papaioannou, S.; Blazquez, J.; Cendron, L.; Brenk, R.; Tondi, D. In silico identification and experimental validation of hits active against KPC-2 β-lactamase. PLoS ONE 2018, 13, e0203241. [Google Scholar] [CrossRef]
- Labute, P. Protonate3D: Assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins Struct. Funct. Bioinform. 2009, 75, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Brenk, R.; Irwin, J.J.; Shoichet, B. Here Be Dragons: Docking and Screening in an Uncharted Region of Chemical Space. J. Biomol. Screen. 2005, 10, 667–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mysinger, M.M.; Shoichet, B.K. Rapid context-dependent ligand desolvation in molecular docking. J. Chem. Inf. Model. 2010, 50, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Lorber, D.M.; Shoichet, B.K. Flexible ligand docking using conformational ensembles. Protein Sci. 1998, 7, 938–950. [Google Scholar] [CrossRef]
- Wei, B.Q.; Baase, W.A.; Weaver, L.H.; Matthews, B.W.; Shoichet, B.K. A Model Binding Site for Testing Scoring Functions in Molecular Docking. J. Mol. Biol. 2002, 322, 339–355. [Google Scholar] [CrossRef]
- Quotadamo, A.; Linciano, P.; Davoli, P.; Tondi, D.; Costi, M.P.; Venturelli, A. An Improved Synthesis of CENTA, a Chromogenic Substrate for β-Lactamases. Synlett 2016, 27, 2447–2450. [Google Scholar]
- Feng, B.Y.; Shoichet, B.K. A detergent-based assay for the detection of promiscuous inhibitors. Nat. Protoc. 2006, 1, 550–553. [Google Scholar] [CrossRef]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Celenza, G.; Vicario, M.; Bellio, P.; Linciano, P.; Perilli, M.; Oliver, A.; Blazquez, J.; Cendron, L.; Tondi, D. Phenyl boronic acids development led to validated leads active in clinical strains overexpressing KPC-2: A step against bacterial resistance. ChemMedChem 2018, 13, 713–724. [Google Scholar] [CrossRef]
- Erlanson, D.A.; Fesik, S.W.; Hubbard, R.E.; Jahnke, W.; Jhoti, H. Twenty years on: The impact of fragments on drug discovery. Nat. Rev. Drug Discov. 2016, 15, 605–619. [Google Scholar] [CrossRef]

| Code | Ki [mM] vs. GES-5 (LE) [a] | Ki [mM] vs. KPC-2 (LE) [a] | Chemical Structure | Predicted Binding Mode in GES-5 |
|---|---|---|---|---|
| 8 | 0.89 (0.27) | 0.35 (0.30) |  |  |
| 12 | 1.01 (0.22) | 1.38 (0.21) | 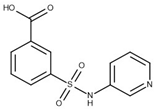 | 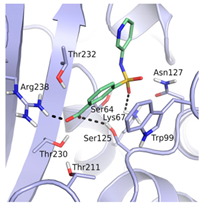 |
| 13 | 0.81 (0.29) | 0.28 (0.33) | 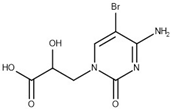 |  |
| 43 | 0.66 (0.32) | 0.45 (0.33) | 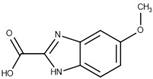 |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, R.; Cendron, L.; Montanari, M.; Bellio, P.; Celenza, G.; Maso, L.; Tondi, D.; Brenk, R. Targeting the Class A Carbapenemase GES-5 via Virtual Screening. Biomolecules 2020, 10, 304. https://doi.org/10.3390/biom10020304
Klein R, Cendron L, Montanari M, Bellio P, Celenza G, Maso L, Tondi D, Brenk R. Targeting the Class A Carbapenemase GES-5 via Virtual Screening. Biomolecules. 2020; 10(2):304. https://doi.org/10.3390/biom10020304
Chicago/Turabian StyleKlein, Raphael, Laura Cendron, Martina Montanari, Pierangelo Bellio, Giuseppe Celenza, Lorenzo Maso, Donatella Tondi, and Ruth Brenk. 2020. "Targeting the Class A Carbapenemase GES-5 via Virtual Screening" Biomolecules 10, no. 2: 304. https://doi.org/10.3390/biom10020304
APA StyleKlein, R., Cendron, L., Montanari, M., Bellio, P., Celenza, G., Maso, L., Tondi, D., & Brenk, R. (2020). Targeting the Class A Carbapenemase GES-5 via Virtual Screening. Biomolecules, 10(2), 304. https://doi.org/10.3390/biom10020304






