Diosgenin Loaded Polymeric Nanoparticles with Potential Anticancer Efficacy
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Polymer and Nanoparticles Synthesis
2.3. In Silico Optimization of Parameters Using Box-Behnken Design (BBD)
2.4. Physicochemical Characterisation
2.5. Drug Loading and Encapsulation Efficiency
2.6. In Vitro Drug Release Kinetics
2.7. Apoptosis Analysis through DAPI Staining
2.8. Acridine Orange/Ethidium Bromide Staining for Apoptotic Analysis
2.9. Cytotoxicity Assay on A549 Cells
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Diosgenin Loaded Polymeric Nanoparticles
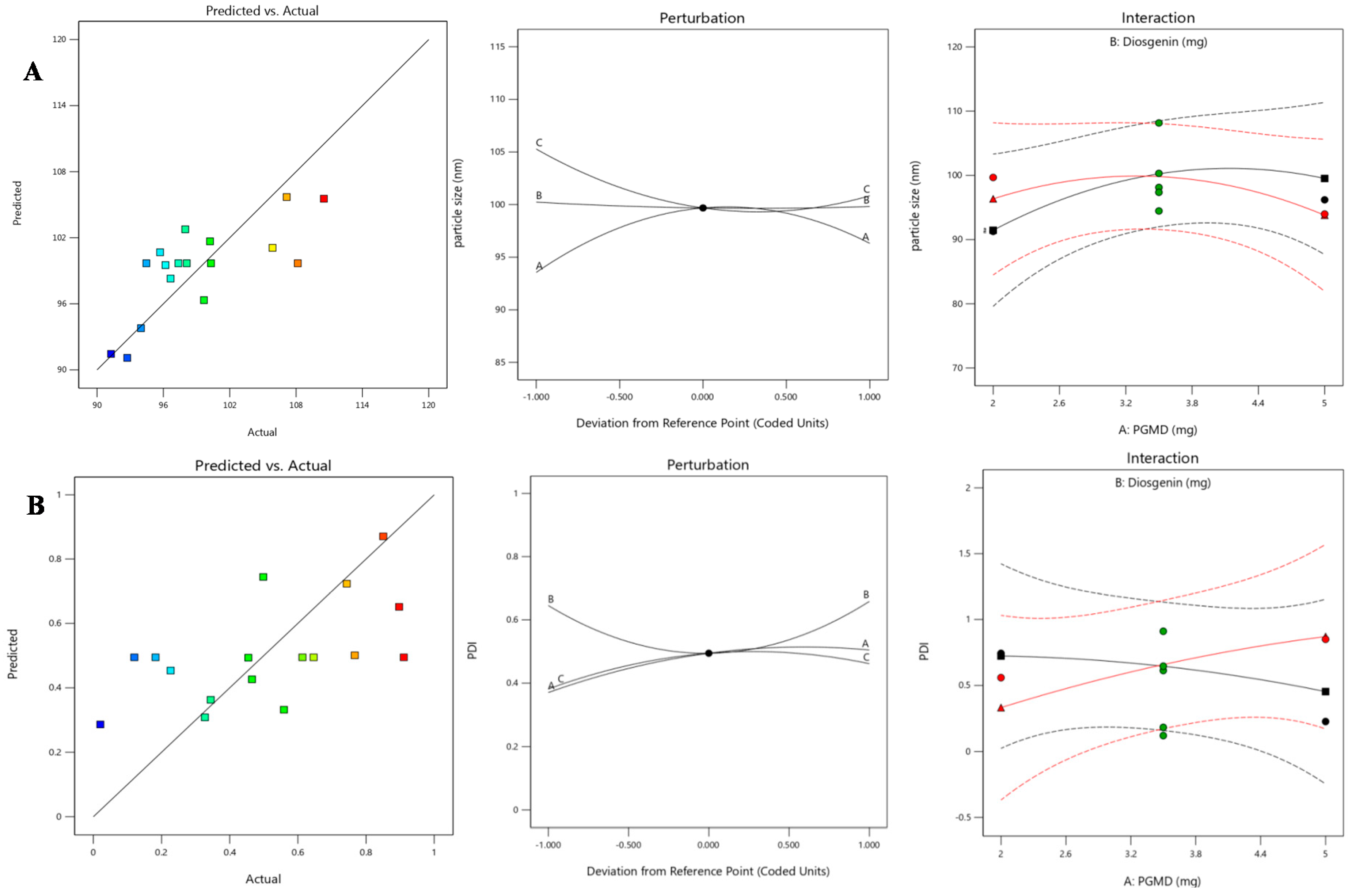
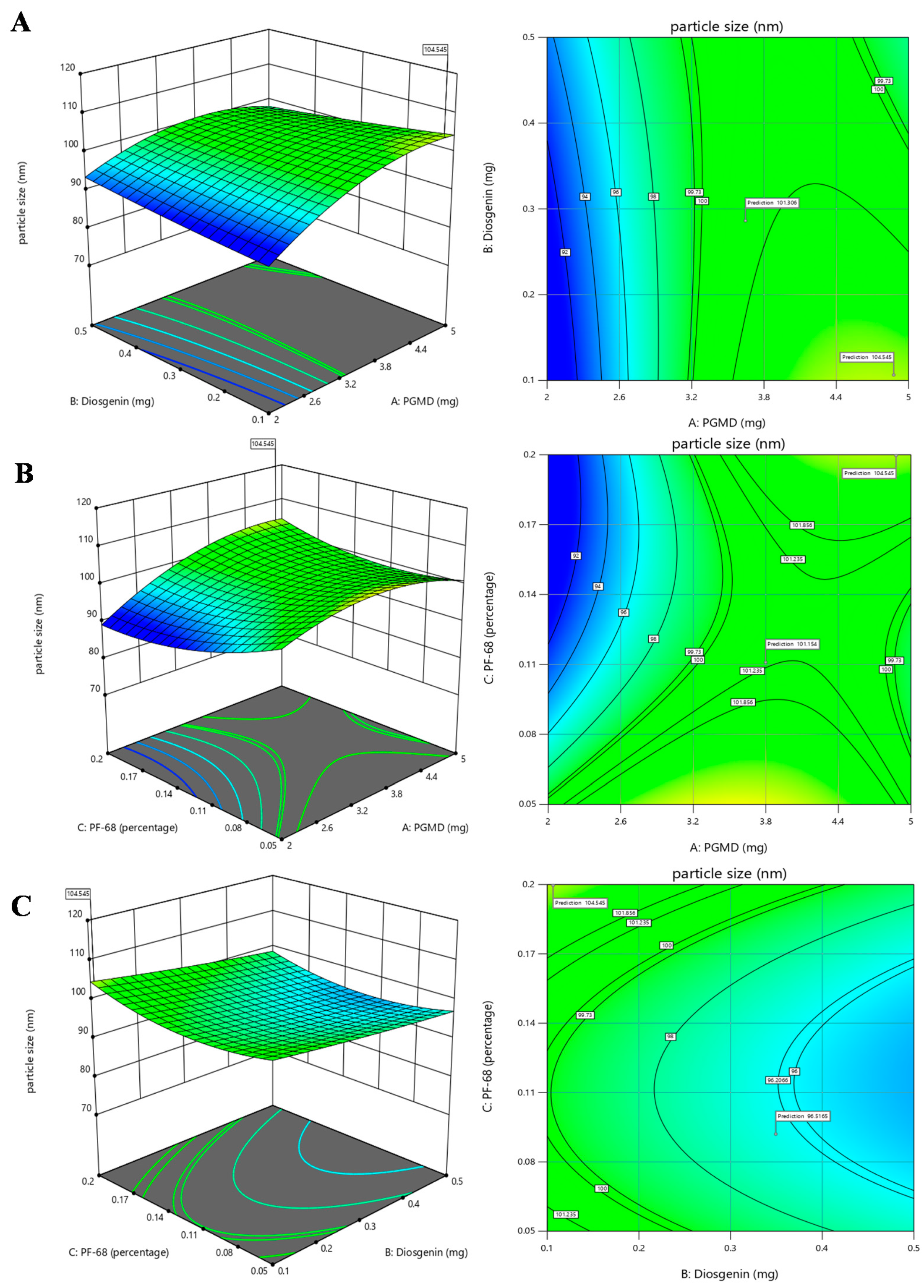
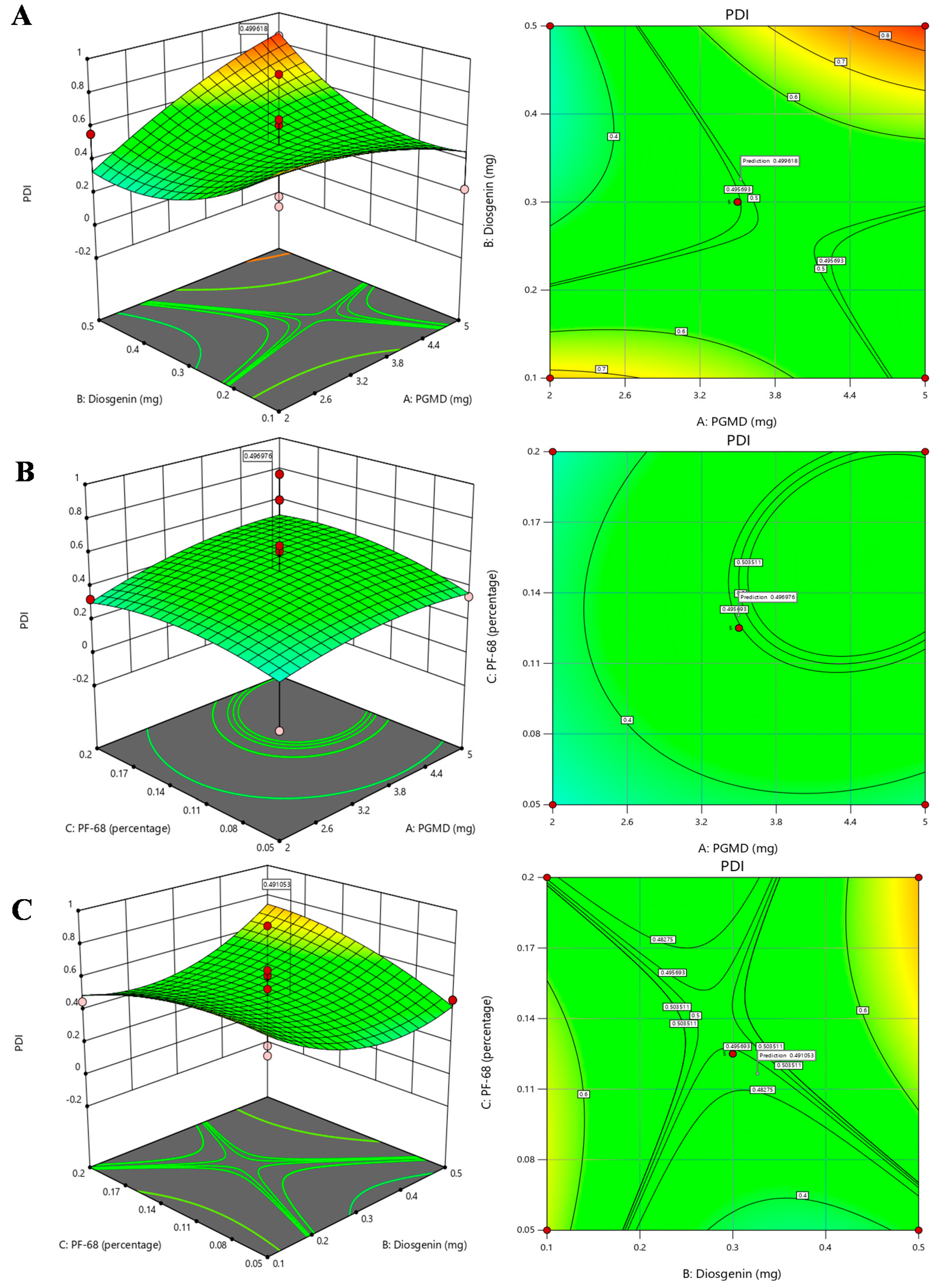
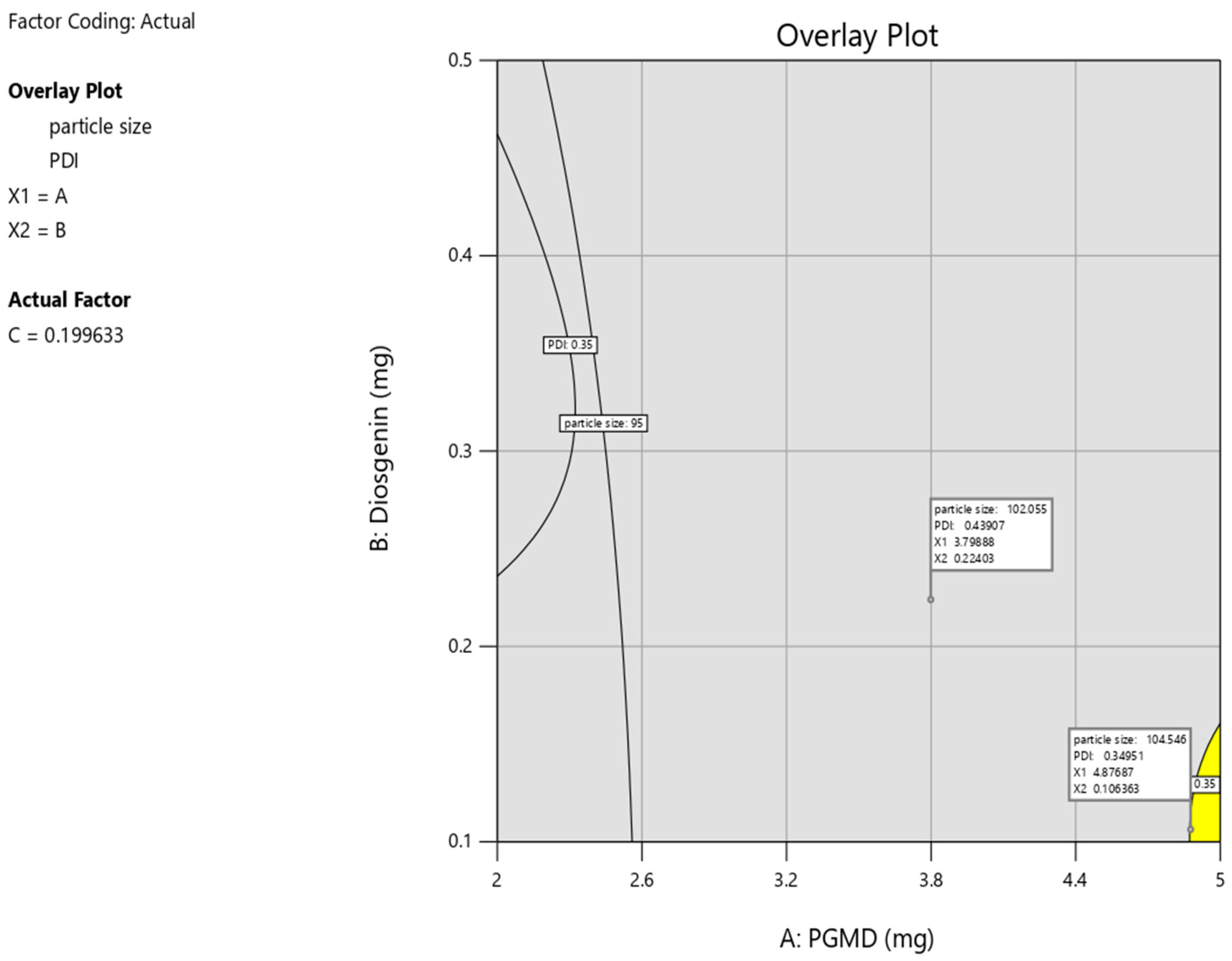
3.2. Response Surface Method by Box-Behnken Design
3.3. Investigation for Optimized Polymeric Nanoparticles
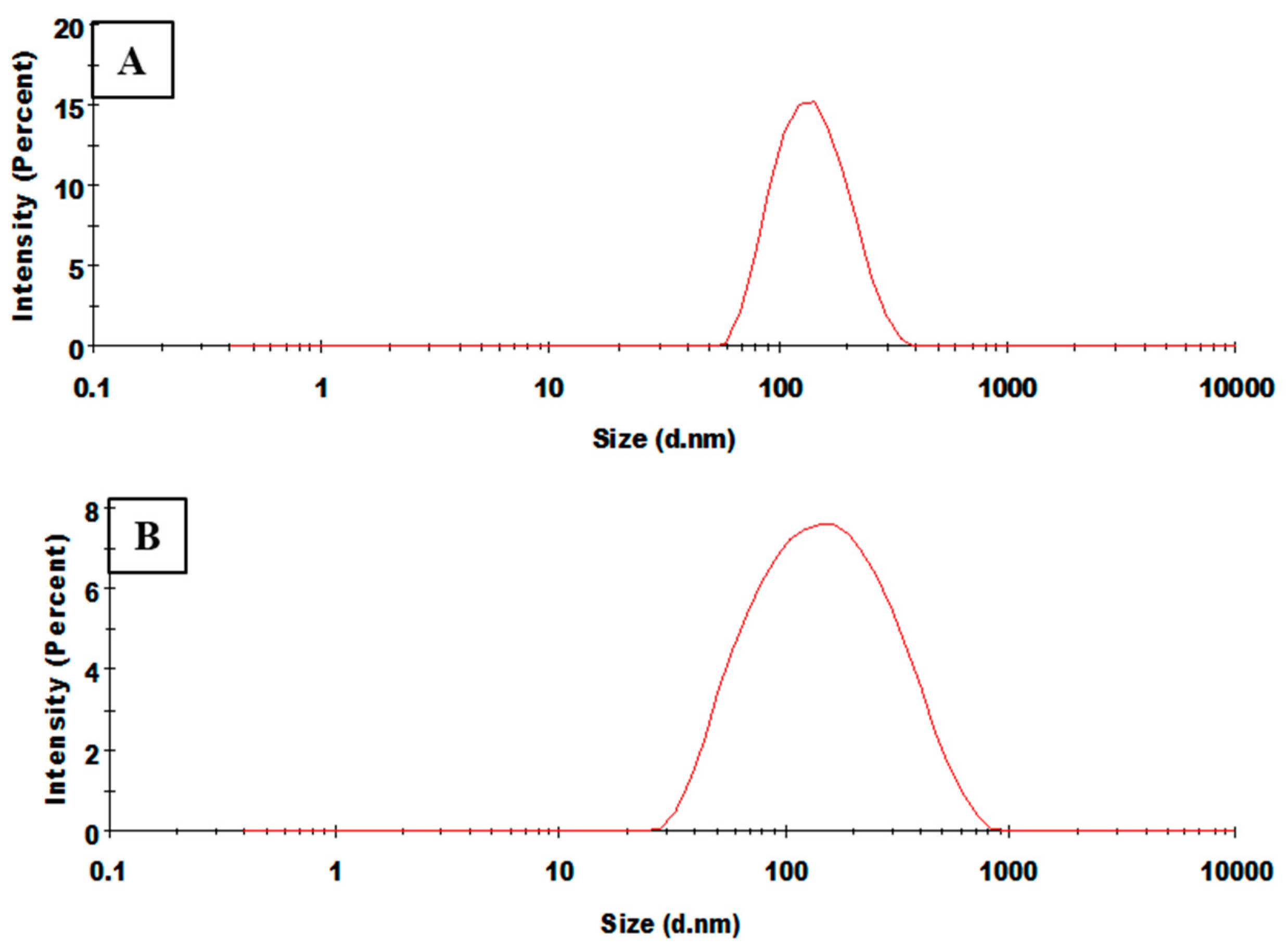
3.4. Physicochemical Characterisation of Nanoparticles
3.5. Percentage Drug Loading and Encapsulation Efficiency
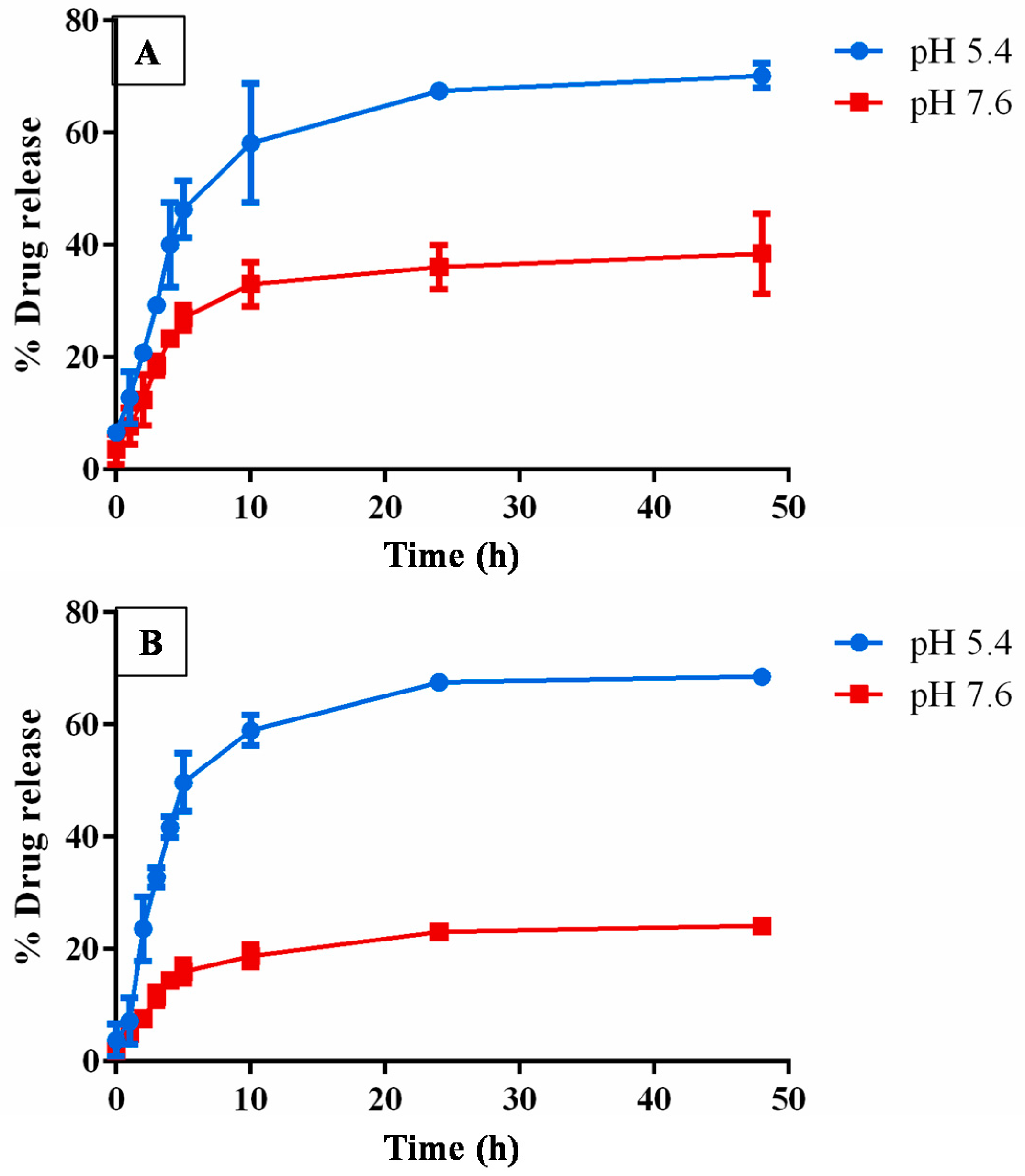
3.6. In Vitro Drug Release Analysis
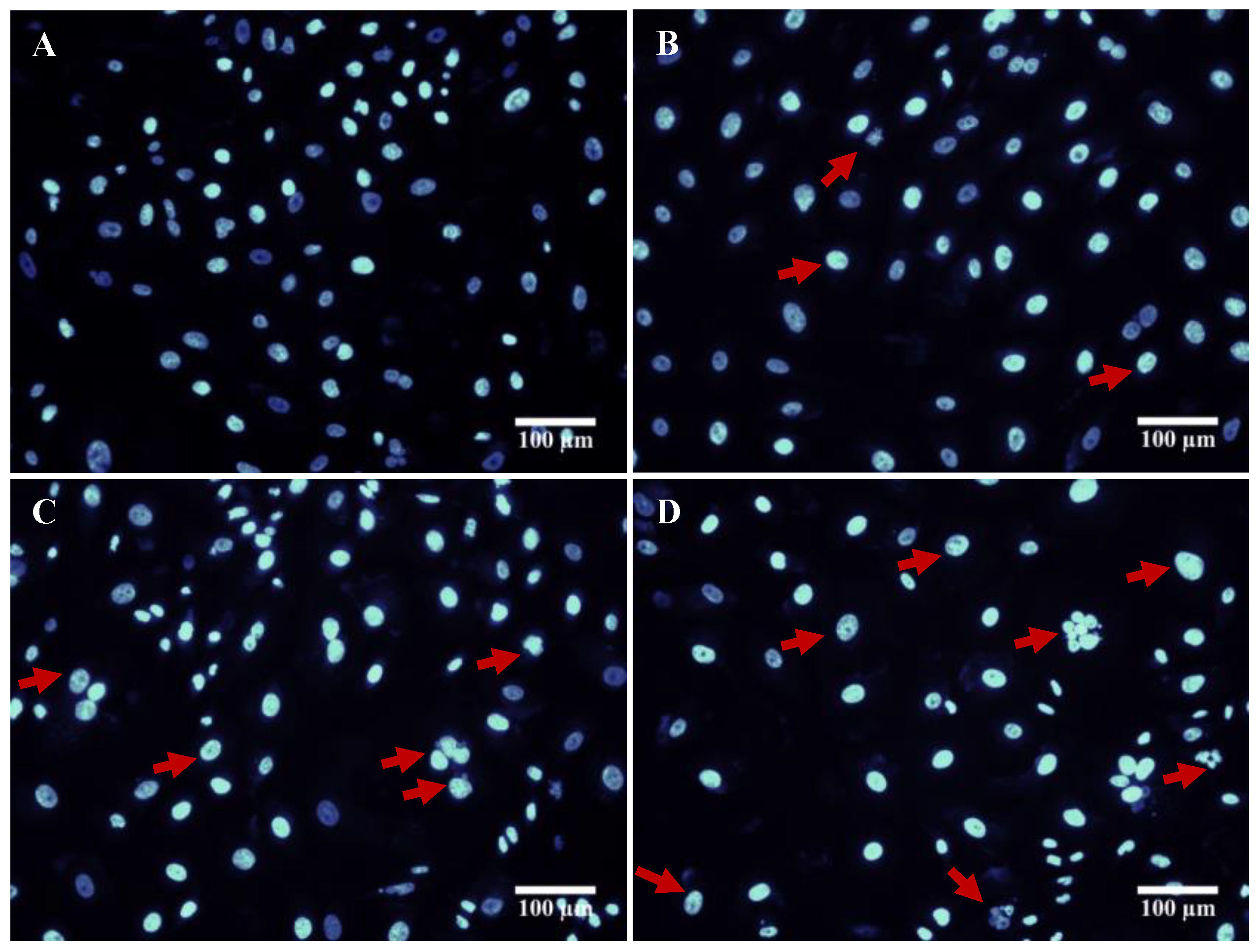
3.7. DAPI Staining
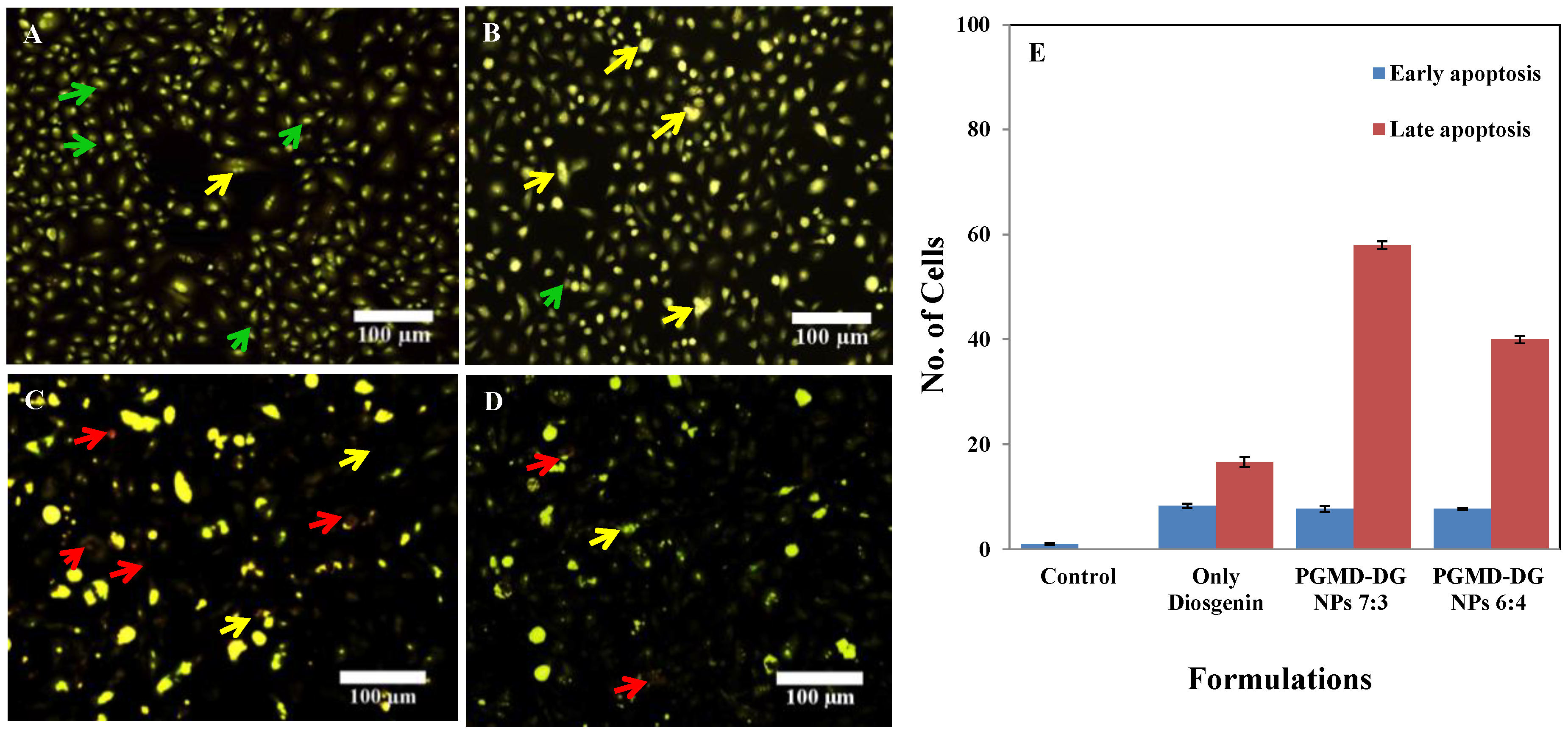
3.8. Acridine Orange/Ethidium Bromide Staining
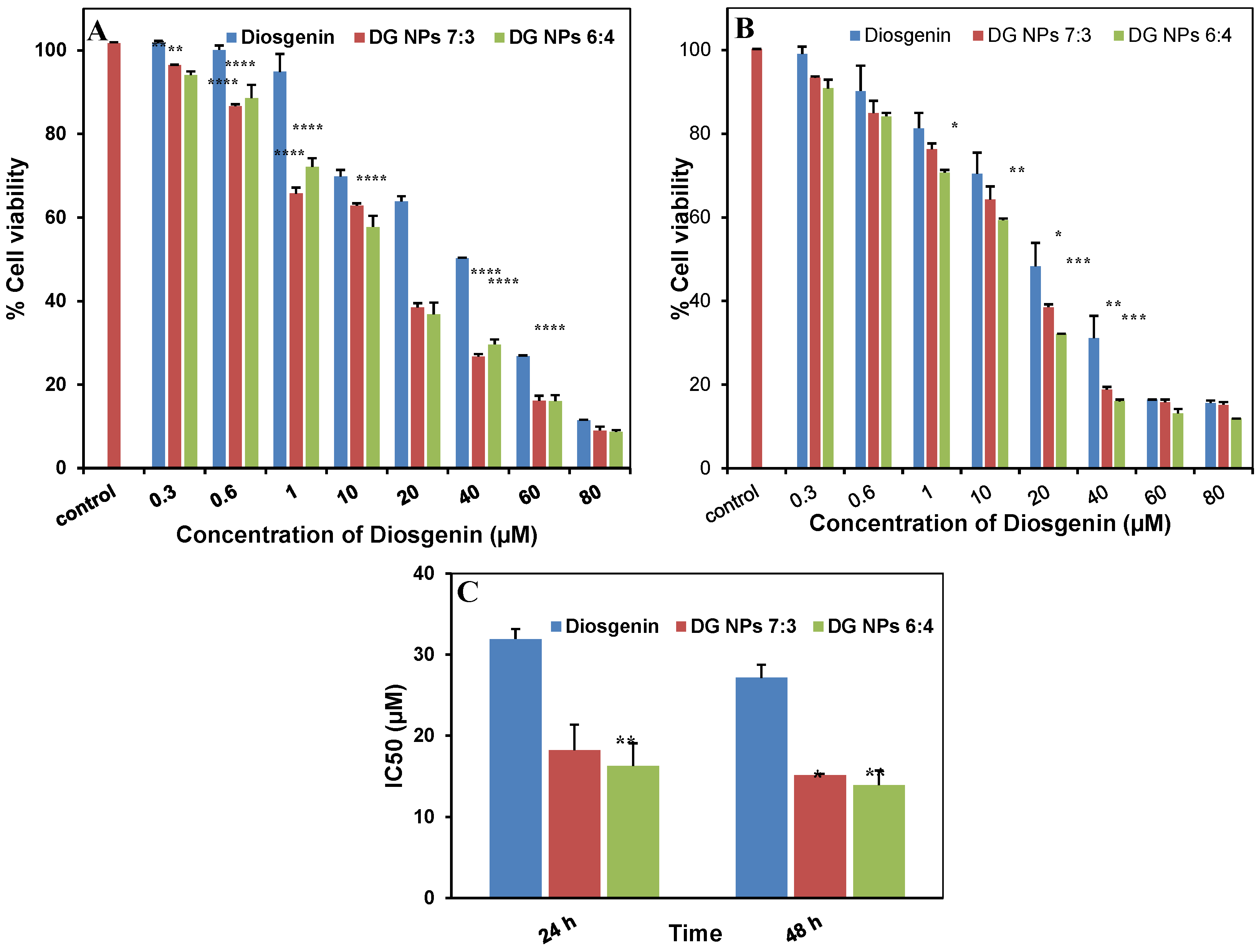
3.9. Cytotoxicity Studies
3.10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint; Cancer Drug Resistance; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–18. [Google Scholar]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Mazumder, A.; Cerella, C.; Diederich, M. Natural scaffolds in anticancer therapy and precision medicine. Biotechnol. Adv. 2018, 36, 1563–1585. [Google Scholar] [CrossRef]
- Selim, S.; Al Jaouni, S. Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complementary Altern. Med. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Parvaz, N.; Barani, M.; Khoshdel, A.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. Diosgenin-loaded niosome as an effective phytochemical nanocarrier: Physicochemical characterization, loading efficiency, and cytotoxicity assay. DARU J. Pharm. Sci. 2019, 27, 329–339. [Google Scholar] [CrossRef]
- Nie, C.; Zhou, J.; Qin, X.; Shi, X.; Zeng, Q.; Liu, J.; Yan, S.; Zhang, L. Diosgenininduced autophagy and apoptosis in a human prostate cancer cell line. Mol. Med. Rep. 2016, 14, 4349–4359. [Google Scholar] [CrossRef]
- He, Z.; Chen, H.; Li, G.; Zhu, H.; Gao, Y.; Zhang, L.; Sun, J. Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 871–876. [Google Scholar] [CrossRef]
- Raju, J.; Rao, C.V. Diosgenin, a steroid saponin constituent of yams and fenugreek: Emerging evidence for applications in medicine. Bioact. Compd. Phytomed. 2012, 125, 143. [Google Scholar]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Silvestre, S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.M.; Zhou, P.; Li, S.Y.; Zhang, X.Y.; Shen, J.X.; Chen, Q.X.; Zhuang, J.X.; Shen, D.Y. Diosgenin Suppresses Cholangiocarcinoma Cells Via Inducing Cell Cycle Arrest And Mitochondria-Mediated Apoptosis. OncoTargets Ther. 2019, 12, 9093–9104. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Kydd, J.; Piel, B.; Rai, P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int. J. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Lei, T.; Manchanda, R.; Fernandez-Fernandez, A.; Huang, Y.-C.; Wright, D.; McGoron, A.J. Thermal and pH sensitive multifunctional polymer nanoparticles for cancer imaging and therapy. RSC Adv. 2014, 4, 17959–17968. [Google Scholar] [CrossRef]
- Migneco, F.; Huang, Y.-C.; Birla, R.K.; Hollister, S.J. Poly (glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials 2009, 30, 6479–6484. [Google Scholar] [CrossRef]
- Shet, M.S.; Fisher, C.W.; Holmans, P.L.; Estabrook, R.W. The Omega-Hydroxylation of Lauric Acid: Oxidation of 12-Hydroxylauric Acid to Dodecanedioic Acid by a Purified Recombinant Fusion Protein Containing P450 4A1 and NADPH–P450 Reductase. Arch. Biochem. Biophys. 1996, 330, 199–208. [Google Scholar] [CrossRef]
- Lei, T.; Manchanda, R.; Huang, Y.-C.; Fernandez-Fernandez, A.; Bunetska, K.; Milera, A.; Sarmiento, A.; McGoron, A.J. Near-infrared imaging loaded polymeric nanoparticles: In vitro and in vivo studies. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications V, San Francisco, CA, USA, 4–6 February 2013; International Society for Optics and Photonics: Bellingham, DC, USA, 2013; Volume 8596. [Google Scholar] [CrossRef]
- Lei, T.; Fernandez-Fernandez, A.; Manchanda, R.; Huang, Y.-C.; McGoron, A.J. Near-infrared dye loaded polymeric nanoparticles for cancer imaging and therapy and cellular response after laser-induced heating. Beilstein J. Nanotechnol. 2014, 5, 313–322. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Yildiz, U. Diosgenin-conjugated PCL–MPEG polymeric nanoparticles for the co-delivery of anticancer drugs: Design, optimization, in vitro drug release and evaluation of anticancer activity. New J. Chem. 2019, 43, 6622–6635. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, H.; Xin, G.; Zeng, Z.; Li, S.; Ming, Y.; Zhang, X.; Xing, Z.; Li, L.; Li, Y.; et al. A pH-Sensitive Prodrug Nanocarrier Based on Diosgenin for Doxorubicin Delivery to Efficiently Inhibit Tumor Metastasis. Int. J. Nanomed. 2020, 15, 6545. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot4493. [Google Scholar] [CrossRef]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly (glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Hoa, L.T.M.; Chi, N.T.; Nguyen, L.H.; Chien, D.M. Preparation and characterisation of nanoparticles containing ketoprofen and acrylic polymers prepared by emulsion solvent evaporation method. J. Exp. Nanoscience. 2012, 7, 189–197. [Google Scholar] [CrossRef]
- Ansari, M. Factors affecting preparation and properties of nanoparticles by nanoprecipitation method. Indo Am. J. Pharm. Sci. 2017, 4, 4854–4858. [Google Scholar]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Kiafar, F.; Jelvehgari, M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res. Pharm. Sci. 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Hu, S.-H. Functional nanoparticles for tumor penetration of therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr 2 O 3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef]
- Bodmeier, R.; McGinity, J.W. The preparation and evaluation of drug-containing poly (dl-lactide) microspheres formed by the solvent evaporation method. Pharm. Res. 1987, 4, 465–471. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Fréchet, J.M. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjugate Chem. 2005, 16, 361–368. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.M.; Schwendeman, S.P. Characterization of the initial burst release of a model peptide from poly (D, L-lactide-co-glycolide) microspheres. J. Control. Release 2002, 82, 289–307. [Google Scholar] [CrossRef]
- Sharma, N.; Kumari, R.M.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. Poly-(Lactic-co-Glycolic) Acid Nanoparticles for Synergistic Delivery of Epirubicin and Paclitaxel to Human Lung Cancer Cells. Molecules 2020, 25, 4243. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.K.; Kshirsagar, R.; Patil, S. Mathematical models for drug release characterization: A review. World J. Pharm. Pharm. Sci. 2015, 4, 324–338. [Google Scholar]
- Grassi, M.; Grassi, G. Mathematical modelling and controlled drug delivery: Matrix systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Budiasih, S.; Jiyauddin, K.; Logavinod, N.; Kaleemullah, M.; Jawad, A.; Samer, A.; Fadli, A.; Eddy, Y. Optimization of polymer concentration for designing of oral matrix controlled release dosage form. UK J. Pharm. Biosci. 2014, 2, 54. [Google Scholar] [CrossRef]
- Jagadeesan, J.; Nandakumar, N.; Rengarajan, T.; Balasubramanian, M.P. Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU-induced breast carcinoma. J. Environ. Pathol. Toxicol. Oncol. 2012, 31. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of cell death in mammalian cells. Curr. Protoc. Pharmacol. 2004, 25, 12.8.1–12.8.22. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeon, B.K.; Lee, Y.E.; Woo, W.H.; Mun, Y.J. Diosgenin induces apoptosis in HepG2 cells through generation of reactive oxygen species and mitochondrial pathway. Evid. Based Complementary Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Moalic, S.; Liagre, B.; Corbière, C.; Bianchi, A.; Dauça, M.; Bordji, K.; Beneytout, J.L. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001, 506, 225–230. [Google Scholar] [CrossRef]
- Ćurčić, M.G.; Stanković, M.S.; Mrkalić, E.M.; Matović, Z.D.; Banković, D.D.; Cvetković, D.M.; Đačić, D.S.; Marković, S.D. Antiproliferative and proapoptotic activities of methanolic extracts from Ligustrum vulgare L. as an individual treatment and in combination with palladium complex. Int. J. Mol. Sci. 2012, 13, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Attari, F.; Sepehri, H.; Delphi, L.; Goliaei, B. Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran. Biomed. J. 2009, 13, 229–236. [Google Scholar]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. Bioimpacts 2018, 8, 185–194. [Google Scholar] [CrossRef]
- Mirunalini, S.; Arulmozhi, V.; Shahira, R. Diosgenin: A plant steroid induced apoptosis in human laryngeal carcinoma (Hep2) cells. J. Pharm. Res. 2011, 4, 2610–2614. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MA, USA, 2016.
- Fonseca, C.; Simoes, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef]
- Betancourt, T.; Brown, B.; Brannon-Peppas, L. Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: Preparation, characterization and in vitro evaluation. Nanomedicine 2007. [Google Scholar] [CrossRef]
| Factors | Unit | Low | High |
|---|---|---|---|
| PGMD | mg | 2 | 5 |
| Diosgenin | mg | 0.1 | 0.5 |
| PF-68 | percentage | 0.05 | 0.2 |
| Run | A:PGMD | B:Diosgenin | C:PF-68 | Particle size | PDI |
|---|---|---|---|---|---|
| mg | mg | percentage | nm | ||
| 1 | 3.5 | 0.3 | 0.125 | 97.3579 | 0.182443 |
| 2 | 5 | 0.3 | 0.05 | 96.6539 | 0.344201 |
| 3 | 2 | 0.1 | 0.125 | 91.2672 | 0.743494 |
| 4 | 3.5 | 0.5 | 0.05 | 107.153 | 0.46536 |
| 5 | 2 | 0.3 | 0.2 | 92.7336 | 0.327228 |
| 6 | 3.5 | 0.3 | 0.125 | 94.4605 | 0.910793 |
| 7 | 3.5 | 0.3 | 0.125 | 100.307 | 0.12029 |
| 8 | 5 | 0.3 | 0.2 | 105.873 | 0.766865 |
| 9 | 3.5 | 0.3 | 0.125 | 98.1165 | 0.613436 |
| 10 | 3.5 | 0.3 | 0.125 | 108.157 | 0.646145 |
| 11 | 2 | 0.5 | 0.125 | 99.6713 | 0.559112 |
| 12 | 3.5 | 0.1 | 0.05 | 110.518 | 0.896998 |
| 13 | 3.5 | 0.1 | 0.2 | 100.219 | 0.454666 |
| 14 | 5 | 0.1 | 0.125 | 96.1892 | 0.22674 |
| 15 | 5 | 0.5 | 0.125 | 93.9755 | 0.850414 |
| 16 | 2 | 0.3 | 0.05 | 97.9802 | 0.0205136 |
| 17 | 3.5 | 0.5 | 0.2 | 95.7049 | 0.498521 |
| Formulations | Polymer: Drug Ratio | Encapsulation Efficiency (%EE) | Loading Content (%DL) |
|---|---|---|---|
| PGMD-DG 7:3 NPs | 10:1 | 83.34 ± 3.67 | 12.68 ± 1.01 |
| PGMD-DG 6:4 NPs | 10:1 | 77.16 ± 2.61 | 10.95 ± 0.37 |
| Drug Formulation | R2 Values | |||
|---|---|---|---|---|
| Zero Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | |
| PGMD-DG 7:3 NPs | 0.724 | 0.839 | 0.907 | 0.931 |
| PGMD-DG 6:4 NPs | 0.656 | 0.685 | 0.886 | 0.939 |
| Formulations | IC50 (µM) | |
|---|---|---|
| 24 h | 48 h | |
| Diosgenin only | 31.92 ± 1.237 | 27.14 ± 1.597 |
| PGMD-DG NPs 7:3 | 18.23 ± 3.159 | 15.15 ± 0.174 |
| PGMD-DG NPs 6:4 | 16.27 ± 2.793 | 13.91 ± 1.803 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Singhal, M.; Kumari, R.M.; Gupta, N.; Manchanda, R.; Syed, A.; Bahkali, A.H.; Nimesh, S. Diosgenin Loaded Polymeric Nanoparticles with Potential Anticancer Efficacy. Biomolecules 2020, 10, 1679. https://doi.org/10.3390/biom10121679
Sharma N, Singhal M, Kumari RM, Gupta N, Manchanda R, Syed A, Bahkali AH, Nimesh S. Diosgenin Loaded Polymeric Nanoparticles with Potential Anticancer Efficacy. Biomolecules. 2020; 10(12):1679. https://doi.org/10.3390/biom10121679
Chicago/Turabian StyleSharma, Nikita, Monisha Singhal, R. Mankamna Kumari, Nidhi Gupta, Romila Manchanda, Asad Syed, Ali H. Bahkali, and Surendra Nimesh. 2020. "Diosgenin Loaded Polymeric Nanoparticles with Potential Anticancer Efficacy" Biomolecules 10, no. 12: 1679. https://doi.org/10.3390/biom10121679
APA StyleSharma, N., Singhal, M., Kumari, R. M., Gupta, N., Manchanda, R., Syed, A., Bahkali, A. H., & Nimesh, S. (2020). Diosgenin Loaded Polymeric Nanoparticles with Potential Anticancer Efficacy. Biomolecules, 10(12), 1679. https://doi.org/10.3390/biom10121679





