Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer
Abstract
1. Introduction
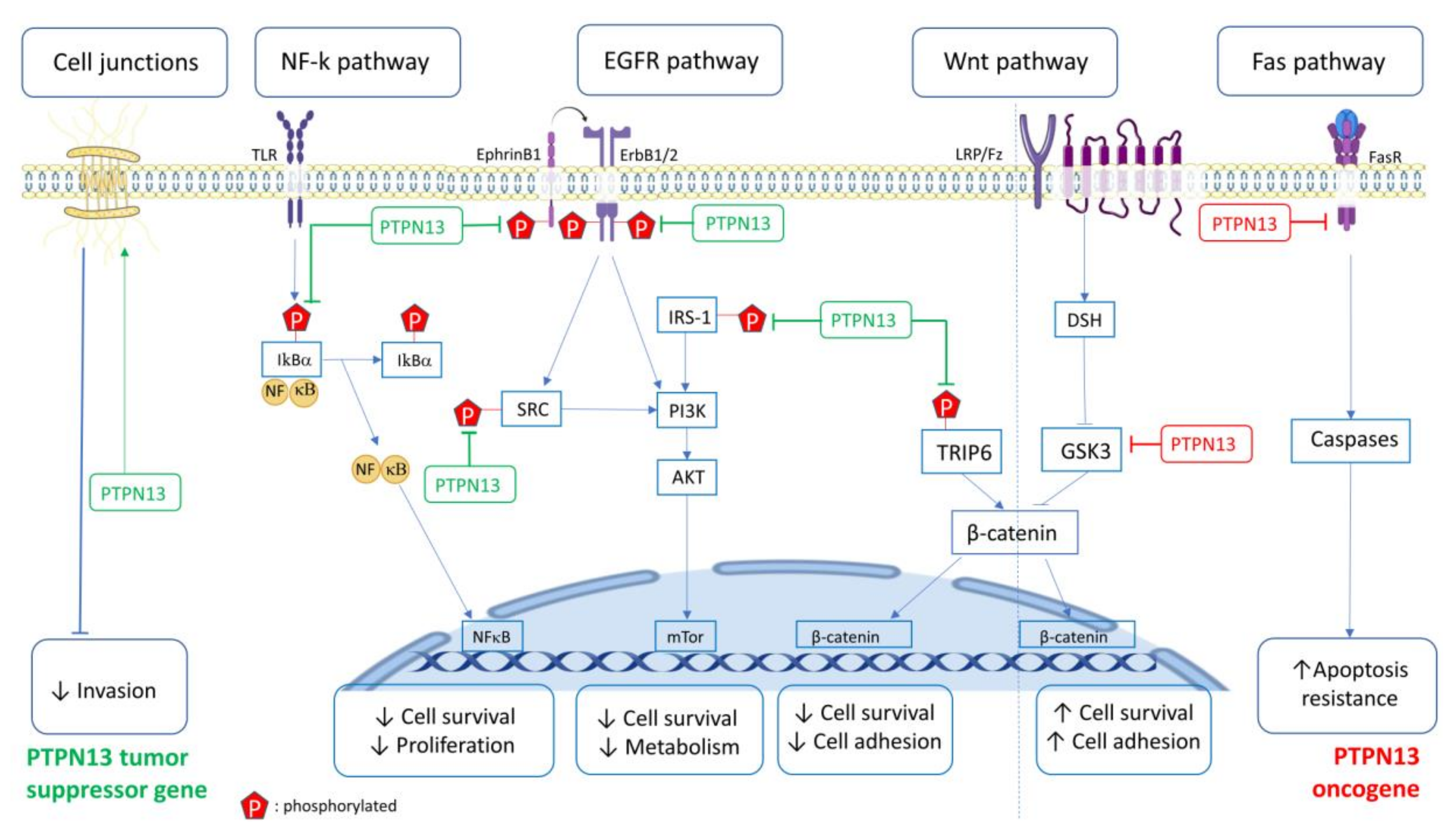
2. Signaling Pathways and Biological Activities Affected by PTPN13
2.1. FAS Pathway
2.2. SRC, Ephrin, ErbB Pathways
2.3. PTPN13 Is Implicated in HPV16 Carcinogenicity
2.4. NF-κB Pathway
2.5. EMT, Cell Migration and Invasion
2.6. PI3K/PTEN Pathway
3. Transcriptionnal, Post-Transcriptional, Genetic, and Epigenetic Regulation of PTPN13
3.1. Transcriptional Regulation
3.1.1. Transcriptional Regulation Mediated by Transcription Factors
3.1.2. Transcriptional Regulation Mediated by PTPN13 Promoter Methylation
3.2. Post-Transcriptional Regulation
3.2.1. Post-Transcriptional Regulation by microRNAs
3.2.2. Post-Transcriptional Regulation by Alternative Splicing
3.3. Post-Translational Regulation
4. Medical Implication of PTPN13
4.1. Prognostic Marker of Survival
4.2. PTPN13 and Drug Sensitivity
4.3. PTPN13 Gene Alterations
4.3.1. Loss of Heterozygosity (LOH)
4.3.2. Single Nucleotide Polymorphisms (SNP)
4.4. Bio-Informatic Analysis of the PTPN13 Gene Regulatory Network
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Maekawa, K.; Imagawa, N.; Nagamatsu, M.; Harada, S. Molecular cloning of a novel protein-tyrosine phosphatase containing a membrane-binding domain and GLGF repeats. FEBS Lett. 1994, 337, 200–206. [Google Scholar] [CrossRef]
- Banville, D.; Ahmad, S.; Stocco, R.; Shen, S.H. A novel protein-tyrosine phosphatase with homology to both the cytoskeletal proteins of the band 4.1 family and junction-associated guanylate kinases. J. Biol. Chem. 1994, 269, 22320–22327. [Google Scholar] [PubMed]
- Saras, J.; Claesson-Welsh, L.; Heldin, C.H.; Gonez, L.J. Cloning and characterization of PTPL1, a protein tyrosine phosphatase with similarities to cytoskeletal-associated proteins. J. Biol. Chem. 1994, 269, 24082–24089. [Google Scholar] [PubMed]
- Sato, T.; Irie, S.; Kitada, S.; Reed, J.C. FAP-1: A protein tyrosine phosphatase that associates with Fas. Science 1995, 268, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.G.; Dubé, N.; Hardy, S.; Tremblay, M.L. Inside the human cancer tyrosine phosphatome. Nat. Rev. Cancer 2011, 11, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Blanchetot, C.; Chagnon, M.; Dube, N.; Halle, M.; Tremblay, M.L. Substrate-trapping techniques in the identification of cellular PTP targets. Methods 2005, 35, 44–53. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, M.S.; Park, W.S.; Kim, S.Y.; Kim, H.S.; Lee, J.-H.; Han, S.Y.; Lee, H.K.; Park, J.Y.; Oh, R.R.; et al. Immunohistochemical localization of FAP-1, an inhibitor of Fas-mediated apoptosis, in normal and neoplastic human tissues. APMIS 1999, 107, 1101–1108. [Google Scholar] [CrossRef]
- Herrmann, L.; Dittmar, T.; Erdmann, K.S. The Protein Tyrosine Phosphatase PTP-BL Associates with the Midbody and Is Involved in the Regulation of Cytokinesis V. Mol. Biol. Cell 2003, 14, 230–240. [Google Scholar] [CrossRef]
- Maagdenberg, A.M.J.M.V.D.; Weghuis, D.O.; Rijss, J.; Merkx, G.F.; Wieringa, B.; Van Kessel, A.G.; Hendriksa, W. The gene (PTPN13) encoding the protein tyrosine phosphatase PTP-BL/PTP-BAS is located in mouse chromosome region 5E/F and human chromosome region 4q21. Cytogenet. Genome Res. 1996, 74, 153–155. [Google Scholar] [CrossRef]
- Mangeat, P.; Roy, C.; Martin, M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999, 9, 187–192. [Google Scholar] [CrossRef]
- Hendriks, W.J.; Schepens, J.; Bächner, D.; Rijss, J.; Zeeuwen, P.L.J.M.; Zechner, U.; Hameister, H.; Wieringa, B. Molecular cloning of a mouse epithelial protein-tyrosine phosphatase with similarities to submembranous proteins. J. Cell. Biochem. 1995, 59, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Cuppen, E.; Wijers, M.; Schepens, J.; Fransen, J.; Wieringa, B.; Hendriks, W. A FERM domain governs apical confinement of PTP-BL in epithelial cells. J. Cell Sci. 1999, 112, 3299–3308. [Google Scholar] [PubMed]
- Bompard, G.; Martin, M.; Roy, C.; Vignon, F.; Freiss, G. Membrane targeting of protein tyrosine phosphatase PTPL1 through its FERM domain via binding to phosphatidylinositol 4,5-biphosphate. J. Cell Sci. 2003, 116, 2519–2530. [Google Scholar] [CrossRef]
- Kimber, W.A.; Deak, M.; Prescott, A.; Alessi, D.R. Interaction of the protein tyrosine phosphatase PTPL1 with the PtdIns(3,4)P2-binding adaptor protein TAPP1. Biochem. J. 2003, 376, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Ackermann, N.; Christmann, J.; Brier, S.; Yu, F.; Erdmann, K.S. The serologically defined colon cancer antigen-3 interacts with the protein tyrosine phosphatase PTPN13 and is involved in the regulation of cytokinesis. Oncogene 2012, 32, 4602–4613. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Van Itallie, C.M. PDZ domains: Fundamental building blocks in the organization of protein complexes at the plasma membrane. J. Clin. Investig. 1999, 103, 767–772. [Google Scholar] [CrossRef]
- Freiss, G.; Chalbos, D. PTPN13/PTPL1: An Important Regulator of Tumor Aggressiveness. Anti Cancer Agents Med. Chem. 2011, 11, 78–88. [Google Scholar] [CrossRef]
- Kawano, S.; Ikeda, W.; Kishimoto, M.; Ogita, H.; Takai, Y. Silencing of ErbB3/ErbB2 Signaling by Immunoglobulin-like Necl-2. J. Biol. Chem. 2009, 284, 23793–23805. [Google Scholar] [CrossRef]
- Cuppen, E.; Van Ham, M.; Pepers, B.; Wieringa, B.; Hendriks, W.J. Identification and molecular characterization of BP75, a novel bromodomain-containing protein. FEBS Lett. 1999, 459, 291–298. [Google Scholar] [CrossRef]
- Maekawa, K.; Imagawa, N.; Naito, A.; Harada, S.; Yoshie, O.; Takagi, S. Association of protein-tyrosine phosphatase PTP-BAS with the transcription- factor-inhibitory protein IκBα through interaction between the PDZ1 domain and ankyrin repeats. Biochem. J. 1999, 337. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, Q.; Conrad, K.; Wozney, J.; Cheung, J.Y.; Miller, B.A. Regulation of TRP channel TRPM2 by the tyrosine phosphatase PTPL1. Am. J. Physiol. Physiol. 2007, 292, C1746–C1758. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Weight, C.M.; Luissint, A.-C.; Hilgarth, R.S.; Brazil, J.C.; Ettel, M.; Nusrat, A.; Parkos, C.A. Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation. Mol. Biol. Cell 2019, 30, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Cuppen, E.; Van Ham, M.; Wansink, D.G.; De Leeuw, A.; Wieringa, B.; Hendriks, W. The zyxin-related protein TRIP6 interacts with PDZ motifs in the adaptor protein RIL and the protein tyrosine phosphatase PTP-BL. Eur. J. Cell Biol. 2000, 79, 283–293. [Google Scholar] [CrossRef]
- Irie, S.; Hachiya, T.; Rabizadeh, S.; Maruyama, W.; Mukai, J.; Li, Y.; Reed, J.C.; Bredesen, D.E.; Sato, T.-A. Functional interaction of Fas-associated phosphatase-1 (FAP-1) with p75NTR and their effect on NF-κB activation. FEBS Lett. 1999, 460, 191–198. [Google Scholar] [CrossRef]
- Erdmann, K.S.; Kuhlmann, J.; Lessmann, V.; Herrmann, L.; Eulenburg, V.; Müller, O.; Heumann, R. The Adenomatous Polyposis Coli-protein (APC) interacts with the protein tyrosine phosphatase PTP-BL via an alternatively spliced PDZ domain. Oncogene 2000, 19, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Cuppen, E.; Gerrits, H.; Pepers, B.; Wieringa, B.; Hendriks, W.J. PDZ Motifs in PTP-BL and RIL Bind to Internal Protein Segments in the LIM Domain Protein RIL. Mol. Biol. Cell 1998, 9, 671–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saras, J.; Engström, U.; Góñez, L.J.; Heldin, C.-H. Characterization of the interactions between PDZ domains of the protein-tyrosine phosphatase PTPL1 and the carboxyl-terminal tail of Fas. J. Biol. Chem. 1997, 272, 20979–20981. [Google Scholar] [CrossRef]
- Sotelo, N.S.; Schepens, J.T.; Valiente, M.; Hendriks, W.J.; Pulido, R. PTEN–PDZ domain interactions: Binding of PTEN to PDZ domains of PTPN13. Methods 2015, 77–78, 147–156. [Google Scholar] [CrossRef]
- Gross, C.; Heumann, R.; Erdmann, K.S. The protein kinase C-related kinase PRK2 interacts with the protein tyrosine phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Lett. 2001, 496, 101–104. [Google Scholar] [CrossRef]
- Wang, Y.; Hall, R.A.; Lee, M.; Kamgar-Parsi, A.; Bi, X.; Baudry, M. The tyrosine phosphatase PTPN13/FAP-1 links calpain-2, TBI and tau tyrosine phosphorylation. Sci. Rep. 2017, 7, 11771. [Google Scholar] [CrossRef]
- Saras, J.; Franzén, P.; Aspenström, P.; Hellman, U.; Gonez, L.J.; Heldin, C.-H. A Novel GTPase-activating Protein for Rho Interacts with a PDZ Domain of the Protein-tyrosine Phosphatase PTPL1. J. Biol. Chem. 1997, 272, 24333–24338. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Gish, G.D.; Songyang, Z.; Pawson, T. The Carboxyl Terminus of B Class Ephrins Constitutes a PDZ Domain Binding Motif. J. Biol. Chem. 1999, 274, 3726–3733. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, M.; Tanaka, T.; Robson, B.E.; Mizgerd, J.P.; Grusby, M.J. Regulation of Signal Transducer and Activator of Transcription Signaling by the Tyrosine Phosphatase PTP-BL. Immunity 2007, 26, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Glondu-Lassis, M.; Dromard, M.; Chavey, C.; Puech, C.; Fajas, L.; Hendriks, W.J.; Freiss, G. Downregulation of protein tyrosine phosphatase PTP-BL represses adipogenesis. Int. J. Biochem. Cell Biol. 2009, 41, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Wansink, D.G.; Peters, W.; Schaafsma, I.; Sutmuller, R.P.M.; Oerlemans, F.; Adema, G.J.; Wieringa, B.; Van Der Zee, C.E.E.M.; Hendriks, W. Mild impairment of motor nerve repair in mice lacking PTP-BL tyrosine phosphatase activity. Physiol. Genom. 2004, 19, 50–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dromard, M.; Bompard, G.; Glondu-Lassis, M.; Puech, C.; Chalbos, D.; Freiss, G. The Putative Tumor Suppressor GenePTPN13/PTPL1Induces Apoptosis through Insulin Receptor Substrate-1 Dephosphorylation. Cancer Res. 2007, 67, 6806–6813. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Chen, R.; Yi, W.; Cantin, G.T.; Fearns, C.; Yang, Y.; Yates, J.R.; Lee, J.-D. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene 2007, 27, 2525–2531. [Google Scholar] [CrossRef][Green Version]
- Glondu-Lassis, M.; Dromard, M.; Lacroix-Triki, M.; Nirdé, P.; Puech, C.; Knani, D.; Chalbos, D.; Freiss, G. PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase. Cancer Res. 2010, 70, 5116–5126. [Google Scholar] [CrossRef]
- Abaan, O.D.; Hendriks, W.J.; Üren, A.; Toretsky, J.A.; Erkizan, H.V.P. Valosin containing protein (VCP/p97) is a novel substrate for the protein tyrosine phosphatase PTPL1. Exp. Cell Res. 2012, 319, 1–11. [Google Scholar] [CrossRef]
- Palmer, A.; Zimmer, M.; Erdmann, K.S.; Eulenburg, V.; Porthin, A.; Heumann, R.; Deutsch, U.; Klein, R. EphrinB Phosphorylation and Reverse Signaling. Mol. Cell 2002, 9, 725–737. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Lin, W.-C.; Lin, F.-T. PTPL1/FAP-1 negatively regulates TRIP6 function in lysophosphatidic acid-induced cell migration. J. Biol. Chem. 2007, 282, 24381–24387. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Irie, S.; Sato, T.-A. Identification of IκBα as a substrate of Fas-associated phosphatase-1. JBIC J. Biol. Inorg. Chem. 2000, 267, 7170–7175. [Google Scholar] [CrossRef] [PubMed]
- Sardina, J.L.; López-Ruano, G.; Prieto-Bermejo, R.; Sánchez-Sánchez, B.; Pérez-Fernández, A.; Sánchez-Abarca, L.I.; Pérez-Simón, J.A.; Quintales, L.; Sánchez-Yagüe, J.; Llanillo, M.; et al. PTPN13 regulates cellular signalling and β-catenin function during megakaryocytic differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2886–2899. [Google Scholar] [CrossRef] [PubMed]
- Bidère, N.; Su, H.C.; Lenardo, M.J. Genetic Disorders of Programmed Cell Death in the Immune System. Annu. Rev. Immunol. 2006, 24, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.N.; Bergami, P.L.; Maulit, G.; Sato, T.-A.; Sassoon, D.; Ronai, Z.A. FAP-1 Association with Fas (Apo-1) Inhibits Fas Expression on the Cell Surface. Mol. Cell. Biol. 2003, 23, 3623–3635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kanki, H.; Hachiya, T.; Ohyama, T.; Irie, S.; Tang, G.-L.; Mukai, J.; Sato, T.-A. Negative regulation of Fas-mediated apoptosis by FAP-1 in human cancer cells. Int. J. Cancer 2000, 87, 473–479. [Google Scholar] [CrossRef]
- Yanagisawa, J.; Takahashi, M.; Kanki, H.; Yano-Yanagisawa, H.; Tazunoki, T.; Sawa, E.; Nishitoba, T.; Kamishohara, M.; Kobayashi, E.; Kataoka, S.; et al. The Molecular Interaction of Fas and FAP-1. J. Biol. Chem. 1997, 272, 8539–8545. [Google Scholar] [CrossRef]
- Yao, H.; Song, E.; Chen, J.; Hamar, P. Expression of FAP-1 by human colon adenocarcinoma: Implication for resistance against Fas-mediated apoptosis in cancer. Br. J. Cancer 2004, 91, 1718–1725. [Google Scholar] [CrossRef]
- Myc, A.; Arscott, P.L.; Bretz, J.D.; Thompson, N.W.; Baker, J.R. Characterization of FAP-1 Expression and Function in Thyroid Follicular Cells. Endocrinology 1999, 140, 5431–5434. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Kataoka, S. Development of anti cancer drugs targeted on Fas-mediated apoptosis signal. Cancer Chemother. 1997, 24, 222–228. [Google Scholar]
- Huang, W.; Bei, L.; Eklund, E.A. Inhibition of Fas associated phosphatase 1 (Fap1) facilitates apoptosis of colon cancer stem cells and enhances the effects of oxaliplatin. Oncotarget 2018, 9, 25891–25902. [Google Scholar] [CrossRef] [PubMed]
- Gagnoux-Palacios, L.; Awina, H.; Audebert, S.; Rossin, A.; Mondin, M.; Borgese, F.; Planas-Botey, C.; Mettouchi, A.; Borg, J.-P.; Hueber, A.-O. Cell polarity and adherens junction formation inhibit epithelial Fas cell death receptor signaling. J. Cell Biol. 2018, 217, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Hamyeh, M.; Bernex, F.; Larive, R.M.; Naldi, A.; Urbach, S.; Simony-Lafontaine, J.; Puech, C.; Bakhache, W.; Solassol, J.; Coopman, P.J.; et al. PTPN13 induces cell junction stabilization and inhibits mammary tumor invasiveness. Theranostics 2020, 10, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Ungefroren, P.D.R.N.H.; Kruse, M.L.; Trauzold, A.; Roeschmann, S.; Roeder, C.; Arlt, A.; Henne-Bruns, D.; Kalthoff, H. FAP-1 in pancreatic cancer cells: Functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J. Cell Sci. 2001, 114, 2735–2746. [Google Scholar] [PubMed]
- Xiao, Z.-Y.; Wu, W.; Eagleton, N.; Chen, H.-Q.; Shao, J.; Teng, H.; Liu, T.-H.; Jiang, Z.-M.; Yao, H.-R. Silencing Fas-associated phosphatase 1 expression enhances efficiency of chemotherapy for colon carcinoma with oxaliplatin. World J. Gastroenterol. 2010, 16, 112–118. [Google Scholar] [PubMed]
- Schickel, R.; Park, S.-M.; Murmann, A.E.; Peter, M.E. mir-200c Regulates Induction of Apoptosis through CD95 by Targeting FAP-1. Mol. Cell 2010, 38, 908–915. [Google Scholar] [CrossRef]
- Neznanov, N.; Neznanova, L.; Angres, B.; Gudkov, A.V. Serologically Defined Colon Cancer Antigen 3 Is Necessary for the Presentation of TNF Receptor 1 on Cell Surface. DNA Cell Biol. 2005, 24, 777–785. [Google Scholar] [CrossRef]
- Sharma, S.; Carmona, A.; Skowronek, A.; Yu, F.; Collins, M.O.; Naik, S.; Murzeau, C.M.; Tseng, P.-L.; Erdmann, K.S. Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Huang, W.; Bei, L.; Eklund, E.A. Fas-associated phosphatase 1 mediates Fas resistance in myeloid progenitor cells expressing the Bcr–abl oncogene. Leuk. Lymphoma 2013, 54, 619–630. [Google Scholar] [CrossRef]
- Huang, W.; Bei, L.; Eklund, E.A. Fas-associated Phosphatase 1 (Fap1) Influences βCatenin Activity in Myeloid Progenitor Cells Expressing the Bcr-abl Oncogene. J. Biol. Chem. 2013, 288, 12766–12776. [Google Scholar] [CrossRef]
- Huang, W.; Luan, C.-H.; Hjort, E.E.; Bei, L.; Mishra, R.; Sakamoto, K.M.; Platanias, L.C.; Eklund, E.A. The role of Fas-associated phosphatase 1 in leukemia stem cell persistence during tyrosine kinase inhibitor treatment of chronic myeloid leukemia. Leukemia 2016, 30, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Castilla, C.; Chinchón, D.; Medina, R.; Torrubia, F.J.; Japón, M.A.; Sáez, C. PTPL1 and PKCδ contribute to proapoptotic signalling in prostate cancer cells. Cell Death Dis. 2013, 4, e576. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, B.J.; Arlt, A.; Duttmann, A.; Ungefroren, H.; Schäfer, H.; Kalthoff, H.; Kruse, M.-L. Characterisation of FAP-1 expression and CD95 mediated apoptosis in the A818-6 pancreatic adenocarcinoma differentiation system. Differentiation 2012, 83, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 2009, 223, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Holen, H.L.; Shadidi, M.; Narvhus, K.; Kjøsnes, O.; Tierens, A.; Aasheim, H.-C. Signaling through ephrin-A ligand leads to activation of Src-family kinases, Akt phosphorylation, and inhibition of antigen receptor-induced apoptosis. J. Leukoc. Biol. 2008, 84, 1183–1191. [Google Scholar] [CrossRef]
- Scrima, M.; De Marco, C.; De Vita, F.; Fabiani, F.; Franco, R.; Pirozzi, G.; Rocco, G.; Malanga, D.; Viglietto, G. The Nonreceptor-Type Tyrosine Phosphatase PTPN13 Is a Tumor Suppressor Gene in Non–Small Cell Lung Cancer. Am. J. Pathol. 2012, 180, 1202–1214. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, Y.; Zhao, J.; Chen, K.; Wu, C. Reversion-induced LIM interaction with Src reveals a novel Src inactivation cycle. J. Cell Biol. 2009, 184, 785–792. [Google Scholar] [CrossRef]
- Lucci, M.A.; Orlandi, R.; Triulzi, T.; Tagliabue, E.; Balsari, A.; Villa-Moruzzi, E. Expression Profile of Tyrosine Phosphatases in HER2 Breast Cancer Cells and Tumors. Cell. Oncol. 2010, 32, 361–372. [Google Scholar]
- Vermeer, P.D.; Bell, M.; Lee, K.; Vermeer, D.W.; Wieking, B.G.; Bilal, E.; Bhanot, G.; Drapkin, R.I.; Ganesan, S.; Klingelhutz, A.J.; et al. ErbB2, EphrinB1, Src Kinase and PTPN13 Signaling Complex Regulates MAP Kinase Signaling in Human Cancers. PLoS ONE 2012, 7, e30447. [Google Scholar] [CrossRef]
- Vermeer, P.D.; Colbert, P.L.; Wieking, B.G.; Vermeer, D.W.; Lee, J.H. Targeting ERBB Receptors Shifts Their Partners and Triggers Persistent ERK Signaling through a Novel ERBB/EFNB1 Complex. Cancer Res. 2013, 73, 5787–5797. [Google Scholar] [CrossRef] [PubMed]
- Grandis, J.R.; Tweardy, D.J. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993, 53, 3579–3584. [Google Scholar] [PubMed]
- Spanos, W.C.; Hoover, A.; Harris, G.F.; Wu, S.; Strand, G.L.; Anderson, M.E.; Klingelhutz, A.J.; Hendriks, W.; Bossler, A.D.; Lee, J.H. The PDZ Binding Motif of Human Papillomavirus Type 16 E6 Induces PTPN13 Loss, Which Allows Anchorage-Independent Growth and Synergizes with Ras for Invasive Growth. J. Virol. 2008, 82, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, T.; Yang, Z.; Li, Y.; Li, W.; Wang, T.; Wang, S.; Jia, L.; Zhang, S.; Li, S.-Q. miR-26a desensitizes non-small cell lung cancer cells to tyrosine kinase inhibitors by targeting PTPN13. Oncotarget 2016, 7, 45687–45701. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Shou, T.; Li, K.; Gao, C.; Duan, L.; Fang, L.; Zhang, Q.; Chen, Z.; Zhang, C.; Yang, S.; et al. MicroRNA-30e-5p promotes cell growth by targetingPTPN13and indicates poor survival and recurrence in lung adenocarcinoma. J. Cell. Mol. Med. 2017, 21, 2852–2862. [Google Scholar] [CrossRef]
- Yu, M.; Maden, S.K.; Stachler, M.; Kaz, A.M.; Ayers, J.; Guo, Y.; Carter, K.T.; Willbanks, A.; Heinzerling, T.J.; O’Leary, R.M.; et al. Subtypes of Barrett’s oesophagus and oesophageal adenocarcinoma based on genome-wide methylation analysis. Gut 2018, 68, 389–399. [Google Scholar] [CrossRef]
- Yamada, A.; Inoue, E.; Deguchi-Tawarada, M.; Matsui, C.; Togawa, A.; Nakatani, T.; Ono, Y.; Takai, Y. Necl-2/CADM1 interacts with ErbB4 and regulates its activity in GABAergic neurons. Mol. Cell. Neurosci. 2013, 56, 234–243. [Google Scholar] [CrossRef]
- Sugiyama, H.; Mizutani, K.; Kurita, S.; Okimoto, N.; Shimono, Y.; Takai, Y. Interaction of Necl-4/CADM4 with ErbB3 and integrin α6 β4 and inhibition of ErbB2/ErbB3 signaling and hemidesmosome disassembly. Genes Cells 2013, 18, 519–528. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef]
- Hausen, H.Z. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Gardiol, D.; Kühne, C.; Glaunsinger, B.; Lee, S.S.; Javier, R.; Banks, L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 1999, 18, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.C.; Spanos, W.C.; Harris, G.F.; Anderson, M.E.; Klingelhutz, A.J.; Lee, J.H. The Role of Human Papillomavirus 16 E6 in Anchorage-Independent and Invasive Growth of Mouse Tonsil Epithelium. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wieking, B.G.; Vermeer, D.W.; Spanos, W.C.; Lee, K.M.; Vermeer, P.; Lee, W.T.; Xu, Y.; Gabitzsch, E.S.; Balcaitis, S.; Balint, J.P.; et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012, 19, 667–674. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Schoonbroodt, S.; Ferreira, V.; Best-Belpomme, M.; Boelaert, J.R.; Legrand-Poels, S.; Korner, M.; Piette, J. Crucial Role of the Amino-Terminal Tyrosine Residue 42 and the Carboxyl-Terminal PEST Domain of IκBα in NF-κB Activation by an Oxidative Stress. J. Immunol. 2000, 164, 4292–4300. [Google Scholar] [CrossRef]
- Fan, C.; Yang, J.; Engelhardt, J.F. Temporal pattern of NFκB activation influences apoptotic cell fate in a stimuli-dependent fashion. J. Cell Sci. 2002, 115, 4843–4853. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Huang, T.; Li, J. Protein tyrosine phosphatase L1 inhibits high-grade serous ovarian carcinoma progression by targeting IκBα. OncoTargets Ther. 2018, 11, 7603–7612. [Google Scholar] [CrossRef]
- Zhan, H.; Jiang, J.; Luo, C.; Sun, Q.; Ke, A.; Sun, C.; Hu, J.; Hu, Z.; Hu, B.; Zhu, K.; et al. Tumour-suppressive role of PTPN13 in hepatocellular carcinoma and its clinical significance. Tumor Biol. 2016, 37, 9691–9698. [Google Scholar] [CrossRef]
- Ranković, B.; Zidar, N.; Žlajpah, M.; Boštjančič, E. Epithelial-Mesenchymal Transition-Related MicroRNAs and Their Target Genes in Colorectal Cancerogenesis. J. Clin. Med. 2019, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Liang, J.Q.; Zhang, L.; Chen, H.; Zhang, Y.; Li, R.; Wang, X.; Ji, J.; Tong, J.H.; To, K.-F.; et al. TTPAL Promotes Colorectal Tumorigenesis by Stabilizing TRIP6 to Activate Wnt/β-Catenin Signaling. Cancer Res. 2019, 79, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Castilla, C.; Flores, M.L.; Conde, J.M.; Medina, R.; Torrubia, F.J.; Japón, M.A.; Sáez, C. Downregulation of protein tyrosine phosphatase PTPL1 alters cell cycle and upregulates invasion-related genes in prostate cancer cells. Clin. Exp. Metastasis 2012, 29, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Yatim, S.M.J.; Peng, S.; Gunaratne, J.; Hunziker, W.; Ludwig, A. The Mammalian Crumbs Complex Defines a Distinct Polarity Domain Apical of Epithelial Tight Junctions. Curr. Biol. 2020, 30, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- López-Ruano, G.; Prieto-Bermejo, R.; Ramos, T.L.; San-Segundo, L.; Sánchez-Abarca, L.I.; Sanchezguijo, F.M.; Pérez-Simón, J.A.; Sánchez-Yagüe, J.; Llanillo, M.; Hernández-Hernández, Á. PTPN13 and β-Catenin Regulate the Quiescence of Hematopoietic Stem Cells and Their Interaction with the Bone Marrow Niche. Stem Cell Rep. 2015, 5, 516–531. [Google Scholar] [CrossRef]
- Kuchay, S.; Duan, S.; Schenkein, E.; Peschiaroli, A.; Saraf, A.; Florens, L.; Washburn, M.P.; Pagano, M. FBXL2- and PTPL1-mediated degradation of p110-free p85β regulatory subunit controls the PI(3)K signalling cascade. Nat. Cell Biol. 2013, 15, 472–480. [Google Scholar] [CrossRef]
- Bompard, G.; Puech, C.; Prébois, C.; Vignon, F.; Freiss, G. Protein-tyrosine Phosphatase PTPL1/FAP-1 Triggers Apoptosis in Human Breast Cancer Cells. J. Biol. Chem. 2002, 277, 47861–47869. [Google Scholar] [CrossRef]
- Freiss, G.; Puech, C.; Vignon, F. Extinction of Insulin-Like Growth Factor-I Mitogenic Signaling by Antiestrogen-Stimulated Fas-Associated Protein Tyrosine Phosphatase-1 in Human Breast Cancer Cells. Mol. Endocrinol. 1998, 12, 568–579. [Google Scholar] [CrossRef][Green Version]
- Long, Q.; Sun, J.; Lv, J.; Liang, Y.; Li, H.; Li, X. PTPN13 acts as a tumor suppressor in clear cell renal cell carcinoma by inactivating Akt signaling. Exp. Cell Res. 2020, 396, 112286. [Google Scholar] [CrossRef]
- Bruurs, L.J.M.; Van Der Net, M.C.; Zwakenberg, S.; Huber, A.K.M.R.; Post, A.; Zwartkruis, F.J.; Bos, J.L. The Phosphatase PTPL1 Is Required for PTEN-Mediated Regulation of Apical Membrane Size. Mol. Cell. Biol. 2018, 38, e00102–e00118. [Google Scholar] [CrossRef]
- Han, X.-J.; Xue, L.; Gong, L.; Zhu, S.-J.; Yao, L.; Wang, S.-M.; Lan, M.; Zhang, W.; Li, Y.-H. Stat3 Inhibits PTPN13 Expression in Squamous Cell Lung Carcinoma through Recruitment of HDAC5. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Zhou, J.X.; Calvet, J.P.; Godwin, A.K.; Jensen, R.A.; Li, X. Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Fan, L.X.; Zhou, J.X.; Grantham, J.J.; Calvet, J.P.; Sage, J.; Li, X. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2017, 127, 2751–2764. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, P.; Mao, K.; He, C.; Xu, Q.; Zhang, M.; Liu, H.; Zhou, Z.; Zhou, Q.; Zhou, Q.; et al. Anti-oncogene PTPN13 inactivation by hepatitis B virus X protein counteracts IGF2BP1 to promote hepatocellular carcinoma progression. Oncogene 2020, 1–18. [Google Scholar] [CrossRef]
- Hao, S.X.; Ren, R.; Jahn, T.; Seipel, P.; Urschel, S.; Peschel, C.; Duyster, J. Expression of Interferon Consensus Sequence Binding Protein (ICSBP) Is Downregulated in Bcr-Abl-Induced Murine Chronic Myelogenous Leukemia-Like Disease, and Forced Coexpression of ICSBP Inhibits Bcr-Abl-Induced Myeloproliferative Disorder. Mol. Cell. Biol. 2000, 20, 1149–1161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.; Zhu, C.; Wang, H.; Horvath, E.; Eklund, E.A. The Interferon Consensus Sequence-binding Protein (ICSBP/IRF8) RepressesPTPN13Gene Transcription in Differentiating Myeloid Cells. J. Biol. Chem. 2008, 283, 7921–7935. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, L.; Bei, L.; Hjort, E.; Eklund, E.A. The Leukemia-associated Fusion Protein Tel-Platelet-derived Growth Factor Receptor β (Tel-PdgfRβ) Inhibits Transcriptional Repression ofPTPN13Gene by Interferon Consensus Sequence Binding Protein (Icsbp). J. Biol. Chem. 2012, 287, 8110–8125. [Google Scholar] [CrossRef]
- Abaan, O.D.; Levenson, A.; Khan, O.; Furth, P.A.; Üren, A.; Toretsky, J.A. PTPL1 is a direct transcriptional target of EWS-FLI1 and modulates Ewing’s Sarcoma tumorigenesis. Oncogene 2005, 24, 2715–2722. [Google Scholar] [CrossRef]
- Kalyani, R.; Lee, J.Y.; Min, H.; Yoon, H.; Kim, M.H. Genes Frequently Coexpressed with Hoxc8 Provide Insight into the Discovery of Target Genes. Mol. Cells 2016, 39, 395–402. [Google Scholar] [CrossRef]
- Ying, J.; Li, H.; Cui, Y.; Wong, A.H.Y.; Langford, C.; Tao, Q. Epigenetic disruption of two proapoptotic genes MAPK10/JNK3 and PTPN13/FAP-1 in multiple lymphomas and carcinomas through hypermethylation of a common bidirectional promoter. Leukemia 2006, 20, 1173–1175. [Google Scholar] [CrossRef]
- Yeh, S.-H.; Wu, D.-C.; Tsai, C.-Y.; Kuo, T.-J.; Yuan-Shau, C.; Chang, Y.-S.; Chen, C.-L.; Chang, C.-F.; Chen, D.-S.; Chen, P.-J. Genetic Characterization of Fas-Associated Phosphatase-1 as a Putative Tumor Suppressor Gene on Chromosome 4q21.3 in Hepatocellular Carcinoma. Clin. Cancer Res. 2006, 12, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Li, M.; Ying, J.; Jing, H. 5-Azacitidine induces demethylation of PTPL1 and inhibits growth in non-Hodgkin lymphoma. Int. J. Mol. Med. 2015, 36, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Li, Z.; Zhu, M.; Zhang, Z.; Wang, Y.; Jing, H.-M. Promoter hypermethylation of PTPL1, PTPN6, DAPK, p16 and 5-azacitidine inhibits growth in DLBCL. Oncol. Rep. 2015, 35, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, S.; Hu, L.; Jia, L.; Wang, H.; Guo, M.; Chen, C.; Liu, Y.; Xu, L. miR-30 Family: A Promising Regulator in Development and Disease. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, H.; He, H.; Tong, W.; Wang, B.; Liao, G.; Chen, Z.; Du, C. Up-regulation of microRNA in bladder tumor tissue is not common. Int. Urol. Nephrol. 2009, 42, 95–102. [Google Scholar] [CrossRef]
- Daiuto, F.; Callari, M.; Dugo, M.; Merlino, G.; Musella, V.; Miodini, P.; Paolini, B.; Cappelletti, V.; Daidone, M.G. miR-30e* is an independent subtype-specific prognostic marker in breast cancer. Br. J. Cancer 2015, 113, 290–298. [Google Scholar] [CrossRef]
- D’Angelo, E.; Fassan, M.; Maretto, I.; Pucciarelli, S.; Zanon, C.; Digito, M.; Rugge, M.; Nitti, D.; Agostini, M. Serum miR-125b is a non-invasive predictive biomarker of the pre-operative chemoradiotherapy responsiveness in patients with rectal adenocarcinoma. Oncotarget 2016, 7, 28647–28657. [Google Scholar] [CrossRef]
- Cinpolat, O.; Unal, Z.N.; Ismi, O.; Gorur, A.; Unal, M. Comparison of microRNA profiles between benign and malignant salivary gland tumors in tissue, blood and saliva samples: A prospective, case-control study. Braz. J. Otorhinolaryngol. 2017, 83, 276–284. [Google Scholar] [CrossRef]
- Cinegaglia, N.C.; Andrade, S.C.S.; Tokar, T.; Pinheiro, M.; Severino, F.E.; Oliveira, R.A.; Hasimoto, E.N.; Cataneo, D.C.; Cataneo, A.J.M.; Defaveri, J.; et al. Integrative transcriptome analysis identifies deregulated microRNA-transcription factor networks in lung adenocarcinoma. Oncotarget 2016, 7, 28920–28934. [Google Scholar] [CrossRef]
- Peter, M.E. Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle 2009, 8, 843–852. [Google Scholar] [CrossRef]
- Liu, H.; Brannon, A.R.; Reddy, A.; Alexe, G.; Seiler, M.W.; Arreola, A.; Oza, J.H.; Yao, M.; Juan, D.; Liou, L.S.; et al. Identifying mRNA targets of microRNA dysregulated in cancer: With application to clear cell Renal Cell Carcinoma. BMC Syst. Biol. 2010, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- GeneCards PTPN13 Gene. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PTPN13 (accessed on 11 December 2020).
- Bowler, E.; Porazinski, S.; Uzor, S.; Thibault, P.; Durand, M.; Lapointe, E.; Rouschop, K.M.A.; Hancock, J.; Wilson, I.; Ladomery, M. Hypoxia leads to significant changes in alternative splicing and elevated expression of CLK splice factor kinases in PC3 prostate cancer cells. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meinhold-Heerlein, I.; Stenner-Liewen, F.; Liewen, H.; Kitada, S.; Krajewska, M.; Krajewski, S.; Zapata, J.M.; Monks, A.; Scudiero, D.A.; Bauknecht, T.; et al. Expression and Potential Role of Fas-Associated Phosphatase-1 in Ovarian Cancer. Am. J. Pathol. 2001, 158, 1335–1344. [Google Scholar] [CrossRef][Green Version]
- D’Hondt, V.; Lacroix-Triki, M.; Jarlier, M.; Boissiere-Michot, F.; Puech, C.; Coopman, P.; Katsaros, D.; Freiss, G. High PTPN13 expression in high grade serous ovarian carcinoma is associated with a better patient outcome. Oncotarget 2017, 8, 95662–95673. [Google Scholar] [CrossRef] [PubMed]
- Révillion, F.; Puech, C.; Rabenoelina, F.; Chalbos, D.; Peyrat, J.-P.; Freiss, G. Expression of the putative tumor suppressor genePTPN13/PTPL1is an independent prognostic marker for overall survival in breast cancer. Int. J. Cancer 2009, 124, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xue, L.; Zhou, L.; Gong, L.; Zhu, S.; Yao, L.; Wang, S.; Lan, M.; Li, Y.-H.; Zhang, W. The role of PTPN13 in invasion and metastasis of lung squamous cell carcinoma. Exp. Mol. Pathol. 2013, 95, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Eckloff, B.W.; Deng, B.; Wang, Y.; Wampfler, J.; Jang, J.S.; Wieben, E.D.; Jen, J.; You, M.; et al. Conserved recurrent gene mutations correlate with pathway deregulation and clinical outcomes of lung adenocarcinoma in never-smokers. BMC Med. Genom. 2014, 7, 486. [Google Scholar] [CrossRef]
- Borinstein, S.C.; Barkauskas, N.A.; Krailo, M.; Scher, D.; Scher, L.; Schlottmann, S.; Kallakury, B.; Dickman, P.S.; Pawel, B.R.; West, D.C.; et al. Investigation of the insulin-like growth factor-1 signaling pathway in localized Ewing sarcoma: A report from the Children’s Oncology Group. Cancer 2011, 117, 4966–4976. [Google Scholar] [CrossRef]
- Foehr, E.D.; Lorente, G.; Vincent, V.; Nikolich, K.; Urfer, R. FAS Associated Phosphatase (FAP-1) Blocks Apoptosis of Astrocytomas through Dephosphorylation of FAS. J. Neuro Oncol. 2005, 74, 241–248. [Google Scholar] [CrossRef]
- O’Connell, J.; Bennett, M.W.; O’Sullivan, G.C.; Roche, D.; Kelly, J.; Collins, J.K.; Shanahan, F. Fas ligand expression in primary colon adenocarcinomas: Evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J. Pathol. 1998, 186, 240–246. [Google Scholar] [CrossRef]
- Colbert, P.L.; Vermeer, D.W.; Wieking, B.G.; Lee, J.H.; Vermeer, P.D. EphrinB1: Novel microtubule associated protein whose expression affects taxane sensitivity. Oncotarget 2014, 6, 953–968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Gan, Y. Cancer-derived IgG involved in cisplatin resistance through PTP-BAS/Src/PDK1/AKT signaling pathway. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wrage, M.; Ruosaari, S.; Eijk, P.P.; Kaifi, J.T.; Hollmén, J.; Yekebas, E.F.; Izbicki, J.R.; Brakenhoff, R.H.; Streichert, T.; Riethdorf, S.; et al. Genomic Profiles Associated with Early Micrometastasis in Lung Cancer: Relevance of 4q Deletion. Clin. Cancer Res. 2009, 15, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Gorringe, K.L.; Ramakrishna, M.; Williams, L.H.; Sridhar, A.; Boyle, S.E.; Bearfoot, J.L.; Li, J.; Anglesio, M.S.; Campbell, I.G. Are there any more ovarian tumor suppressor genes? A new perspective using ultra high-resolution copy number and loss of heterozygosity analysis. Genes Chromosom. Cancer 2009, 48, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Pungliya, M.S.; Choi, J.Y.; Jiang, R.; Sun, X.J.; Salisbury, B.A.; Stephens, J.C. DNA variability of human genes. Mech. Ageing Dev. 2003, 124, 17–25. [Google Scholar] [CrossRef]
- Mita, Y.; Yasuda, Y.; Sakai, A.; Yamamoto, H.; Toyooka, S.; Gunduz, M.; Tanabe, S.; Naomoto, Y.; Ouchida, M.; Shimizu, K. Missense polymorphisms of PTPRJ and PTPN13 genes affect susceptibility to a variety of human cancers. J. Cancer Res. Clin. Oncol. 2009, 136, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Laczmanska, I.; Karpiński, P.; Gil, J.; Laczmanski, L.; Makowska, I.; Bebenek, M.; Ramsey, D.; Sąsiadek, M.M. ThePTPN13Y2081D (T>G) (rs989902) polymorphism is associated with an increased risk of sporadic colorectal cancer. Color. Dis. 2017, 19, O272–O278. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Huang, Y.-J.; Wang, L.-E.; Sturgis, E.M.; Wei, P. Genetic polymorphisms in the PTPN13 gene and risk of squamous cell carcinoma of head and neck. Carcinogenesis 2009, 30, 2053–2058. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Jiang, M.; Luo, L.; Li, Z.; Wang, P.; Dong, W. Colorectal cancer susceptibility variants alter risk of breast cancer in a Chinese Han population. Genet. Mol. Res. 2013, 12, 6268–6274. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Kim, C.; Kim, J.-H.; Kwon, W.S.; Lee, W.S.; Park, J.Y.; Kim, H.S.; Park, K.H.; Kim, T.S.; Park, J.-L.; et al. Genetic alterations and their clinical implications in gastric cancer peritoneal carcinomatosis revealed by whole-exome sequencing of malignant ascites. Oncotarget 2016, 7, 8055–8066. [Google Scholar] [CrossRef]
- Musolf, A.M.; Moiz, B.A.; Sun, H.; Pikielny, C.W.; Bossé, Y.; Mandal, D.; De Andrade, M.; Gaba, C.; Yang, P.; Li, Y.; et al. Whole Exome Sequencing of Highly Aggregated Lung Cancer Families Reveals Linked Loci for Increased Cancer Risk on Chromosomes 12q, 7p, and 4q. Cancer Epidemiol. Biomark. Prev. 2020, 29, 434–442. [Google Scholar] [CrossRef]
- Jeong, E.G.; Lee, S.H.; Yoo, N.J.; Lee, S.H. Mutational analysis of FLASH and PTPN13 genes in colorectal carcinomas. Pathology 2008, 40, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.C.; Strand, G.L.; Nowicki, P.N.; Anderson, M.E.; Vermeer, P.D.; Klingelhutz, A.J.; Bossler, A.D.; Pottala, J.V.; Hendriks, W.J.A.J.; Lee, J.H. Impaired PTPN13 phosphatase activity in spontaneous or HPV-induced squamous cell carcinomas potentiates oncogene signaling through the MAP kinase pathway. Oncogene 2009, 28, 3960–3970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, D.; Parsons, D.W.; Bardelli, A.; Sager, J.; Szabo, S.; Ptak, J.; Silliman, N.; Peters, B.A.; Van Der Heijden, M.S.; et al. Mutational Analysis of the Tyrosine Phosphatome in Colorectal Cancers. Science 2004, 304, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Son, W.; Lim, J.; Xiao, G. Statistical completion of a partially identified graph with applications for the estimation of gene regulatory networks. Biostatistics 2015, 16, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M. Calpain-1 and Calpain-2 in the Brain: Dr. Jekill and Mr Hyde? Curr. Neuropharmacol. 2019, 17, 823–829. [Google Scholar] [CrossRef]
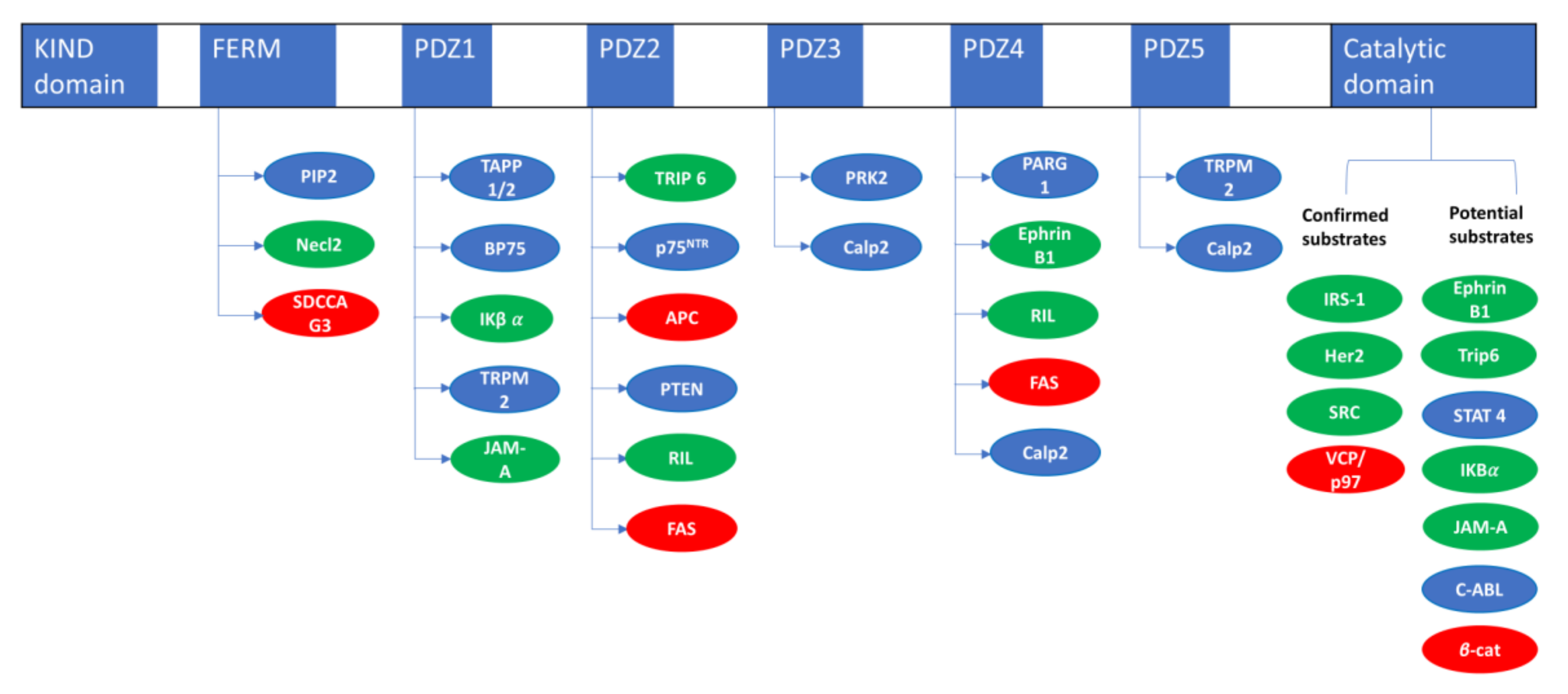
| PTPN13 Interacting Proteins | ||
|---|---|---|
| Name | Interacting Domain | Reference |
| Necl2 | FERM | [18] |
| SDCCAG3/ENTR1 | FERM | [15] |
| TAPP 1/2 | PDZ1 | [14] |
| BP75 | PDZ1 | [19] |
| IκBα | PDZ1 | [20] |
| TRPM2 | PDZ15 | [21] |
| JAM-A | PDZ-1 | [22] |
| TRIP6/ZRP1 | PDZ2 | [23] |
| P75NTR | PDZ2 | [24] |
| APC | PDZ2 | [25] |
| RIL | PDZ2-4 | [26] |
| FAS | PDZ2-4 | [27] |
| PTEN | PDZ2 | [28] |
| PRK2 | PDZ3 | [29] |
| Calp2 | PDZ3-4-5 | [30] |
| PARG1 | PDZ4 | [31] |
| EphrinB1 | PDZ4 | [32] |
| PTPN13 Substrates Evidences | |||
|---|---|---|---|
| Name | Dephosphorylation | Substrate Trapping | Reference |
| IRS1 | In vitro/in cellulo | In vitro/in cellulo | [36] |
| HER2 | In cellulo | In cellulo | [37] |
| SRC | In vitro/in cellulo | In vitro/in cellulo | [38] |
| VCP/P97 | In vitro/in cellulo | In vitro/in cellulo | [39] |
| EphrinB1 | In vitro/in cellulo | [40] | |
| Trip6 | In vitro/in cellulo | [41] | |
| STAT 4 | In vitro/in cellulo | [33] | |
| IκBα | In vitro/in cellulo | [42] | |
| JAM-A | In vitro | [22] | |
| C-ABL | In cellulo | [30] | |
| β-catenin | In cellulo | [43] | |
| Transcription Factors and Co-Repressors Regulating PTPN13 | |||
|---|---|---|---|
| Name | Expression/Tumor Type | Effect | Reference |
| STAT3/HDAC5 |  Lung cancers Lung cancers |  PTPN13 PTPN13 | [101] |
| SMYD2 |  Polycystic kidney disease Polycystic kidney disease Breast cancer cell lines Breast cancer cell lines |  PTPN13 PTPN13 PTPN13 PTPN13 | [103] [102] |
| HBx/DNMT3A |  Liver cancer Liver cancer |  PTPN13 PTPN13 | [104] |
| ICSBP |  Chronic myeloid leukemia Chronic myeloid leukemia |  PTPN13 PTPN13 | [106] |
| EWS-FLI1 |  Ewing’s sarcomas Ewing’s sarcomas |  PTPN13 PTPN13 | [108] |
| Hox-C8 |  Cell differentiation Cell differentiation |  PTPN13 PTPN13 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mcheik, S.; Aptecar, L.; Coopman, P.; D’Hondt, V.; Freiss, G. Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer. Biomolecules 2020, 10, 1659. https://doi.org/10.3390/biom10121659
Mcheik S, Aptecar L, Coopman P, D’Hondt V, Freiss G. Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer. Biomolecules. 2020; 10(12):1659. https://doi.org/10.3390/biom10121659
Chicago/Turabian StyleMcheik, Soha, Leticia Aptecar, Peter Coopman, Véronique D’Hondt, and Gilles Freiss. 2020. "Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer" Biomolecules 10, no. 12: 1659. https://doi.org/10.3390/biom10121659
APA StyleMcheik, S., Aptecar, L., Coopman, P., D’Hondt, V., & Freiss, G. (2020). Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer. Biomolecules, 10(12), 1659. https://doi.org/10.3390/biom10121659






