Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Obesity and Metabolic Disorders
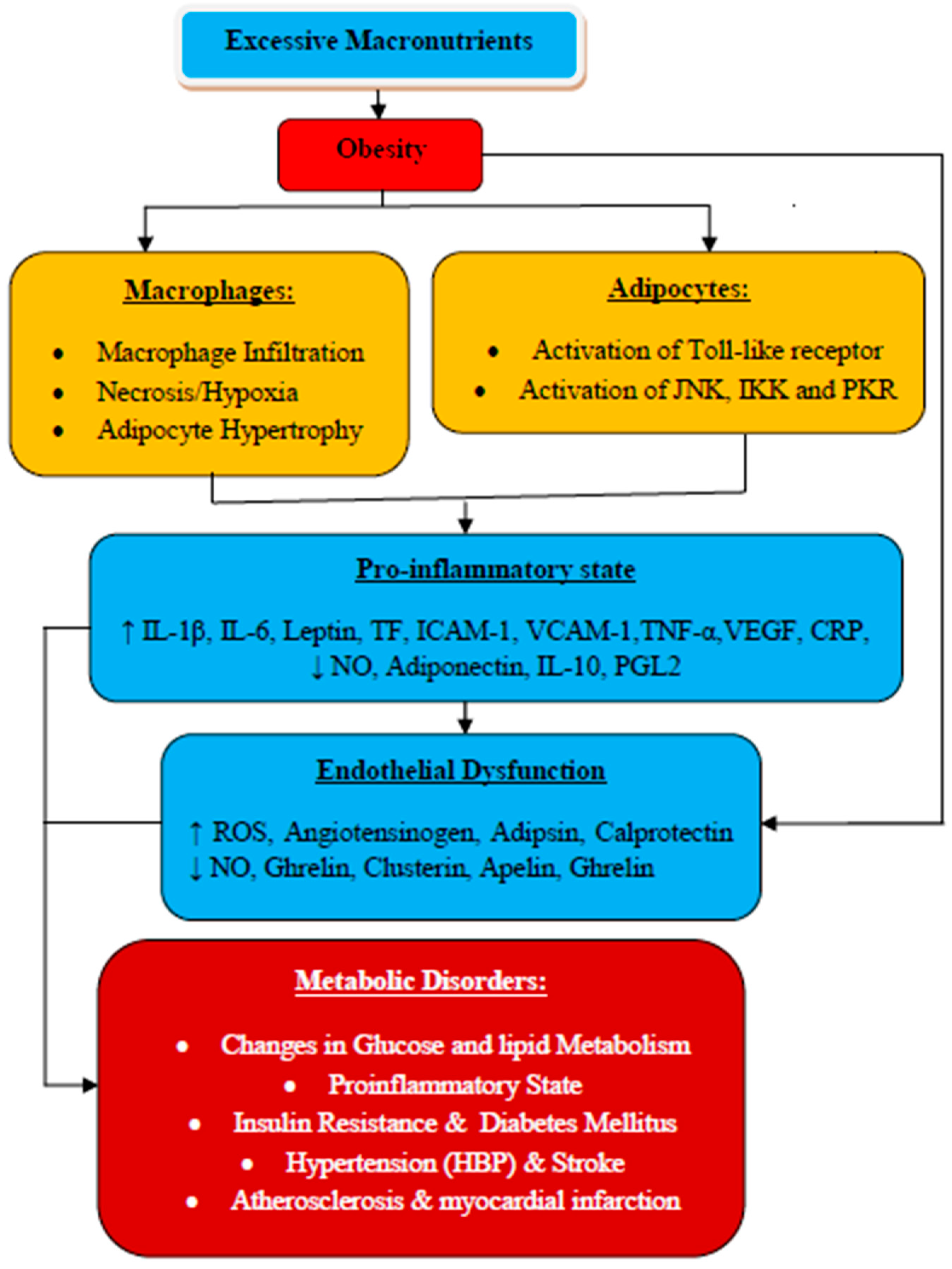
2.1. Impact of Obesity on Adipose Tissue
2.2. Obesity, Inflammation, and Related Metabolic Syndrome: The Linking Mechanisms
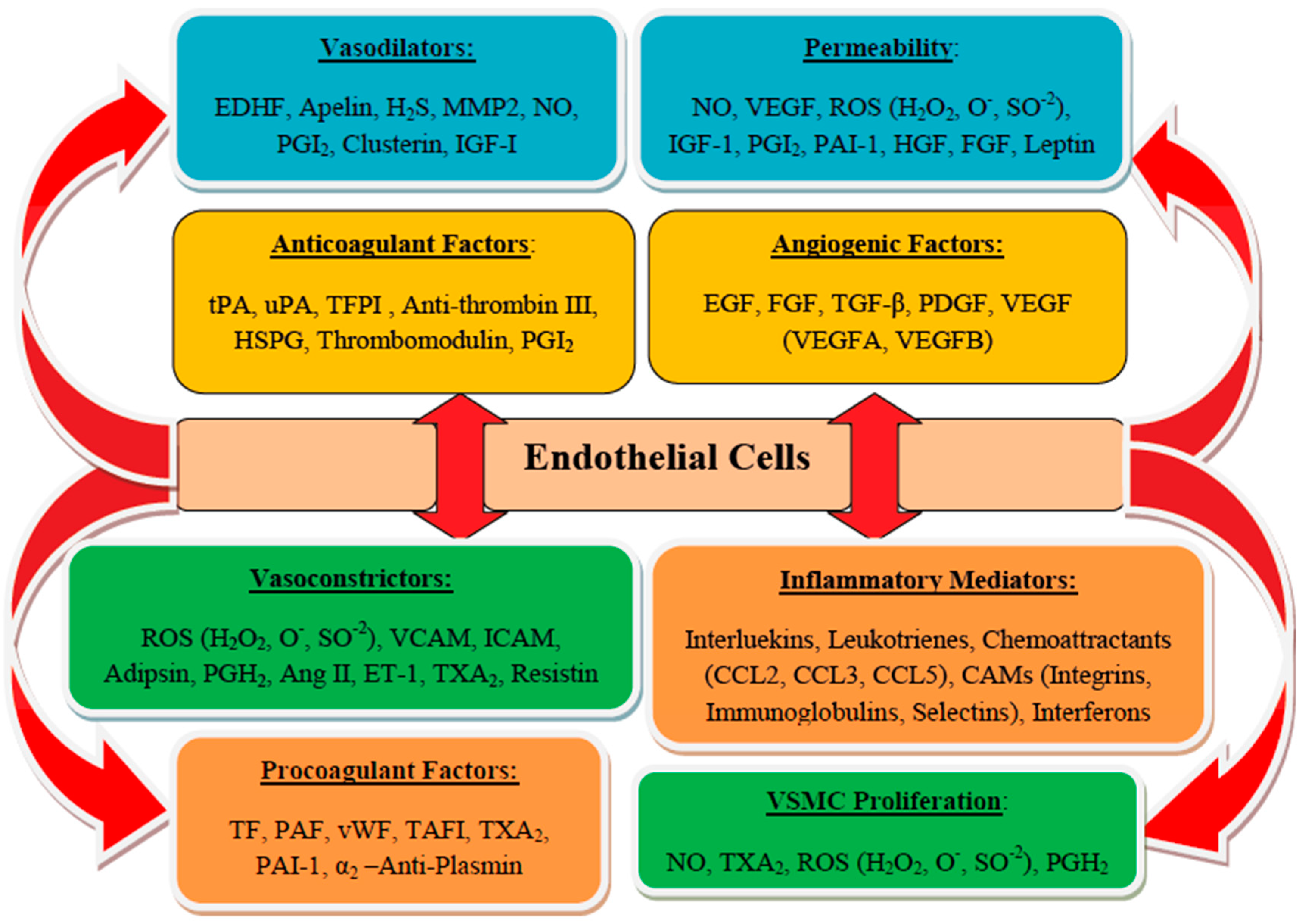
3. Endothelium and Endothelial Cells Functions
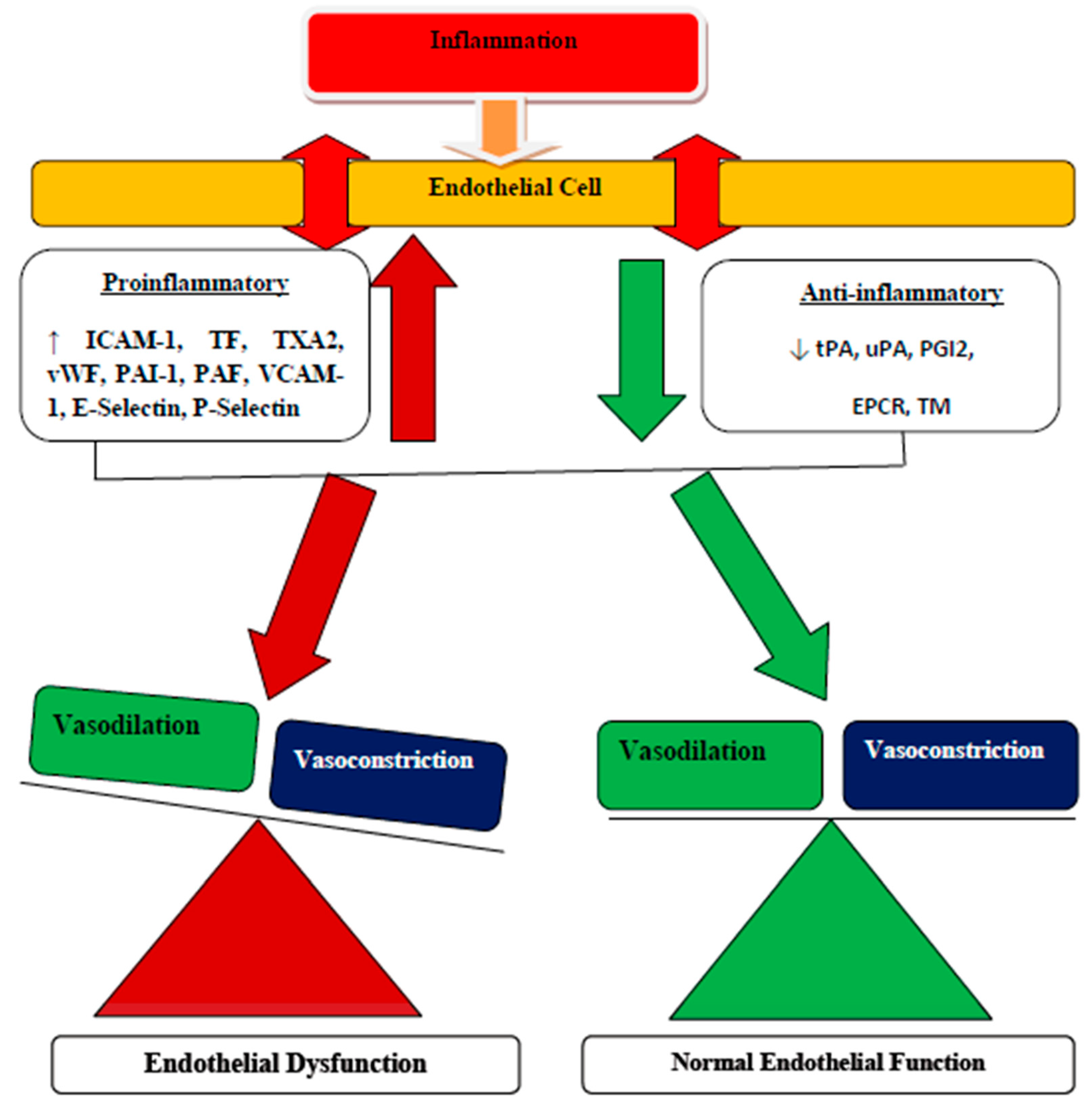
3.1. Endothelial Dysfunction
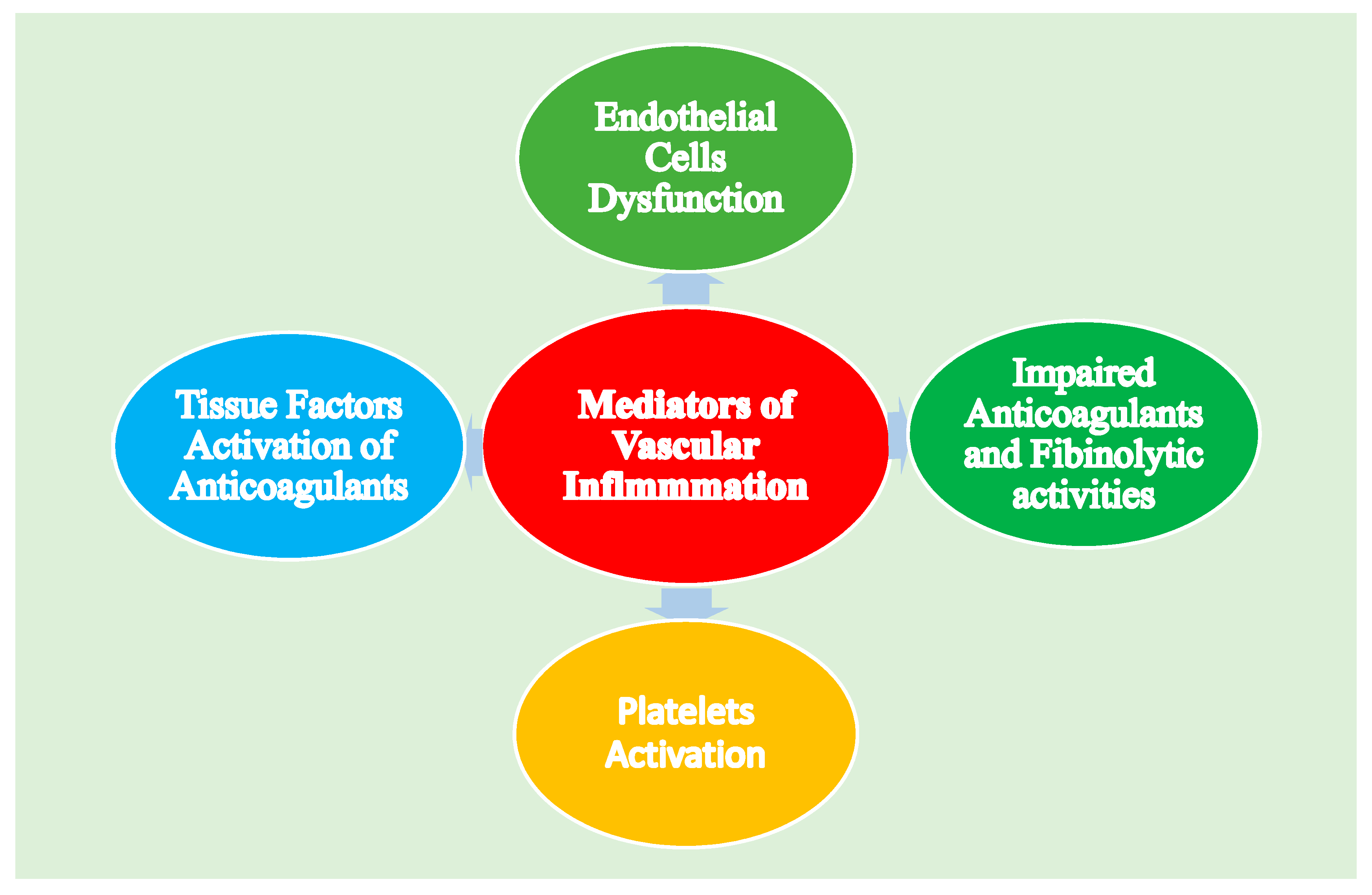
3.2. Coagulation System and Endothelial Dysfunction
3.3. Endothelial Dysfunction during Vascular Aging and Cellular Senescence
3.4. Endothelial Dysfunction and Epigenetic Modifications
3.5. Endothelial Dysfunction Influenced by Reactive Oxygen Species (ROS)
3.6. Endothelial Dysfunction and Vascular Calcification
3.7. Impact of Human Gut Microbiota on Vascular Endothelim
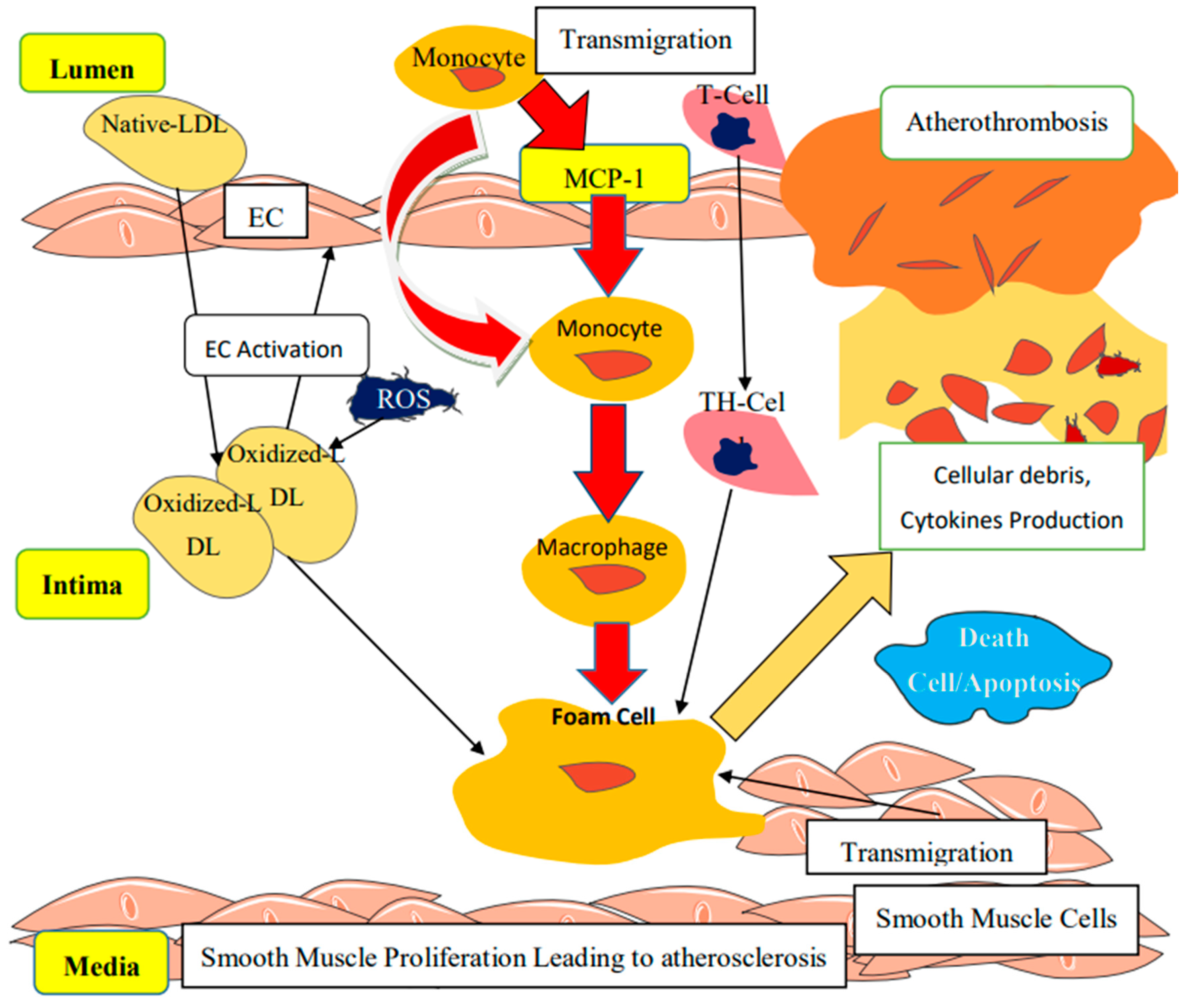
4. MCP-1: A Biochemical Marker Associated with Endothelial Dysfunction
5. Clinical Implication of Obesity-Induced Endothelial Dysfunction
Molecular Mechanisms Linked to the Progression of Atherosclerosis
6. Therapeutic Approaches Targeting Endothelial Dysfunction
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| VCAM-I | Vascular Cell Adhesion Molecule-1 |
| JNK–c-Jun | N-Terminal Kinase |
| TF | Tissue Factor |
| IL-10 | Interleukin-10 |
| ROS | Reactive Oxygen Species |
| VEGF | Vascular Endothelial Growth Factor |
| TNF-α | Tumour Necrosis Factor α |
| PGL2 | prostacyclin |
| IL-6 | Interleukin-6 |
| CCL | Chemokine (C-C Motif) Ligand, |
| EC | Endothelial Cells |
| ET1 | Endothelin-1 |
| FGF | Fibroblast Growth Factor |
| H2S | Hydrogen Sulfide |
| PGH2 | Prostaglandin H2 |
| PGI2 | prostacyclin |
| t-PA | Tissue Plasminogen Activator |
| VSMC | Vascular Smooth Muscle Cells |
| SO2 | Superoxide |
| Upa | Urokinase Plasminogen Activator |
| EPCR | Endothelial Protein C Receptor |
| TH | T-Helper |
| NO | Nitric oxide, |
| IKK | Inhibitor of K- Kinase |
| PKR | Protein Kinase-R |
| CRP | C-Reactive Protein |
| HGF | Hepatocyte Growth Factor |
| IGF-1 | Insulin-Like Growth Factor-1 |
| MMP2 | Matrix MetalloProteinase-2 |
| TAFI | Thrombin-Activatable Fibrinolytic Inhibitor |
| TFPI | Tissue Factor Pathway Inhibitor |
| CAMs | Cells Adhesion Molecules |
| EDHF | Endothelium-Derived Hyperbolizing Factor |
| EGF | Epidermal Growth Factor |
| HSPG | Heparan Sulfate Proteoglycans |
| PAF | Platelet-Activating Factor |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PDGF | Platelet-Derived Growth Factor |
| TGF-β | Transforming Growth Factor-β |
| TXA2 | Thromboxane A2, |
| vWF | von Willebrand Factor |
| H2O2 | Hydrogen Peroxide |
| TM | Thrombomodulin, |
| A2-AP | α2-Antiplasmin |
References
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.R.; Hernández, L.E.S.; Ramírez, G.R.; Reyes, R.M.A. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int. J. Endocrinol. 2013, 2013, 11. [Google Scholar]
- Fleming, N.T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; Abraham, J.P.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Carrilho, F.; Seiça, R.M. Endothelial Dysfunction in Type 2 Diabetes: Targeting Inflammation. Endothelial Dysfunction—Old Concepts and New Challenges, Helena Lenasi, IntechOpen. 2018. Available online: https://www.intechopen.com/books/endothelial-dysfunction-old-concepts-and-new-challenges/endothelial-dysfunction-in-type-2-diabetes-targeting-inflammation (accessed on 17 January 2020). [CrossRef]
- Dhananjayan, R.; Koundinya, K.S.S.; Malati, T.; Kutala, V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016, 31, 372–379. [Google Scholar] [CrossRef]
- Takada, Y.; Hisamatsu, T.; Kamada, N.; Takayama, T.; Kobayashi, T.; Chinen, H. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J. Immunol. 2010, 184, 2671–2676. [Google Scholar] [CrossRef]
- Darvall, K.A.L.; Sam, R.C.; Silverman, S.H.; Bradbury, A.W.; Adam, D.J. Obesity and Thrombosis. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 223–233. [Google Scholar] [CrossRef]
- Wilson, R.M.; Messaoudi, I. The impact of maternal obesity during pregnancy on offspring immunity. Mol. Cell Endocrinol. 2015, 418, 134–142. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Krzysztoszek, J.; Wierzejska, E.; Zielińska, A. Obesity. An analysis of epidemiological and prognostic research. Arch. Med. Sci. 2015, 11, 24–33. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R.; Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease Find the latest version: Review series Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, I.; Monteiro, R. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediators Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF- κ B Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Hursting, M.J. Growth Signals, Inflammation, and Vascular Perturbations. Arterioscler. Thromb. Vasc. Biol. 2012, 1766–1770. [Google Scholar] [CrossRef]
- Phillips, L.K.; Hons, M.; Prins, J.B. The Link Between Abdominal Obesity and the Metabolic Syndrome. Curr. Hypertens Rep. 2008, 10, 156–164. [Google Scholar] [CrossRef]
- Canale, M.P.; Manca, S.; Martino, G.; Rovella, V.; Noce, A.; De Lorenzo, A.; Di Daniele, N. Obesity-Related Metabolic Syndrome: Mechanisms of Sympathetic Overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Karastergiou, K.; Mohamed-Ali, V. The autocrine and paracrine roles of adipokines. Mol. Cell Endocrinol. 2010, 318, 69–78. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal Obesity, Inflammation, and Developmental Programming. Biomed. Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Reynolds, C.M. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J. Dev. Orig. Health Dis. 2019, 8, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Foster, M.C.; Kalyani, R.R. Vaidya, D1.; Burke, G.L.; Woodward, M.; Anderson, C.A. Obesity severity and duration are associated with incident metabolic syndrome: Evidence against metabolically healthy obesity from the multi-ethnic study of atherosclerosis. J. Clin. Endocrinol. Metab. 2016, 101, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-aicha, S.; Badimon, L. New insights into the role of adipose tissue in thrombosis. Cardiovasc. Res. 2017, 113, 1046–1054. [Google Scholar] [CrossRef]
- Nightingale, T.; Cutler, D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J. Thromb. Haemost. 2013, 11, 192–201. [Google Scholar] [CrossRef]
- Möller, K.; Adolph, O.; Grünow, J.; Elrod, J.; Popa, M.; Ghosh, S.; Schwarz, M.; Schwale, C.; Grässle, S.; Huck, V.; et al. Mechanism and functional impact of CD40 ligand-induced von Willebrand factor release from endothelial cells. Thromb. Haemost. 2015, 113, 1095–1108. [Google Scholar] [CrossRef]
- Carnevale, R.; Raparelli, V.; Nocella, C.; Bartimoccia, S.; Novo, M.; Severino, A.; De Falco, E.; Cammisotto, V.; Pasquale, C.; Crescioli, C.; et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J. Hepatol. 2017, 67, 950–956. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seica, R. The endothelial dysfunction-A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Lee, H.Y.; Youn, S.W.; Cho, H.J.; Kwon, Y.W.; Lee, S.W.; Kim, S.J.; Park, Y.B.; Oh, B.H.; Kim, H.S. FOXO1 impairs whereas statin protects endothelial dysfunction in diabetes through reciprocal regulation of kruppel-like factor2. Cardiovasc. Res. 2013, 97, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Chatterjee, T.K.; Tang, Y.; Hui, D.Y.; Weintraub, N.L. The proinflammatory phenotype of perivascular adipocytes. Atheros. Thromb. Vasc. Biol. 2014, 34, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.; Fernandes, R.; Letra, L.; Seica, R.M. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet. Role of perivascular adipose tissue. Br. J. Pharmacol. 2017, 174, 3514–3526. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Habibi, J.; Bostick, B.P.; Ma, L.; DeMarco, V.G.; Aroor, A.R.; Hayden, M.R.; Whaley-Connell, A.T.; Sowers, J.R. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 2015, 65, 531–539. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Massiera, F.; Quignard-Boulange, A.; Ailhaud, G.; Voy, B.H.; Wasserma, D.H.; Moustaid-Moussa, N. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity 2012, 20, 48–56. [Google Scholar] [CrossRef]
- Roberts, A.C.; Porter, K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab. Vasc. Dis. Res. 2013, 10, 472–482. [Google Scholar] [CrossRef]
- Brady, E.M.; Webb, D.R.; Morris, D.H.; Khunti, K.; Talbot, D.S.; Sattar, N.; Davies, M.J. Investigating endothelial activation and oxidative stress in relation to glycaemic control in a multiethnic population. Exp. Diabetes Res. 2012, 2012, 386041. [Google Scholar] [CrossRef]
- Sprague, A.H.; Kalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-kappaB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome complexes. Emerging mechanisms and effector functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Consolidated and emerging inflammatory markers in coronary artery disease. World J. Exp. Med. 2015, 5, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.; Guardado, M.R.; Winner, D.; Fiorentino, T.V.; Pengou, Z.; Cornell, J.; Andreozzi, F.; Jenkinson, C.; Ceersosimo, E.; Federici, M.; et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycaemia in type 2 diabetes mellitus. Acta Diabetol. 2014, 51, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudhason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New perspective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nissen, S.E.; Shao, M.; Elshazly, M.B.; Kataoka, Y.; Kapadia, S.R.; Tuzcu, E.M.; Nicholls, S.J. Non-HDL cholesterol and triglycerides. Implications for coronary atheroma progression and clinical events. Atheroscler. Thromb. Vasc. Biol. 2016, 36, 2220–2228. [Google Scholar] [CrossRef]
- Krintus, M.; Kozinski, M.; Kubica, J.; Sypniewska, G. Critical appraisal of inflammatory markers in cardiovascular risk stratification. Crit. Rev. Clin. Lab. Sci. 2014, 51, 263–279. [Google Scholar] [CrossRef]
- Papapanagiotou, A.; Siasos, G.; Kassi, E.; Gargalionis, A.N.; Papavassiliou, A.G. Novel inflammatory markers in hyperlipidaemia. Clin. Implic. Curr. Med. Chem. 2015, 22, 2727–2743. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; Schultz, M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin. Thromb. Hemost. 2012, 38, 506–514. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, 526–534. [Google Scholar] [CrossRef]
- Margetic, S. Review Inflammation and haemostasis. Biochem. Med. 2012, 22, 49–62. [Google Scholar] [CrossRef]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef]
- Wang, J.C.; Bennett, M. Aging and Atherosclerosis: Mechanisms, Functional Consequences, and Potential Therapeutics for Cellular Senescence. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Khaidakov, M.; Wang, X.; Mehta, J.L. Potential involvement of LOX-1 in functional consequences of endothelial senescence. PLoS ONE 2011, 6, e20964. [Google Scholar] [CrossRef] [PubMed]
- Bolton, E.; Rajkumar, C. The ageing cardiovascular system. Rev. Clin. Gerontol. 2010, 21, 99–109. [Google Scholar] [CrossRef]
- Lund, G.; Zaina, S. Atherosclerosis: An epigenetic balancing act that goes wrong. Curr. Atheroscler. Rep. 2011, 13, 208–214. [Google Scholar] [CrossRef]
- Ventura, A.; Luzi, L.; Pacini, S.; Baldari, C.T.; Pelicci, P.G. The p66Shclongevity gene is silenced through epigenetic modifications of an alternative promoter. J. Biol. Chem. 2002, 277, 22370–22376. [Google Scholar] [CrossRef]
- Willeit, P.; Willeit, J.; Brandstatter, A.; Ehrlenbach, S.; Mayr, A.; Gasperi, A.; Weger, S.; Oberhollenzer, F.; Reindl, M.; Kronenberg., F.; et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1649–1656. [Google Scholar] [CrossRef]
- Panayiotou, A.G.; Nicolaides, A.N.; Griffin, M.; Tyllis, T.; Georgiou, N.; Bond, D.; Martin, R.M.; Hoppensteadt, D.; Fareed, J.; Humphries, S.E. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis 2010, 211, 176–181. [Google Scholar] [CrossRef]
- Gray, K.; Bennett, M. Role of DNA damage in atherosclerosis: Bystander or participant? Biochem. Pharmacol. 2011, 82, 693–700. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Lamers, D.; Schlich, R.; Greulich, S.; Sasson, S.; Sell, H.; Eckel, J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signaling in human vascular smooth muscle cells. J. Cell Mol. Med. 2011, 15, 1177–1188. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E879–E886. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Sin-Malia, Y.; Cohen, G.; Alpert, E.; Gruzman, A.; Eckel, J.; Staels, B.; Guichardant, M.; Sasson, S. The natural protective mechanism against hyperglycemia in vascular endothelial cells: Roles of the lipid peroxidation product 4-hydroxydodecadienal and peroxisome proliferator-activated receptor delta. Diabetes 2010, 59, 808–818. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Kuka, S.; Racay, P.; Lehotsky, J.; Dobrota, D.; Mistuna, D.; Kaplan, P. Effects of ageing on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 2011, 60, 281–289. [Google Scholar] [CrossRef]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef]
- Cohen, G.; Riahi, Y.; Sasson, S. Lipid Peroxidation of Poly-Unsaturated Fatty Acids in Normal and Obese Adipose Tissues. Arch. Physiol. Biochem. 2011, 117, 131–139. [Google Scholar] [CrossRef]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of ageing. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef]
- Goldenberg, L.; Saliba, W.; Hayek, H.; Hasadia, R.; Zeina, A.R. The Impact of Abdominal Fat on Abdominal Aorta Calcification Measured on Non-Enhanced CT. Medicine 2018, 97, e13233. [Google Scholar] [CrossRef]
- Farhat, N.; Thorin-Trescases, N.; Voghel, G.; Villeneuve, L.; Mamarbachi, M.; Perrault, L.P.; Carrier, M.; Thorin, E. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can. J. Physiol. Pharmacol. 2008, 86, 761–769. [Google Scholar] [CrossRef]
- Carmo, L.S.; Burdmann, E.A.; Fessel, M.R.; Almeida, Y.E.; Pescatore, L.A.; Farias-Silva, E.; Gamarra, L.F.; Lopes, G.H.; Aloia, T.P.A.; Liberman, M. Expansive Vascular Remodeling and Increased Vascular Calcification Response to Cholecalciferol in a Murine Model of Obesity and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 200–211. [Google Scholar] [CrossRef]
- Gerhard, M.; Roddy, M.A.; Creager, S.J.; Creager, M.A. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 1996, 27, 849–853. [Google Scholar] [CrossRef]
- Gui, T.; Shimokado, A.; Sun, Y.; Akasaka, T.; Muragaki, Y. Diverse roles of macrophages in atherosclerosis: From inflammatory biology to biomarker discovery. Mediat. Inflamm. 2012. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Kasahara, K.; Tanoue, T.; Yamashita, T.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Mizoguchi, T.; Hayashi, T.; Kitano, N.; Sasaki, N.; et al. Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. J. Lipid Res. 2017, 58, 519–528. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.; Feng, O.; Shenghui, L.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Hui, Z.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, Y.; Kimura, I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci. Microbiota Food Health 2017, 36, 135–140. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018, 16, 171–181. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine- N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zárate, M.L.; Villalobos-Flores, L.E.; García-González, C.; Morales-Hernández, R.M.; Nuñez-Hernández, J.A.; Hernández-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernández-Guerrero, C.; et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Ryu, J.; Han, K.H. Brief report Monocyte chemoattractant protein-1—Induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2019, 105, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Shang, H.; Xu, Y.; Qian, M. Monocyte chemoattractant protein-1 induces endothelial cell apoptosis in vitro through a p53-dependent mitochondrial pathway. Acta Biochim. Biophy. Sin. 2011, 43, 787–795. [Google Scholar] [CrossRef]
- Viedt, C.; Dechend, R.; Fei, J.; Hänsch, G.M.; Kreuzer, J.; Orth, S.R. MCP-1 Induces Inflammatory Activation of Human Tubular Epithelial Cells: Involvement of the Transcription Factors, Nuclear Factor-ΚB and Activating Protein-1. J. Am. Soc. Nephrol. 2002, 13, 1534–1547. [Google Scholar] [CrossRef]
- Tichelaar, Y.I.; Kluin-Nelemans, H.J.; Meijer, K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb. Haemost. 2012, 107, 827–837. [Google Scholar]
- Wang, S.; Ye, Q.; Zeng, X.; Qiao, S. Review Article Functions of Macrophages in the Maintenance of Intestinal Homeostasis. J. Immunol. Res. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.F.; Liao, H.H.; Liu, W.; Liu, J.; Ma, Z.G.; Wu, Q.Q.; Xu, M.; Zhang, N.; Zhang, Y.; et al. Toll-like receptor 5 deficiency attenuates interstitial cardiac fibrosis and dysfunction induced by pressure overload by inhibiting inflammation and the endothelial-mesenchymal transition. Biochim. Biophys. Acta 2015, 1852, 2456–2466. [Google Scholar] [CrossRef]
- Nie, L.; Lyros, O.; Medda, R.; Jovanovic, N.; Schmidt, J.L.; Otterson, M.F.; Johnson, C.P.; Behmaram, B.; Shaker, R.; Rafiee, P. Endothelial-mesenchymal transition in normal human oesophagal endothelial cells cocultured with oesophagal adenocarcinoma cells: Role of IL-1beta and TGF-beta2. Am. J. Physiol. Cell Physiol. 2014, 307, C859–C877. [Google Scholar] [CrossRef]
- Jackson, A.O.; Zhang, J.; Jiang, Z.; Yin, K. Endothelial-to-mesenchymal transition: A novel therapeutic target for cardiovascular diseases. Trends Cardiovasc. Med. 2017, 27, 383–393. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signalling and it’s regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Bussey, C.E.; Withers, S.B.; Aldous, R.G.; Edwards, G.; Heagerty, A.M. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Higashi, Y.; Node, K. Do incretins improve endothelial function? Cardiovasc. Diabetol. 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Berk, K.A.; Oudshoorn, T.P.; Verhoeven, A.J.M.; Mulder, M.T.; Roks, A.J.M.; Dik, W.A.; Timman, R.; Sijbrands, E.J.G. Diet-induced weight loss and markers of endothelial dysfunction and inflammation in treated patients with type 2 diabetes. Clin. Nutr. ESPEN 2016, 15, 101–106. [Google Scholar] [CrossRef]
- Kratzer, A.; Giral, H.; Landmesser, U. High-density lipoproteins as modulators of endothelial cell functions: Alterations in patients with coronary artery disease. Cardiovasc. Res. 2014, 103, 350–361. [Google Scholar] [CrossRef]
- Everett, B.M.; Pradhan, A.D.; Solomon, D.H.; Paynter, N.; MacFadyen, J.; Zaharris, E.; Gupta, M.; Clearfied, M.; Libby, P.; Hasan, A.A.K.; et al. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013, 166, 199–207. [Google Scholar] [CrossRef]
- Nohria, A.; Kinlay, S.; Buck, J.S.; Readline, W.; Copeland-Halperin, R.; Kim, S.; Beckman, J.A. The effect of salsalate therapy on endothelial function in a broad range of subjects. J. Am. Heart Associat. 2014, 3, e000609. [Google Scholar] [CrossRef]
- Van Hout, G.P.; Bosch, L.; Ellenbroek, G.H.; de Haan, J.J.; Van Solinge, W.W.; Cooper, M.A.; Arslan, F.; de Jager, S.C.; Robertson, A.A.; Pasterkamp, G.; et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 2017, 38, 828–836. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. https://doi.org/10.3390/biom10020291
Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules. 2020; 10(2):291. https://doi.org/10.3390/biom10020291
Chicago/Turabian StyleKwaifa, Ibrahim Kalle, Hasnah Bahari, Yoke Keong Yong, and Sabariah Md Noor. 2020. "Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications" Biomolecules 10, no. 2: 291. https://doi.org/10.3390/biom10020291
APA StyleKwaifa, I. K., Bahari, H., Yong, Y. K., & Noor, S. M. (2020). Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules, 10(2), 291. https://doi.org/10.3390/biom10020291







