Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Plant Material
2.3. Preparation of the Aqueous Extract and Its Fractions
2.4. Total Phenolic Content
2.5. Total Flavonoid Content
2.6. Gas Chromatography/Mass Spectrometry Analysis
2.7. Alpha-Amylase Inhibitory Assay
2.8. Determination of Glucose Uptake Capacity by Yeast Cells
2.9. Differentiation of 3T3 Cells into Adipocytes
2.10. Antiproliferative Activities
2.10.1. Primary Carcinoma Cell and Ethical Approval
2.10.2. Cell Culture
2.10.3. CellTiter-Glo Assay
2.11. Molecular Docking
2.11.1. Protein Preparation
2.11.2. Natural Compound Selection
2.11.3. Active Site Prediction
2.11.4. Ligand Preparation
2.11.5. Virtual Screening
2.12. Statistical Analyses
3. Results
3.1. Total Phenolic and Flavonoid Contents
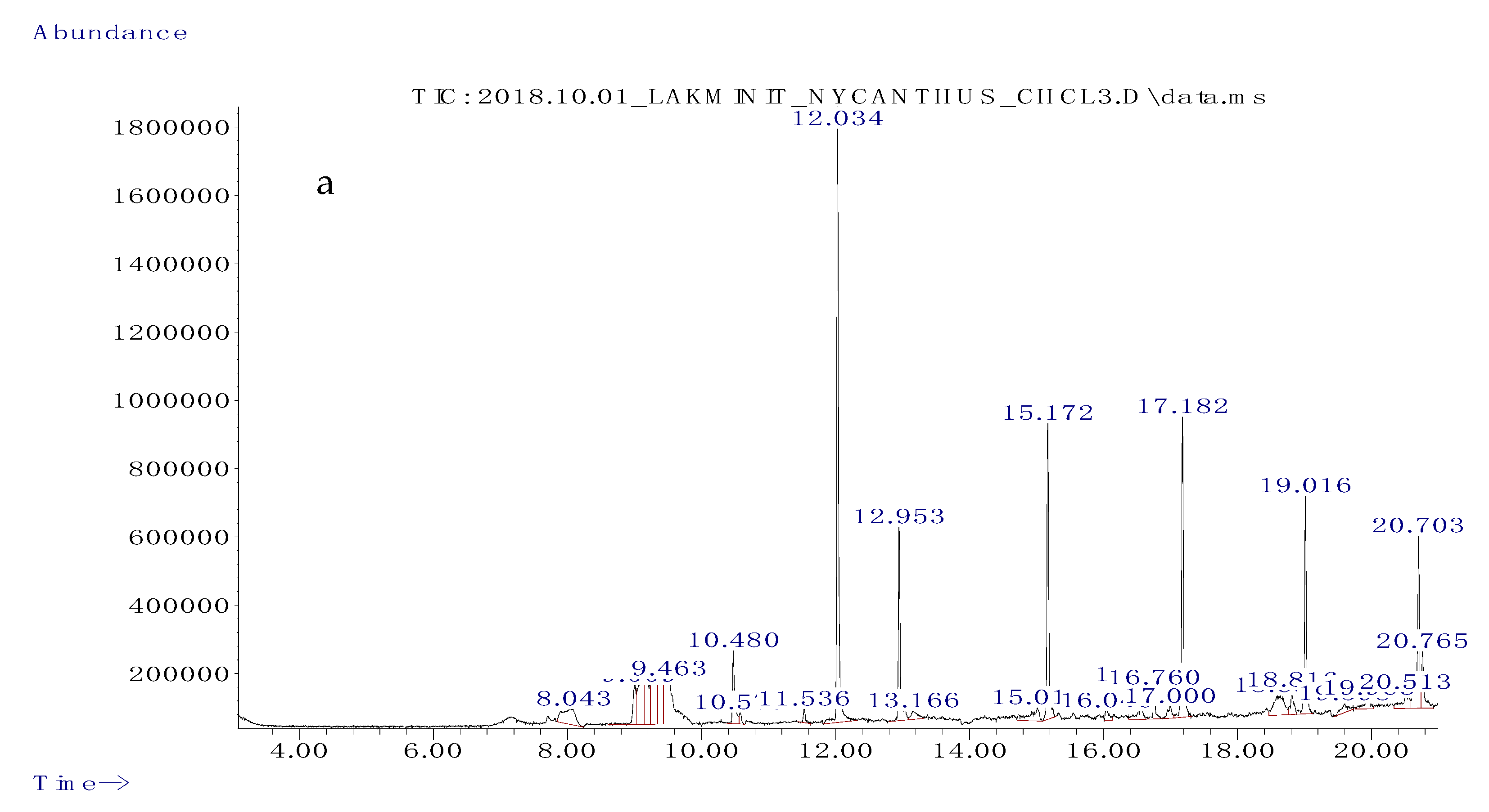
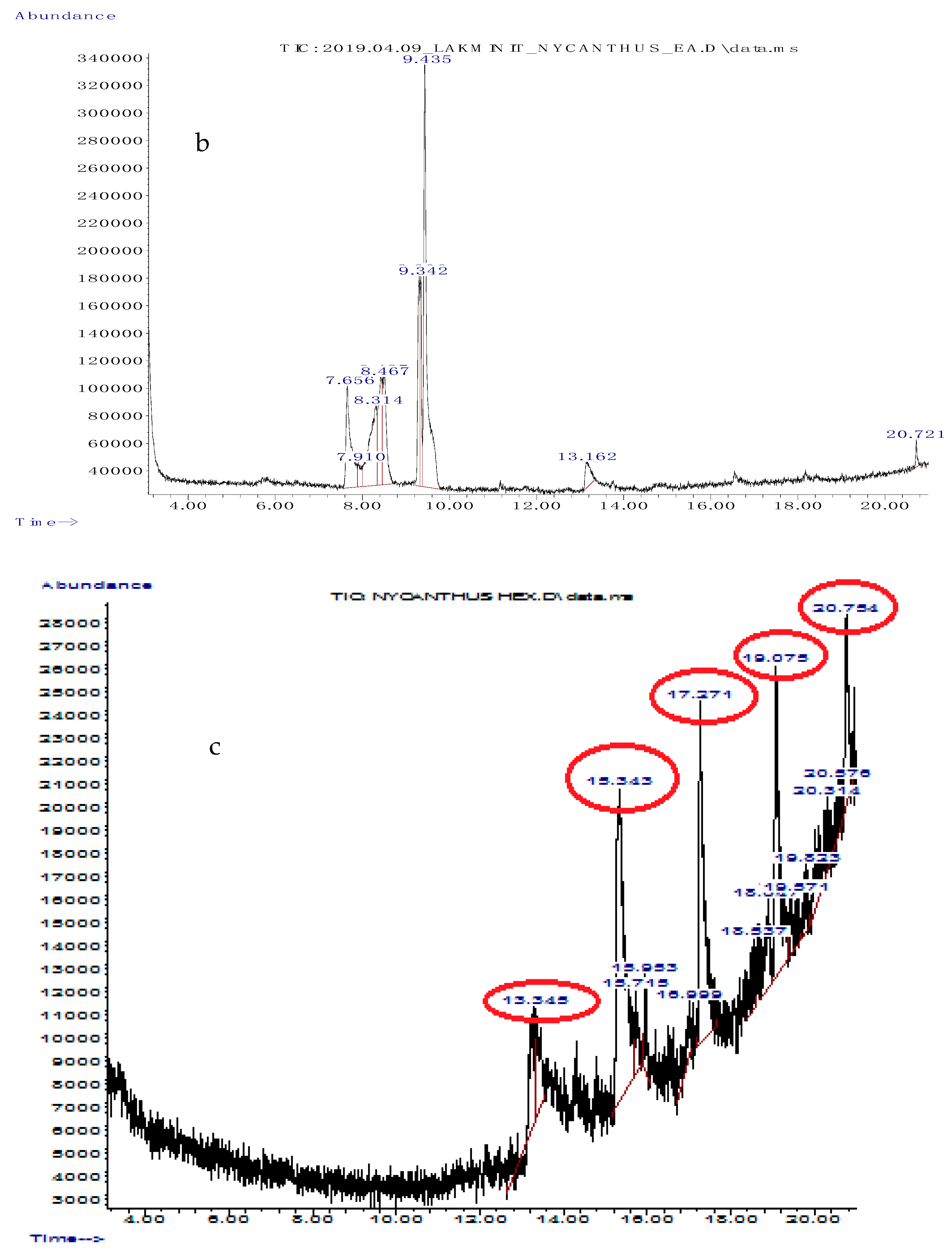
3.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis of Solvent Fractions
3.3. Alpha-Amylase Activity
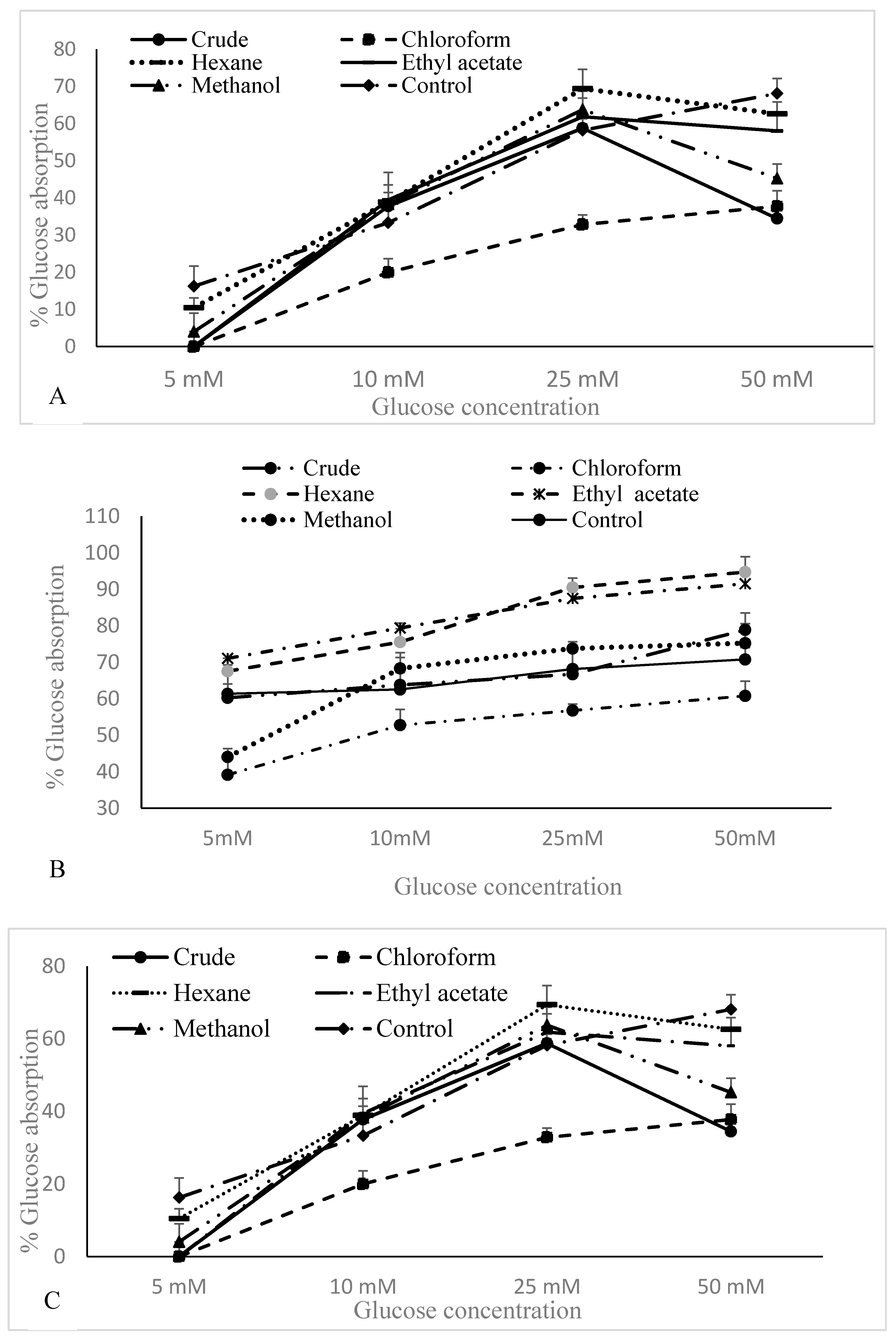
3.4. Glucose Uptake by Yeast Cells
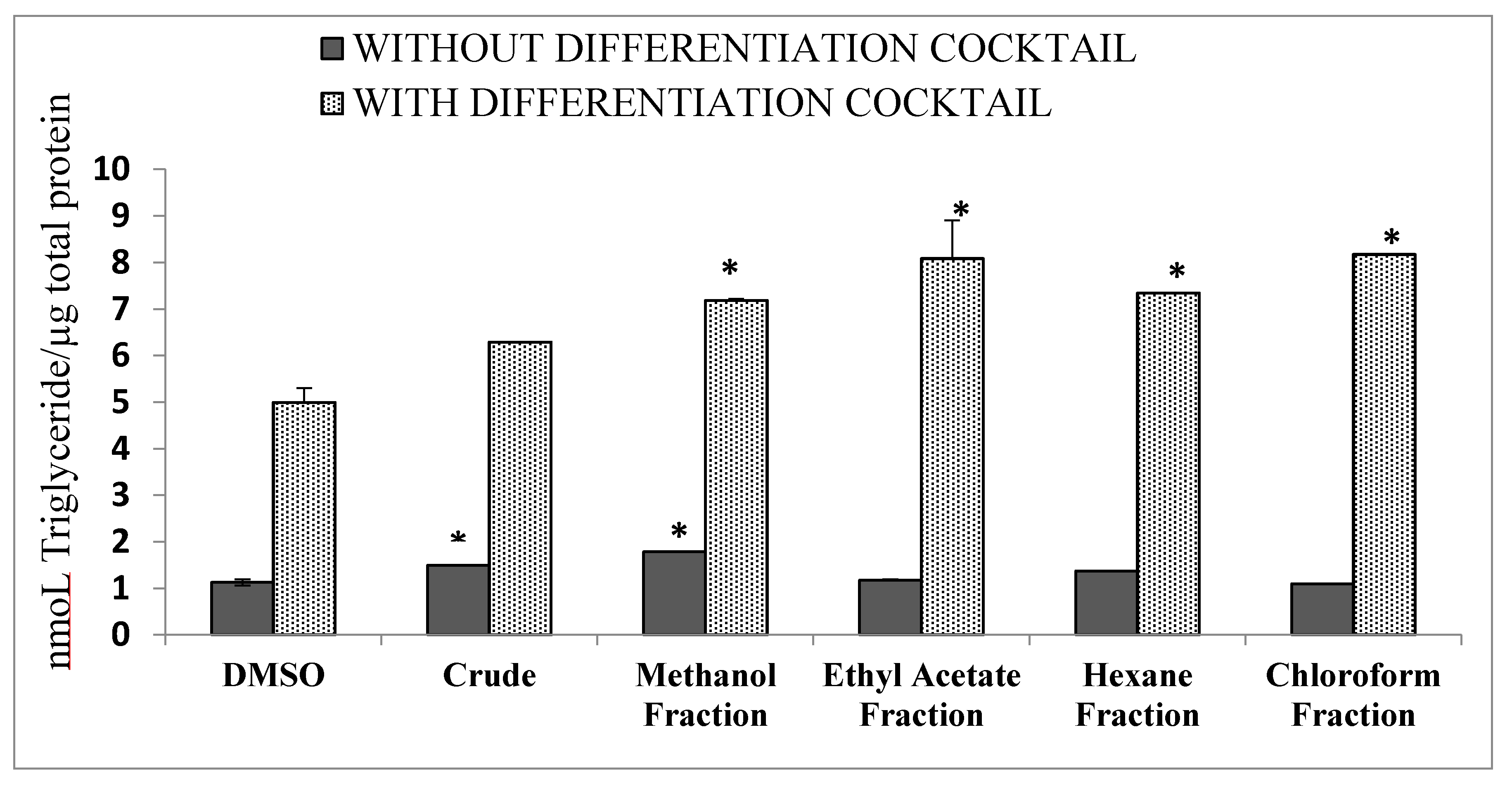
3.5. Differentiation of 3T3 Cells
3.6. Antiproliferative Activity
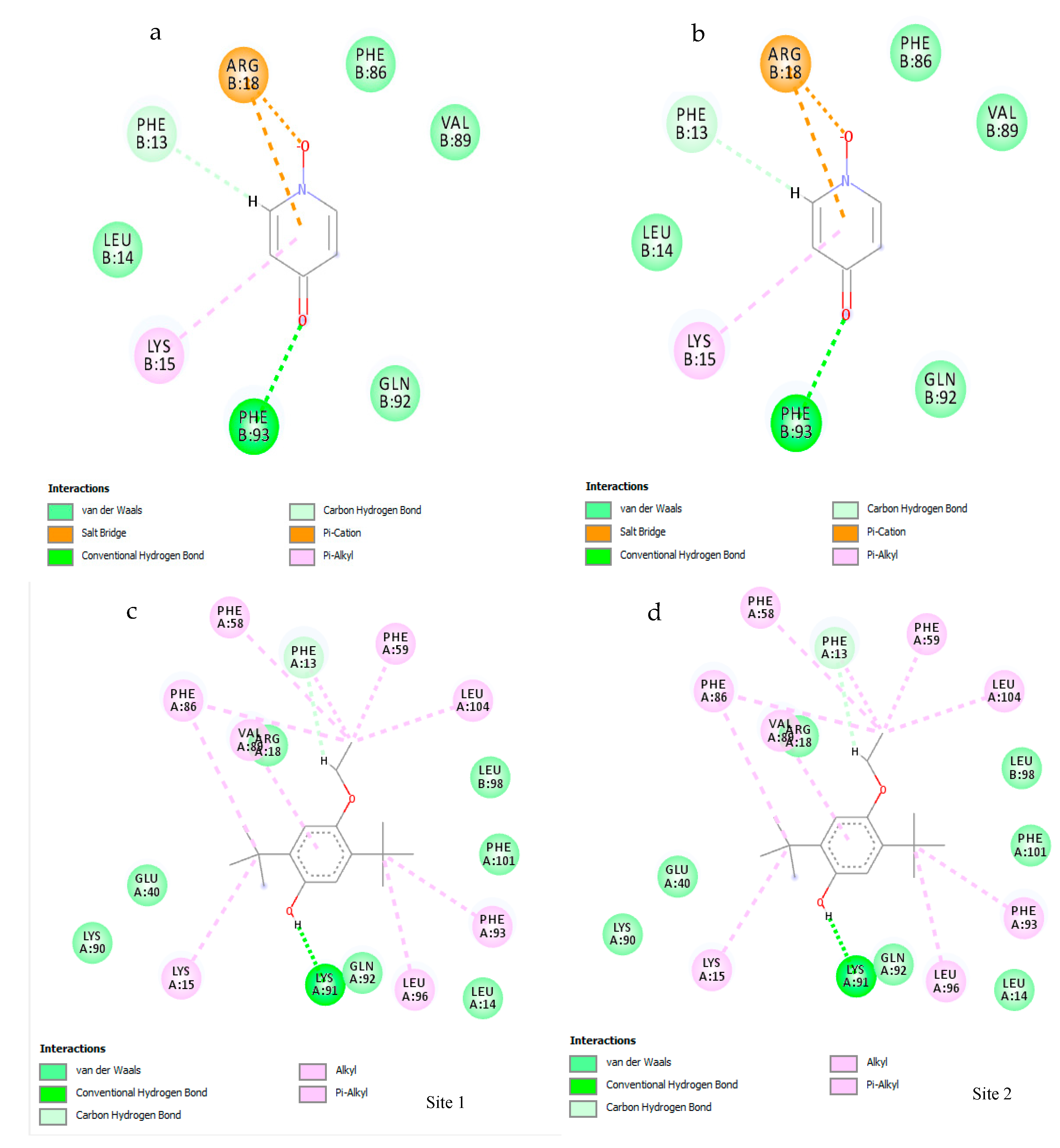
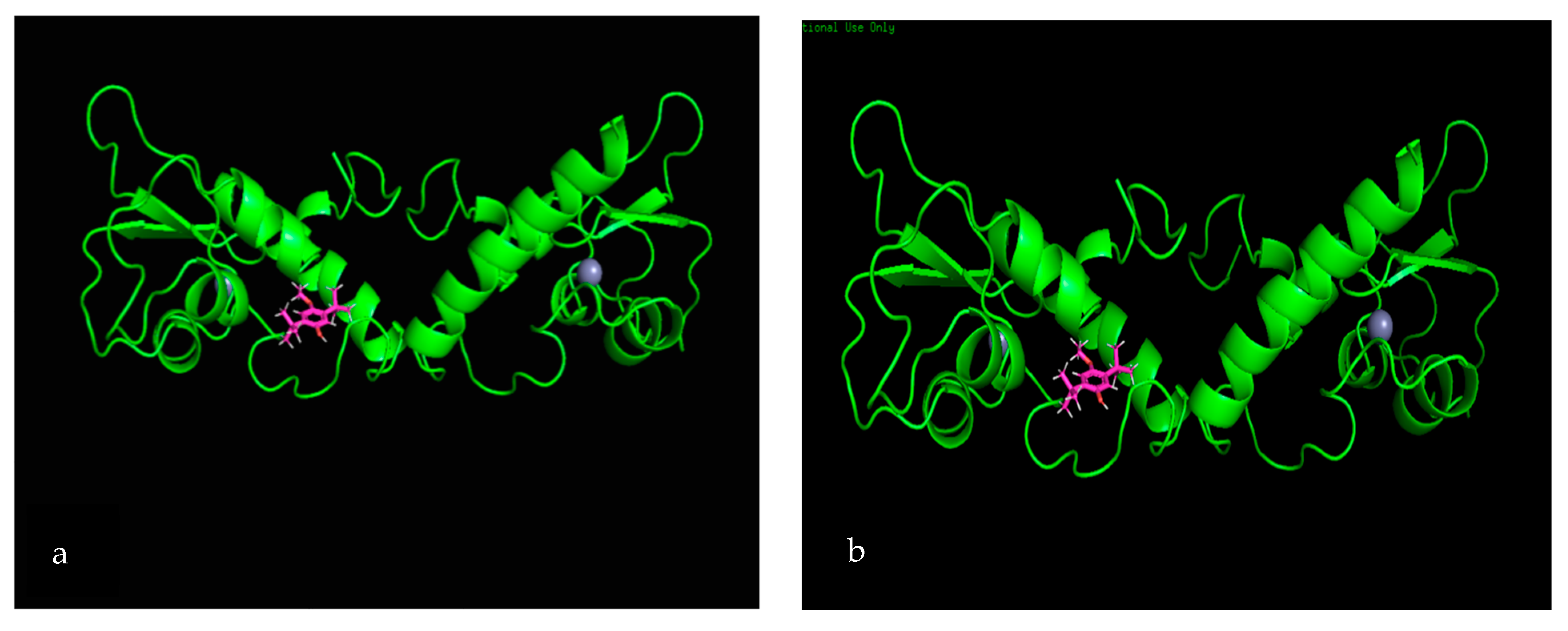
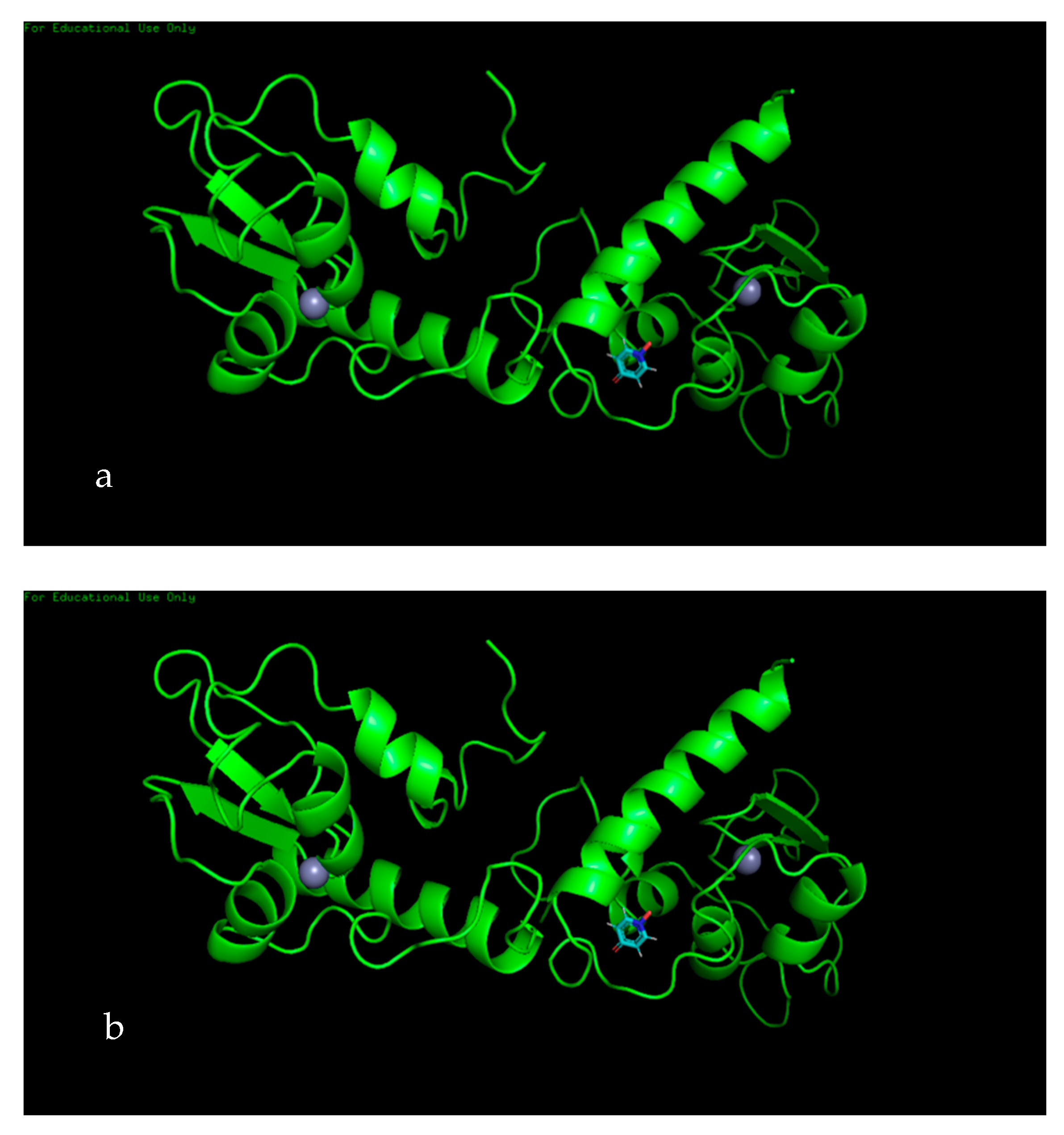
3.7. Molecular Docking
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Porter, P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N. Engl. J. Med. 2008, 358, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Tariq Numa, I.; Hassan, A.F.; Noori Hama, M. Cytotoxic Effect of Different Extracts of Euphorbia Lathyris Seeds on Peripheral Blood Mononuclear Cells in Leukemia. Int. J. Cancer Res. 2018, 14, 86–91. [Google Scholar]
- National Cancer Institute. SEER cancer statistics review. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 29 November 2019).
- National Cancer Control Programmes. Cancer incidence data, Sri Lanka year 2014. Available online: http://www.nccp.health.gov.lk/images/PDF_PUBLICATIONS/Cancer_Incidence_in_Sri_Lanka_2014.pdf (accessed on 29 November 2019).
- Zhao, Y.; Wang, Y.; Ma, S. Racial Differences in Four Leukemia Subtypes: Comprehensive Descriptive Epidemiology. Sci. Rep. 2018, 8, 548. [Google Scholar] [CrossRef]
- Wang, R.; Gao, X.; Yu, L. The prognostic impact of tet oncogene family member 2 mutations in patients with acute myeloid leukemia: A systematic-review and meta-analysis. BMC Cancer 2019, 19, 389. [Google Scholar] [CrossRef]
- Formiguera, X.; Cantón, A. Obesity: Epidemiology and clinical aspects. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 1125–1146. [Google Scholar] [CrossRef]
- Jenum, A.K.; Diep, L.M.; Holmboe-Ottesen, G.; Holme, I.M.K.; Kumar, B.N.; Birkeland, K.I. Diabetes susceptibility in ethnic minority groups from Turkey, Vietnam, Sri Lanka and Pakistan compared with Norwegians–The association with adiposity is strongest for ethnic minority women. BMC Public Health 2012, 12, 150. [Google Scholar] [CrossRef]
- Jayawardena, R.; Swaminathan, S.; Byrne, N.M.; Soares, M.J.; Katulanda, P.; Hills, A.P. Development of a food frequency questionnaire for Sri Lankan adults. Nutr. J. 2012, 11, 63. [Google Scholar] [CrossRef]
- Birari, R.; Javia, V.; Bhutani, K.K. Antiobesity and lipid lowering effects of Murraya koenigii (L.) Spreng leaves extracts and mahanimbine on high fat diet induced obese rats. Fitoterapia 2010, 81, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D.; Nielsen, L.L.; Waninger, A.; Kushner, P. Incretin mimetics and DPP-IV inhibitors: New paradigms for the treatment of type 2 diabetes. J. Am. Board Fam. Med. 2018, 19, 612–620. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S.A. Plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [PubMed]
- Chauhan, A.; Semwal, D.; Mishra, S.; Semwal, R. Ayurvedic research and methodology: Present status and future strategies. AYU (Int. Q. J. Res. Ayurveda) 2015, 36, 364. [Google Scholar]
- Bandara, K.R.V.; Padumadasa, C.; Peiris, D.C. Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves. PeerJ 2018, 6, e4804. [Google Scholar] [CrossRef]
- Rani, N.; Aichem, A.; Schmidtke, G.; Kreft, S.G.; Groettrup, M. FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat. Commun. 2012, 3, 749. [Google Scholar] [CrossRef]
- Verma, R.; Sahu, R.; Lalla, R. Subarachnoid haemorrhage as the initial manifestation of cortical venous thrombosis. Case Reports 2012, 2012, bcr2012006498. [Google Scholar] [CrossRef]
- Khanapur, M.; Avadhanula, R.K.; Setty, O.H. In vitro antioxidant, antiproliferative, and phytochemical study in different extracts of Nyctanthes arbortristis flowers. Biomed Res. Int. 2014, 2014, 291271. [Google Scholar] [CrossRef]
- Rangika, B.S.; Dayananda, P.D.; Peiris, D.C. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nycantus arbor-tristis L. in male mice. BMC Complement. Altern. Med. 2015, 15, 289. [Google Scholar] [CrossRef]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; dos Santos, R.; Oliva, G.; Andricopulo, A. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, H.C.; Samarakoon, K.W.; Lee, W.; Lee, J.-H.; Abeytunga, D.; Lee, H.-S.; Jeon, Y.-J. Anticancer and antioxidant effects of selected Sri Lankan marine algae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 315. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bhutkar, M.; Bhise, S. In vitro hypoglycemic effects of Albizzia lebbeck and Mucuna pruriens. Asian Pac. J. Trop. Biomed. 2013, 3, 866–870. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Turner, P.A.; Gurumurthy, B.; Bailey, J.L.; Elks, C.M.; Janorkar, A. V Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Process Biochem. 2017, 59, 312–320. [Google Scholar] [CrossRef]
- Hannah, R.; Beck, M.; Moravec, R.; Riss, T. CellTiter-GloTM luminescent cell viability assay: A sensitive and rapid method for determining cell viability. Promega Cell Notes 2001, 2, 11–13. [Google Scholar]
- Rivière, C.; Nguyen Thi Hong, V.; Tran Hong, Q.; Chataigné, G.; Nguyen Hoai, N.; Dejaegher, B.; Tistaert, C.; Nguyen Thi Kim, T.; Vander Heyden, Y.; Chau Van, M.; et al. Mallotus species from Vietnamese mountainous areas: Phytochemistry and pharmacological activities. Phytochem. Rev. 2010, 9, 217–253. [Google Scholar] [CrossRef]
- Mfuh, A.M.; Larionov, O.V. Heterocyclic N-Oxides—An Emerging Class of Therapeutic Agents. Curr. Med. Chem. 2015, 22, 2819–2857. [Google Scholar] [CrossRef]
- Myers, M.C.; Napper, A.D.; Motlekar, N.; Shah, P.P.; Chiu, C.-H.; Beavers, M.P.; Diamond, S.L.; Huryn, D.M.; Smith, A.B. Identification and characterization of 3-substituted pyrazolyl esters as alternate substrates for cathepsin B: The confounding effects of DTT and cysteine in biological assays. Bioorg. Med. Chem. Lett. 2007, 17, 4761–4766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melappa, G.; Prakash, B. In vitro antimitotic, antiproliferative and GC-MS studies on the methanolic extract of endophytic fungi, penicillium species of Tabebuia argentea bur & k. Sch. Farmacia 2017, 5, 301–309. [Google Scholar]
- Alagan, V.; Valsala, R.; Rajesh, K. Bioactive Chemical Constituent Analysis, in vitro Antioxidant and Antimicrobial Activity of Whole Plant Methanol Extracts of Ulva lactuca Linn. Br. J. Pharm. Res. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Belakhdar, G.; Benjouad, A.; Abdennebi, E.H. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015, 6, 2778–2783. [Google Scholar]
- Naragani, K.; Mangamuri, U.; Muvva, V.; Poda, S.; Munaganti, R.K. Antimicrobial Potential of Streptomyces Cheonanensis Vuk-A from Mangrove Origin. Int. J. Pharm. Pharm. Sci. 2016, 8, 53–57. [Google Scholar]
- Ben Hsouna, A.; Trigui, M.; Ben Mansour, R.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Das, M.; Himaja, M. Phytochemical screening, GC-MS analysis and biological activity of Ipomoea eriocarpa leaf extracts. Int. J. Pharm. Pharm. Sci. 2014, 6, 4–6. [Google Scholar]
- Olubunmi, A.; Gabriel, O.A.; Stephen, A.O.; Scott, F.O. Antioxidant and Antimicrobial Activity of Cuticular Wax from Kigelia Africana. FABD J. Pharm. Sci. 2009, 34, 187–194. [Google Scholar]
- Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Azevedo, R.A.; Rabaça, A.N.; Farias, C.F.; Pereira, F.V.; Matias, N.S.; Silva, L.P.; et al. Antitumor activity of Kielmeyera coriacea leaf constituents in experimental melanoma, tested in vitro and in vivo in syngeneic mice. Adv. Pharm. Bull. 2014, 4, 429–436. [Google Scholar]
- Govindappa, M.; Cp, C.; Ts, S. In Vitro Antidiabetic Activity of Three Fractions of Methanol Extracts of Loranthus Micranthus, Identification of Phytoconstituents by GC-MS and Possible Mechanism Identified by GEMDOCK Method. Asian J. Biomed. Pharm. Sci. 2014, 4, 34–41. [Google Scholar]
- Mehta, S.; Atpathy, A.; Gupta, R.K. Evaluation of nutritional, phytochemical, antioxidant and antibacterial activity of dried plum (Prunus domestica). J. Pharmaconosy Phytochem. 2014, 3, 166–171. [Google Scholar]
- Eleazu, C.O. Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharm. Biol. 2016, 54, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, N.M.; Mohamed Nor, Z.; Mansor, M.; Azhar, F.; Hasan, M.S.; Kassim, M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement. Altern. Med. 2015, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, C.; Gutiérrez-Salmerón, M.; Chocarro-Calvo, A.; García-Martinez, J.M.; Castaño, A.; De la Vieja, A. From obesity to diabetes and cancer: Epidemiological links and role of therapies. Br. J. Cancer 2016, 114, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.K. The diabetes-cancer link. Diabetes Spectr. 2014, 27, 276–280. [Google Scholar] [CrossRef]
- Probst, Y.C.; Guan, V.X.; Kent, K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open 2017, 7, e013337. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2007, 52, 149–153. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002, 2, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Urooj, A. In vitro studies on the hypoglycemic potential of Ficus racemosa stem bark. J. Sci. Food Agric. 2010, 90, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Abirami, N.; Natarajan, B.; Sagadevan, E. Phytochemical investigation and in vitro evaluation of hypoglycemic potential of Grewia hirsuta. Int. J. Pharma Bio Sci. 2014, 5, 76–83. [Google Scholar]
- Pitchaipillai, R.; Ponniah, T. In Vitro Antidiabetic Activity of Ethanolic Leaf Extract of Bruguiera Cylindrica L.—Glucose Uptake by Yeast Cells Method. Int. Biol. Biomed. J. 2016, 2, 171–175. [Google Scholar]
- Sudasinghe, H.P.; Peiris, D.C. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. PeerJ 2018, 6, e4389. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, M.B.; Ajimire, P.V.; Wagh, A.E.; Mohan, M.; Shivashanmugam, A.T. In vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. Asian J. Plant Sci. Res. 2011, 1, 101–106. [Google Scholar]
- Krentz, A.J. Lipoprotein abnormalities and their consequences for patients with Type 2 diabetes. Diabetes Obes. Metab. 2003, 5, s19–s27. [Google Scholar] [CrossRef]
- Juárez-Rojop, I.E.; Díaz-Zagoya, J.C.; Ble-Castillo, J.L.; Miranda-Osorio, P.H.; Castell-Rodríguez, A.E.; Tovilla-Zárate, C.A.; Rodríguez-Hernández, A.; Aguilar-Mariscal, H.; Ramón-Frías, T.; Bermúdez-Ocaña, D.Y. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 236. [Google Scholar] [CrossRef]
- Lee, J.; Kim, A.-R.; Lee, J.-J. Ramie Leaf Extracts Suppresses Adipogenic Differentiation in 3T3-L1 Cells and Pig Preadipocytes. Asian-Australasian J. Anim. Sci. 2016, 29, 1338–1344. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Xiao, X.-R.; Xu, K.-P.; Li, F. Metabolic profiling of the anti-tumor drug regorafenib in mice. J. Pharm. Biomed. Anal. 2018, 159, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006, 30, 400–418. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [PubMed]
- Quispe, P.A.; Lavecchia, M.J.; León, I.E. On the discovery of a potential survivin inhibitor combining computational tools and cytotoxicity studies. Heliyon 2019, 5, e02238. [Google Scholar] [CrossRef]
| Aqueous Extract and Its Fractions | Total Phenolic Content | Total Flavonoid Content |
|---|---|---|
| mg GAE/g | mg QE/g | |
| Aqueous | 160 ± 0.06 | 600 ± 1.25 |
| Chloroform | 351 ± 0.0004 | 120 ± 0.93 |
| Hexane | 180 ± 0.0003 | 24 ± 0.06 |
| Ethyl acetate | 285 ± 0.001 | 30 ± 0.03 |
| Methanol | 450 ± 0.014 | 528 ± 2.01 |
| Retention Time | Abundance (%) | Name | Molecular Formula | Compound Class | Reported Activity |
|---|---|---|---|---|---|
| 9.094 | 4.261 | 4-hydroxypyridine-1-oxide | C5H5NO2 | Pyridine derivative | Anticancer [32] |
| 9.307 | 4.055 | 2-aminopyrimidine-1-oxide | C4H4BrN3O | Heterocyclic | Antimicrobial [33] |
| 9.387 | 3.430 | 2-thiophenecarbonyl chloride | C5H3ClOS | Hydrazonoyl chloride | Anticancer [34] |
| 9.463 | 7.199 | 5-ethylcyclopent-1-ene-1-carboxylic acid | C8H12O2 | Organic acid | Not reported |
| 12.034 | 17.849 | phenol, 2,5-bis(1,1-dimethyl ethyl) | C14H22O | Phenolic compound | Anticancer [35] |
| 12.953 | 5.836 | 7-hexadecene | C16H32 | Alkene | Antioxidant, antimicrobial [36] |
| 15.172 | 8.088 | 1-octadecene | C18H36 | Alkene | Antibacterial, antioxidant, anticancer [37] |
| 17.182 | 8.372 | 1-eicosene | C20H40 | Alkene | Antimicrobial [38], anticancer [39] |
| 18.636 | 3.091 | hentriacontane | C31H64 | Alkane | Anti-inflammatory [40], anticancer [41] |
| 20.703 | 6.164 | 1-docosene | C29H60 | Alkene | Antibacterial [37], anticancer [42] |
| Retention Time | Abundance (%) | Compound Name | Molecular Formula | Compound Class | Reported Activity |
|---|---|---|---|---|---|
| 7.656 | 11.532 | cyclopentanecarboxamide, N-(2-fluorophenyl) | C12H14FNO | Heterocyclic compound | Not reported |
| 7.910 | 1.918 | 2-thiophenecarboxylic acid, 3,5- dimethylcyclohexyl ester | C13H18O2S | Organic acid | Not reported |
| 8.314 | 13.592 | 2,5-dimethylhexane-2,5-dihydroperoxide | C8H18O4 | Organic compound | Anti-inflammatory, antioxidant [43] |
| 8.467 | 8.925 | adipic acid-ethyl propargyl ester | C11H16O4 | Organic acid | Not reported |
| 9.342 | 7.337 | cyclopentanecarboxamide, N-(2-fluorophenyl) | C12H14FNO | Heterocyclic compound | Not reported |
| 9.435 | 32.655 | succinic acid (3,5-dimethylcyclohexy) ester | C20H34O4 | Organic acid | Not reported |
| 13.162 | 3.071 | diethyl phthalate | C12H14O4 | Diester of phthalic acid | Antimicrobial [44] |
| 20.721 | 7.337 | hexanedioic acid, bis (2-ethylhexyl) ester | C22H42O4 | Organic acid | Antioxidant [45] |
| Retention Time | Abundance (%) | Name | Molecular Formula | Compound Class | Reported Activity |
|---|---|---|---|---|---|
| 13.345 | 4.950 | 2-chloropropionic acid, octadecyl ester | C21H41ClO2 | Organic acid | Antimicrobial, antioxidant [44] |
| 15.343 | 25.685 | 1-octadecene | C18H36 | Alkene | Antibacterial, antioxidant, anticancer [37] |
| 17.271 | 17.759 | pentafluoropropionic acid, hexadecyl ester | C19H33F5O2 | Organic acid | Not reported |
| 19.075 | 14.333 | 1-heptacosanol | C27H56O | Fatty alcohol | Antimicrobial, antioxidant, anticancer [46] |
| 20.754 | 7.135 | pentafluoropropionic acid, hexadecyl ester | C19H33F5O2 | Organic acid | Not reported |
| Extract/Fraction | IC50 (mg/mL) |
|---|---|
| Crude aqueous extract | 2.223 ± 0.02 |
| Chloroform fraction | 12.68 ± 0.09 |
| Hexane fraction | 0.665 ± 0.01 ** |
| Ethyl acetate fraction | 0.718 ± 0.01 ** |
| Methanol fraction | 1.504 ± 0.34 |
| Acarbose (standard) | 2.45 ± 0.23 |
| IC50 (Inhibition of killing) µg/mL | Extract/Fraction | Jurkat Cells | MCF7 | PBMC-AML | PBMC-CLL | Control PBMC |
| Crude aqueous | 987 | 1664 | 1262 | 90.1 | 1588 | |
| Methanol | 733 | 1016 | 1986 | 138 | 6654 | |
| Ethyl acetate | 70500 | 1493 | 2994 | 60.3 | 6179 | |
| Hexane | 1174 | 717.6 | 9710 | 273 | 2520 | |
| Chloroform | 1394 | 38,210 | 6169 | 98.6 | 8894 | |
| Doxorubicin (standard) | 8.19 × 10−2 | 4.62 | 3.39 | 6.08 × 10−2 | 9.00 × 10−2 |
| Extract/Fraction | PBMC-AML (SIA) | PBMC-CLL (SIB) |
|---|---|---|
| Crude aqueous extract | 1.26 | 17.6 |
| Methanol fraction | 3.35 | 48.22 |
| Ethyl acetate fraction | 2.06 | 102.5 |
| Hexane fraction | 0.26 | 9.23 |
| Chloroform fraction | 1.44 | 98.20 |
| Doxorubicin (standard drug) | 0.03 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules 2020, 10, 165. https://doi.org/10.3390/biom10020165
Heendeniya SN, Keerthirathna LR, Manawadu CK, Dissanayake IH, Ali R, Mashhour A, Alzahrani H, Godakumbura P, Boudjelal M, Peiris DC. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules. 2020; 10(2):165. https://doi.org/10.3390/biom10020165
Chicago/Turabian StyleHeendeniya, Saumya Nishanga, Lakshika. Rangi Keerthirathna, Chamalika Kanthini Manawadu, Indeewarie Hemamali Dissanayake, Rizwan Ali, Abdullah Mashhour, Hajar Alzahrani, Pahan Godakumbura, Mohamed Boudjelal, and Dinithi Champika Peiris. 2020. "Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites" Biomolecules 10, no. 2: 165. https://doi.org/10.3390/biom10020165
APA StyleHeendeniya, S. N., Keerthirathna, L. R., Manawadu, C. K., Dissanayake, I. H., Ali, R., Mashhour, A., Alzahrani, H., Godakumbura, P., Boudjelal, M., & Peiris, D. C. (2020). Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Metabolites. Biomolecules, 10(2), 165. https://doi.org/10.3390/biom10020165








