Carotenoids in Cancer Metastasis—Status Quo and Outlook
Abstract
1. Introduction
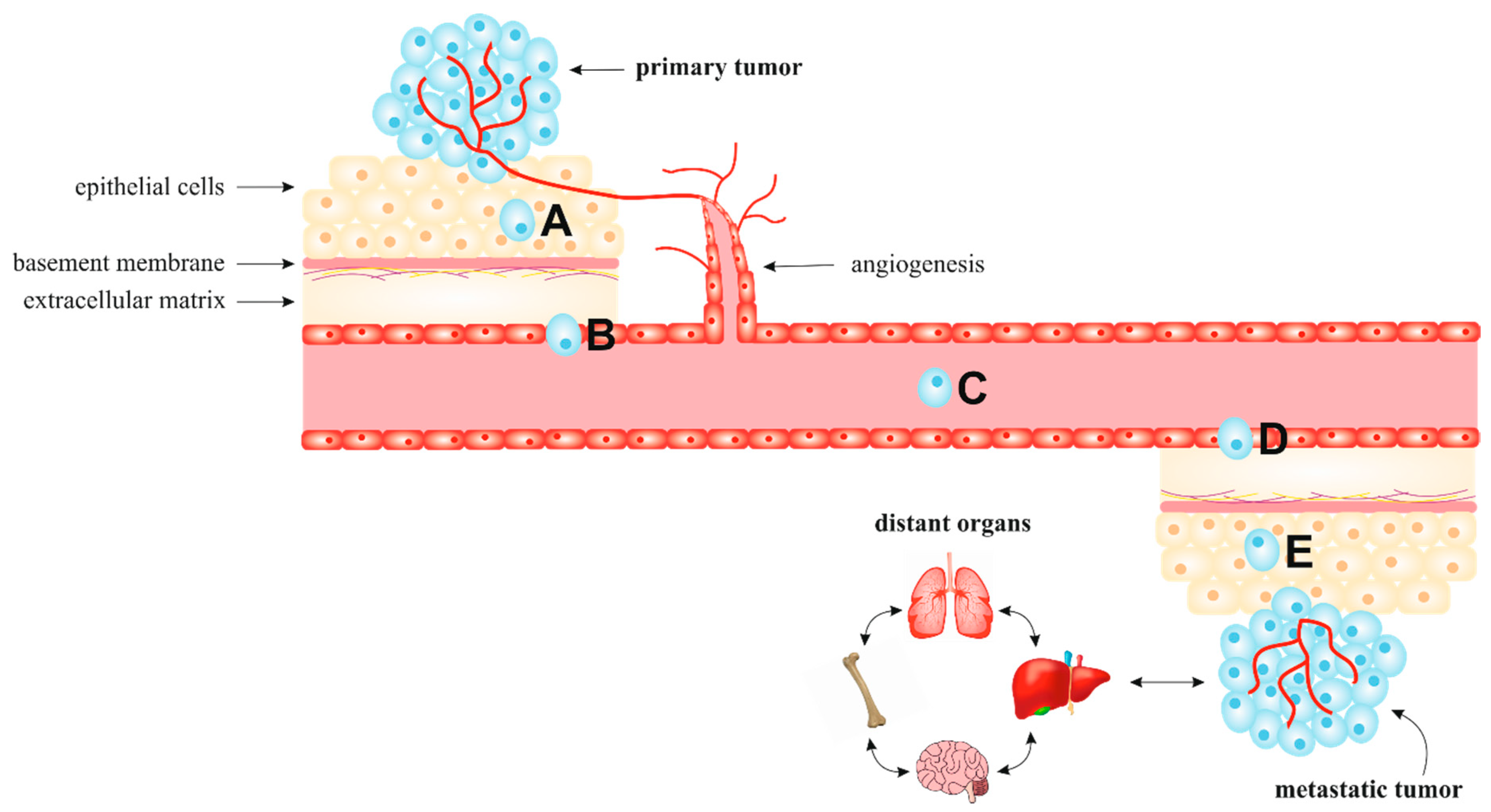
2. Metastatic Process
2.1. Epithelial-Mesenchymal and Mesenchymal-Epithelial Transition
2.2. Extrinsic Microenvironment and Extracellular Matrix
2.3. Regulatory Processes Associated with Metastasis
2.4. Chronic Inflammation
2.5. Genetic and Epigenetic Factors
2.6. Cancer Stem Cells (CSCs)
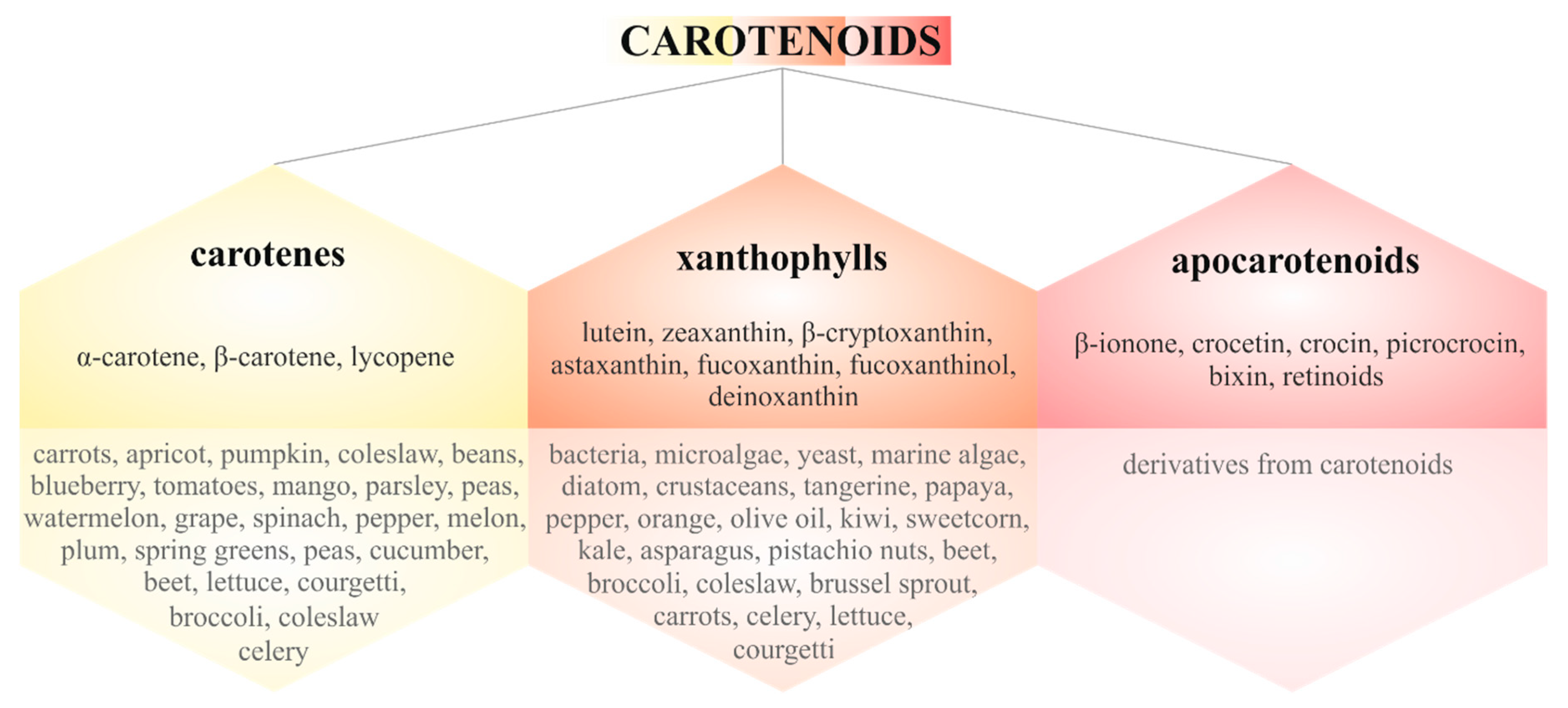
3. Carotenoids in Cancer Metastasis
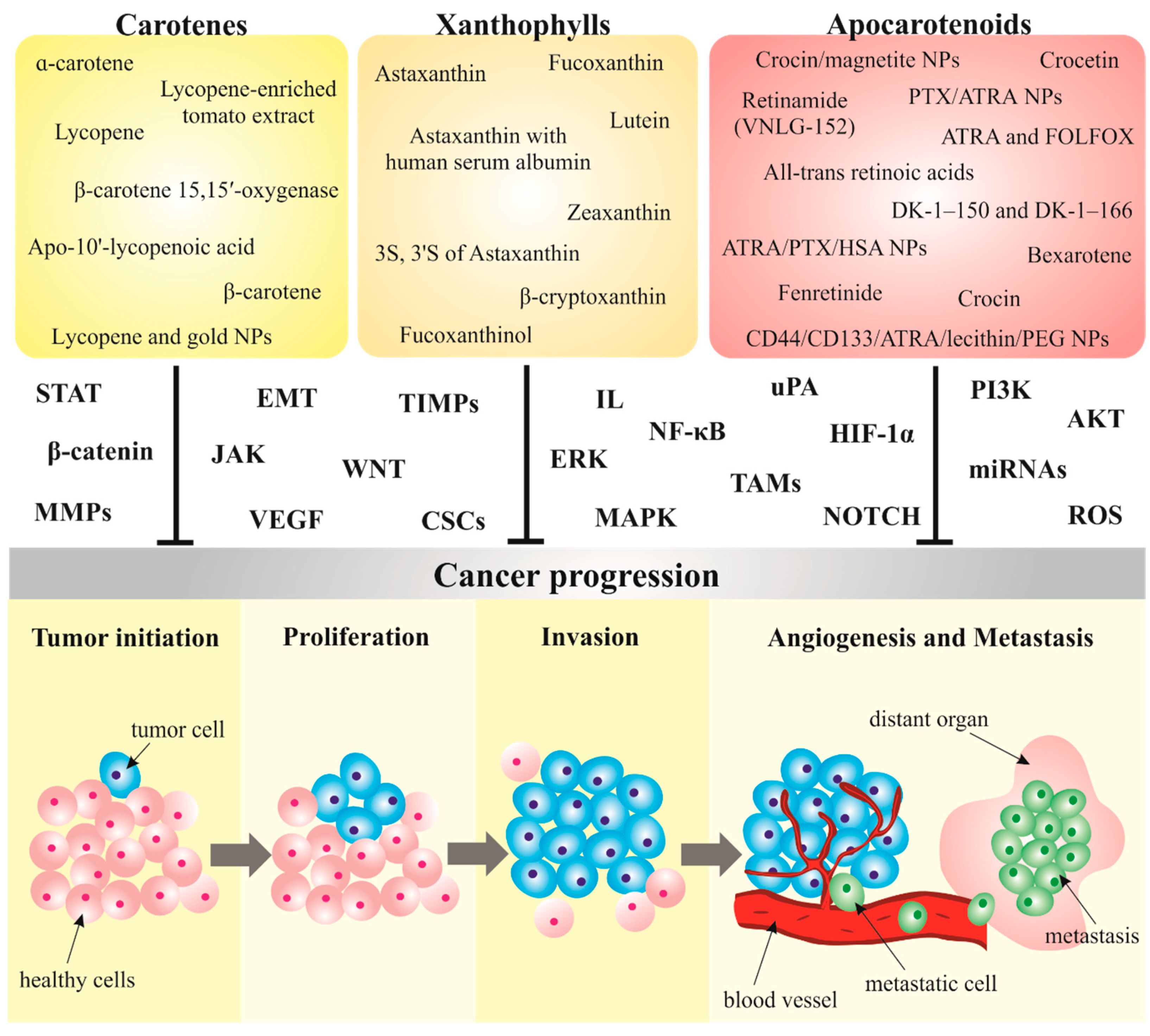
3.1. Carotenoids Modulate Metastatic Processes in Preclinical Research
3.1.1. Carotenes
α-Carotene
β-Carotene
Lycopene
3.1.2. Xanthophylls
Astaxanthin
Fucoxanthin
Other Xanthophylls
3.1.3. Apocarotenoids
All-Trans Retinoic Acids
Crocin and Crocetin
Other Apocarotenoids
3.2. Nanoparticles Conjugated with Carotenoids as a Novel Strategy in Cancer Management
3.3. Carotenoids and Their Anti-Metastatic Effects in Clinical Practice
Limitations of Carotenoids
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Qian, C.-N.; Mei, Y.; Zhang, J. Cancer metastasis: Issues and challenges. Chin. J. Cancer 2017, 36. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Das, S.K.; Minn, I.; Emdad, L.; Wang, X.-Y.; Sarkar, D.; Pomper, M.G.; Fisher, P.B. Detecting Tumor Metastases: The Road to Therapy Starts Here. Adv. Cancer Res. 2016, 132, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Loud, J.; Murphy, J. Cancer screening and early detection in the 21st century. Semin Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Haun, M.W.; Estel, S.; Rücker, G.; Friederich, H.; Villalobos, M.; Thomas, M.; Hartmann, M. Early palliative care for adults with advanced cancer. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro. IJMS 2019, 20, 1749. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Liskova, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Mojžiš, J.; Adamkov, M.; Fialová, S.; Veizerová, L.; Zulli, A.; et al. Oregano demonstrates distinct tumour-suppressive effects in the breast carcinoma model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kapinová, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Výbohová, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojžiš, J.; et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2016, 55, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Kubatka, P.; Liskova, A.; Baranenko, D.; Kruzliak, P.; Matta, M.; Büsselberg, D.; Malicherova, B.; Zulli, A.; Kwon, T.K.; et al. Controlling metastatic cancer: The role of phytochemicals in cell signaling. J Cancer Res. Clin. Oncol. 2019, 145, 1087–1109. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef]
- Soares, N.d.C.P.; Teodoro, A.J.; Lotsch, P.F.; Granjeiro, J.M.; Borojevic, R. Anticancer properties of carotenoids in prostate cancer. A review. Histol. Histopathol. 2015, 30, 1143–1154. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Mezquita, B.; Mezquita, P.; Pau, M.; Gasa, L.; Navarro, L.; Samitier, M.; Pons, M.; Mezquita, C. All-trans-retinoic acid activates the pro-invasive Src-YAP-Interleukin 6 axis in triple-negative MDA-MB-231 breast cancer cells while cerivastatin reverses this action. Sci. Rep. 2018, 8, 7047. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Hoang, T.X.; Kim, J.Y. All-Trans Retinoic Acid Enhances Matrix Metalloproteinase 2 Expression and Secretion in Human Myeloid Leukemia THP-1 Cells. Biomed Res. Int. 2018, 2018, 5971080. [Google Scholar] [CrossRef]
- Middha, P.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Mondul, A.M. β-Carotene Supplementation and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob. Res. 2019, 21, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Nash, S.H.; Till, C.; Song, X.; Lucia, M.S.; Parnes, H.L.; Thompson, I.M.; Lippman, S.M.; Platz, E.A.; Schenk, J. Serum Retinol and Carotenoid Concentrations and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation and tumorigenesis. J. Cell. Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-N.; Zeng, Q.; Wang, H.-Y.; Zhang, B.; Li, S.-T.; Nan, X.; Cao, N.; Fu, C.-J.; Yan, X.-L.; Jia, Y.-L.; et al. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology 2015, 62, 801–815. [Google Scholar] [CrossRef]
- Choi, H.-J.; Park, J.-H.; Park, M.; Won, H.-Y.; Joo, H.-S.; Lee, C.H.; Lee, J.-Y.; Kong, G. UTX inhibits EMT-induced breast CSC properties by epigenetic repression of EMT genes in cooperation with LSD1 and HDAC1. EMBO Rep. 2015, 16, 1288–1298. [Google Scholar] [CrossRef]
- An, H.; Stoops, S.L.; Deane, N.G.; Zhu, J.; Zi, J.; Weaver, C.; Waterson, A.G.; Zijlstra, A.; Lindsley, C.W.; Beauchamp, R.D. Small molecule/ML327 mediated transcriptional de-repression of E-cadherin and inhibition of epithelial-to-mesenchymal transition. Oncotarget 2015, 6, 22934–22948. [Google Scholar] [CrossRef]
- Said, N.A.B.M.; Williams, E.D. Growth factors in induction of epithelial-mesenchymal transition and metastasis. Cells Tissues Organs 2011, 193, 85–97. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Inoue, H.; Nagata, N.; Kurokawa, H.; Yamanaka, S. iPS cells: A game changer for future medicine. EMBO J. 2014, 33, 409–417. [Google Scholar] [CrossRef]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef]
- Capasso, L.L. Antiquity of cancer. Int. J. Cancer 2005, 113, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Goldberg, I.D.; Shi, Y.E. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 2002, 21, 2245–2252. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Chen, J.-K.; Peng, S.-F.; Lai, K.C.; Liu, H.-C.; Huang, Y.-P.; Lin, C.-C.; Huang, A.-C.; Chueh, F.-S.; Chung, J.-G. Fistein Suppresses Human Osteosarcoma U-2 OS Cell Migration and Invasion via Affecting FAK, uPA and NF-ĸB Signaling Pathway In Vitro. In Vivo 2019, 33, 801–810. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Su, C.-W.; Lin, C.-W.; Yang, W.-E.; Yang, S.-F. TIMP-3 as a therapeutic target for cancer. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Thaysen-Andersen, M.; Thøgersen, I.B.; Lademann, U.; Offenberg, H.; Giessing, A.M.B.; Enghild, J.J.; Nielsen, H.J.; Brünner, N.; Højrup, P. Investigating the biomarker potential of glycoproteins using comparative glycoprofiling-application to tissue inhibitor of metalloproteinases-1. Biochim. Biophys. Acta 2008, 1784, 455–463. [Google Scholar] [CrossRef]
- Luparello, C.; Avanzato, G.; Carella, C.; Pucci-Minafra, I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res. Treat. 1999, 54, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Guedez, L.; Stetler-Stevenson, W.G.; Wolff, L.; Wang, J.; Fukushima, P.; Mansoor, A.; Stetler-Stevenson, M. In Vtro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J. Clin. Investig. 1998, 102, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Han, H.; Jensen-Taubman, S.; Gavil, N.; Isaac, B.; Wei, B.; Neckers, L.; Stetler-Stevenson, W.G. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget 2013, 4, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, P.C.; Zhou, B.P. Inflammation Fuels Tumor Progress and Metastasis. Curr. Pharm. Des. 2015, 21, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, O.S.; Spagnuolo, L.; de Visser, K.E. Immune regulation of metastasis: Mechanistic insights and therapeutic opportunities. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.-A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Staller, P.; Sulitkova, J.; Lisztwan, J.; Moch, H.; Oakeley, E.J.; Krek, W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 2003, 425, 307–311. [Google Scholar] [CrossRef]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Patel, S.A.; Vanharanta, S. Epigenetic determinants of metastasis. Mol. Oncol. 2017, 11, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Calin, G.A.; Villanueva, A.; Ropero, S.; Sánchez-Céspedes, M.; Blanco, D.; Montuenga, L.M.; Rossi, S.; Nicoloso, M.S.; Faller, W.J.; et al. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13556–13561. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, S.; Alahari, S.K. miRNA control of tumor cell invasion and metastasis. Int. J. Cancer 2010, 126, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Fuller, M. Stem cells and cancer: Two faces of eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Hassan, G.; Osman, A.; Calle, A.S.; Nawara, H.M.; Zahra, M.H.; El-Ghlban, S.; Mansour, H.; Alam, M.J.; Abu Quora, H.A.; et al. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering 2019, 6, 73. [Google Scholar] [CrossRef]
- Irani, S. Emerging insights into the biology of metastasis: A review article. Iran. J. Basic Med. Sci. 2019, 22, 833–847. [Google Scholar] [CrossRef]
- Nandy, S.B.; Lakshmanaswamy, R. Cancer Stem Cells and Metastasis. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 151, pp. 137–176. ISBN 978-0-12-812772-8. [Google Scholar]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.P.; Hammond, B.R. Possible influences of lutein and zeaxanthin on the developing retina. Clin. Ophthalmol. 2007, 1, 25–35. [Google Scholar] [PubMed]
- Kiokias, S.; Proestos, C.; Varzakas, T. A Review of the Structure, Biosynthesis, Absorption of Carotenoids-Analysis and Properties of their Common Natural Extracts. Curr. Res. Nutr Food Sci. J. 2016, 4, 25–37. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. In Carotenoids in Nature; Stange, C., Ed.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 359–375. ISBN 978-3-319-39124-3. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Rowles, J.L.; Erdman, J.W. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Athreya, K.; Xavier, M.F. Antioxidants in the Treatment of Cancer. Nutr. Cancer 2017, 69, 1099–1104. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Structures and Analysis of Carotenoid Molecules. In Carotenoids in Nature; Stange, C., Ed.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 71–108. ISBN 978-3-319-39124-3. [Google Scholar]
- Liu, Y.-Z.; Yang, C.-M.; Chen, J.-Y.; Liao, J.-W.; Hu, M.-L. Alpha-carotene inhibits metastasis in Lewis lung carcinoma in vitro, and suppresses lung metastasis and tumor growth in combination with taxol in tumor xenografted C57BL/6 mice. J. Nutr. Biochem. 2015, 26, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, J.; Li, M.; Tang, L.; Wu, R.; Jin, L.; Liang, Z. β-carotene reverses tobacco smoke-induced gastric EMT via Notch pathway in vivo. Oncol. Rep. 2018, 39, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, H.-A.; Lim, J.Y.; Kim, Y.; Jung, C.-H.; Yoo, S.-H.; Kim, Y. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Kim, Y.; Kim, Y.S.; Shin, J.-H.; Rubin, L.P.; Kim, Y. β-Carotene exerts anti-colon cancer effects by regulating M2 macrophages and activated fibroblasts. J. Nutr. Biochem. 2020, 82, 108402. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gong, X.; Rubin, L.P.; Choi, S.-W.; Kim, Y. β-Carotene 15,15′-oxygenase inhibits cancer cell stemness and metastasis by regulating differentiation-related miRNAs in human neuroblastoma. J. Nutr. Biochem. 2019, 69, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Jhou, B.-Y.; Song, T.-Y.; Lee, I.; Hu, M.-L.; Yang, N.-C. Lycopene Inhibits Metastasis of Human Liver Adenocarcinoma SK-Hep-1 Cells by Downregulation of NADPH Oxidase 4 Protein Expression. J. Agric. Food Chem. 2017, 65, 6893–6903. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Yu, R. Lycopene Inhibits Epithelial–Mesenchymal Transition and Promotes Apoptosis in Oral Cancer via PI3K/AKT/m-TOR Signal Pathway. Drug Des. Dev. Ther. 2020, 14, 2461–2471. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322–1336. [Google Scholar]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, B.; Hu, K.-Q.; Fu, X.; Wang, X.-D. Apo-10′-lycopenoic acid inhibits cancer cell migration and angiogenesis and induces peroxisome proliferator-activated receptor γ. J. Nutr. Biochem. 2018, 56, 26–34. [Google Scholar] [CrossRef]
- Bhatia, N.; Gupta, P.; Singh, B.; Koul, A. Lycopene Enriched Tomato Extract Inhibits Hypoxia, Angiogenesis, and Metastatic Markers in early Stage N-Nitrosodiethylamine Induced Hepatocellular Carcinoma. Nutr. Cancer 2015, 67, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Bernstein, P.S. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, Y.-M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Siangcham, T.; Vivithanaporn, P.; Sangpairoj, K. Anti-Migration and Invasion Effects of Astaxanthin against A172 Human Glioblastoma Cell Line. Asian Pac. J. Cancer Prev. 2020, 21, 2029–2033. [Google Scholar] [CrossRef]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef]
- Su, X.-Z.; Chen, R.; Wang, C.-B.; Ouyang, X.-L.; Jiang, Y.; Zhu, M.-Y. Astaxanthin Combine with Human Serum Albumin to Abrogate Cell Proliferation, Migration, and Drug-resistant in Human Ovarian Carcinoma SKOV3 Cells. Anticancer Agents Med. Chem. 2019, 19, 792–801. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Lin, Y.-J.; Liu, W.; Lin, H.-Y.; Chou, H.-Y.; Thia, C.; Wu, J.H.; Chang, J.-S.; Wen, Z.-H.; Chang, J.-J.; et al. Metabolic engineering probiotic yeast produces 3S, 3′S-astaxanthin to inhibit B16F10 metastasis. Food Chem. Toxicol. 2020, 135, 110993. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine Carotenoid Fucoxanthin Possesses Anti-Metastasis Activity: Molecular Evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin Activates Apoptosis via Inhibition of PI3K/Akt/mTOR Pathway and Suppresses Invasion and Migration by Restriction of p38-MMP-2/9 Pathway in Human Glioblastoma Cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Terasaki, M.; Mima, M.; Kudoh, S.; Endo, T.; Maeda, H.; Hamada, J.; Osada, K.; Miyashita, K.; Mutoh, M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol. Rep. 2018, 40, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dang, F.; Deng, C. β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.-C.; Hose, N.; Xu, C.-L.; Zhang, C.; Sassoon, J.; Song, E. Nonlethal Levels of Zeaxanthin Inhibit Cell Migration, Invasion, and Secretion of MMP-2 via NF-κB Pathway in Cultured Human Uveal Melanoma Cells. J. Ophthalmol. 2016, 2016. [Google Scholar] [CrossRef]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef]
- Mahima Reji, R.; Grace, V.M. ATRA Entrapped in DSPC Liposome Enhances Anti-metastasis Effect on Lung and Liver during B16F10 Cell Line Metastasis in C57BL6 Mice. Anticancer Agents Med. Chem. 2017, 17, 875–884. [Google Scholar] [CrossRef]
- Shi, G.; Zheng, X.; Wu, X.; Wang, S.; Wang, Y.; Xing, F. All-trans retinoic acid reverses epithelial-mesenchymal transition in paclitaxel-resistant cells by inhibiting nuclear factor kappa B and upregulating gap junctions. Cancer Sci. 2019, 110, 379–388. [Google Scholar] [CrossRef]
- Zuo, L.; Yang, X.; Lu, M.; Hu, R.; Zhu, H.; Zhang, S.; Zhou, Q.; Chen, F.; Gui, S.; Wang, Y. All-Trans Retinoic Acid Inhibits Human Colorectal Cancer Cells RKO Migration via Downregulating Myosin Light Chain Kinase Expression through MAPK Signaling Pathway. Nutr. Cancer 2016, 68, 1225–1233. [Google Scholar] [CrossRef]
- Cui, J.; Gong, M.; He, Y.; Li, Q.; He, T.; Bi, Y. All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int. J. Oncol. 2016, 48, 349–357. [Google Scholar] [CrossRef]
- Arzi, L.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R.; Jafarzadeh, N. A Comparative Study on Anti-Invasion, Antimigration, and Antiadhesion Effects of the Bioactive Carotenoids of Saffron on 4T1 Breast Cancer Cells Through Their Effects on Wnt/β-Catenin Pathway Genes. DNA Cell Biol. 2018, 37, 697–707. [Google Scholar] [CrossRef]
- Arzi, L.; Hoshyar, R.; Jafarzadeh, N.; Riazi, G.; Sadeghizadeh, M. Anti-metastatic properties of a potent herbal combination in cell and mice models of triple negative breast cancer. Life Sci. 2020, 243, 117245. [Google Scholar] [CrossRef] [PubMed]
- Arzi, L.; Farahi, A.; Jafarzadeh, N.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R. Inhibitory Effect of Crocin on Metastasis of Triple-Negative Breast Cancer by Interfering with Wnt/β-Catenin Pathway in Murine Model. DNA Cell Biol. 2018, 37, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Q.; Shang, J.; Lu, L.; Chen, G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J. Cell. Physiol. 2019, 234, 17876–17885. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Starz-Gaiano, M. Drosophila Jak/STAT Signaling: Regulation and Relevance in Human Cancer and Metastasis. Int. J. Mol. Sci. 2018, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, B. Saffron carotenoids inhibit STAT3 activation and promote apoptotic progression in IL-6-stimulated liver cancer cells. Oncol. Rep. 2018, 39, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.P.; Ramalingam, S.; Gediya, L.K.; Njar, V.C.O. The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK–eIF4E signaling pathways to suppress EMT and castration-resistant prostate cancer xenograft growth. FEBS J. 2018, 285, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Long, C.; Nguyen, J.; Kumar, D.; Lee, J. Discovering alkylamide derivatives of bexarotene as new therapeutic agents against triple-negative breast cancer. Bioorganic Med. Chem. Lett. 2018, 28, 420–424. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Shao, D.; Liu, H.; Zhou, Q.; Gui, S.; Wei, W.; Wang, Y. Fenretinide inhibits the proliferation and migration of human liver cancer HepG2 cells by downregulating the activation of myosin light chain kinase through the p38-MAPK signaling pathway. Oncol. Rep. 2018, 40, 518–526. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Wong, H.L. A novel hybrid delivery system: Polymer-oil nanostructured carrier for controlled delivery of highly lipophilic drug all-trans-retinoic acid (ATRA). Int. J. Pharm. 2012, 436, 721–731. [Google Scholar] [CrossRef]
- Zhang, T.; Xiong, H.; Dahmani, F.Z.; Sun, L.; Li, Y.; Yao, L.; Zhou, J.; Yao, J. Combination chemotherapy of doxorubicin, all-trans retinoic acid and low molecular weight heparin based on self-assembled multi-functional polymeric nanoparticles. Nanotechnology 2015, 26, 145101. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, H.; Liu, J.; Min, Y.; Wang, Y.; Wang, A.Z.; Wang, J.; Liu, Y. Co-delivery of all-trans-retinoic acid enhances the anti-metastasis effect of albumin-bound paclitaxel nanoparticles. Chem. Commun. 2017, 53, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-Y.; Jeong, Y.-I.; Lee, S.J.; Lee, E.; Oh, J.S.; Lee, H.C. Combination of paclitaxel- and retinoic acid-incorporated nanoparticles for the treatment of CT-26 colon carcinoma. Arch. Pharm. Res. 2011, 34, 407–417. [Google Scholar] [CrossRef] [PubMed]
- El-Kharrag, R.; Amin, A.; Hisaindee, S.; Greish, Y.; Karam, S.M. Development of a therapeutic model of precancerous liver using crocin-coated magnetite nanoparticles. Int. J. Oncol. 2017, 50, 212–222. [Google Scholar] [CrossRef]
- Huang, R.-F.S.; Wei, Y.-J.; Inbaraj, B.S.; Chen, B.-H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Z.; Zheng, L.; Qin, J.; Li, H.; Xue, X.; Gao, J.; Fang, G. SATB1 siRNA-Encapsulated Immunoliposomes Conjugated with CD44 Antibodies Target and Eliminate Gastric Cancer-Initiating Cells. Available online: https://www.dovepress.com/satb1-sirna-encapsulated-immunoliposomes-conjugated-with-cd44-antibodi-peer-reviewed-article-OTT (accessed on 2 November 2020).
- Li, L.; Cui, D.; Ye, L.; Li, Y.; Zhu, L.; Yang, L.; Bai, B.; Nie, Z.; Gao, J.; Cao, Y. Codelivery of salinomycin and docetaxel using poly(d, l-lactic-co-glycolic acid)-poly(ethylene glycol) nanoparticles to target both gastric cancer cells and cancer stem cells. Anti-Cancer Drugs 2017, 28, 989–1001. [Google Scholar] [CrossRef]
- Chen, H.; Lin, J.; Shan, Y.; Zhengmao, L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed. Pharmacother. 2019, 115, 108857. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z. Properties of encapsulated saffron extracts in maltodextrin using the Büchi B-90 nano spray-dryer. Food Chem. 2018, 266, 458–465. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Recchia, F.; Saggio, G.; Cesta, A.; Candeloro, G.; Di Blasio, A.; Amiconi, G.; Lombardo, M.; Nuzzo, A.; Lalli, A.; Alesse, E.; et al. Phase II study of interleukin-2 and 13-cis-retinoic acid as maintenance therapy in metastatic colorectal cancer. Cancer Immunol. Immunother. 2007, 56, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Recchia, F.; De Filippis, S.; Rosselli, M.; Saggio, G.; Fumagalli, L.; Rea, S. Interleukin-2 and 13-cis retinoic acid in the treatment of minimal residual disease: A phase II study. Int. J. Oncol. 2002, 20, 1275–1282. [Google Scholar] [CrossRef]
- Higginbotham, K.B.; Lozano, R.; Brown, T.; Patt, Y.Z.; Arima, T.; Abbruzzese, J.L.; Thomas, M.B. A phase I/II trial of TAC-101, an oral synthetic retinoid, in patients with advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2008, 134, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Plager, C.; Papadopoulos, N.; Ellerhorst, J.; Smith, T.; Benjamin, R.S. A phase II evaluation of bexarotene (Targretin) capsules in patients with metastatic melanoma. Oncol. Rep. 2000, 7, 883–886. [Google Scholar] [CrossRef]
- Bryan, M.; Pulte, E.D.; Toomey, K.C.; Pliner, L.; Pavlick, A.C.; Saunders, T.; Wieder, R. A pilot phase II trial of all-trans retinoic acid (Vesanoid) and paclitaxel (Taxol) in patients with recurrent or metastatic breast cancer. Investig. New Drugs 2011, 29, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs. orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003, 92, 375–378. [Google Scholar] [CrossRef]
- Hung, R.J.; Zhang, Z.-F.; Rao, J.Y.; Pantuck, A.; Reuter, V.E.; Heber, D.; Lu, Q.-Y. Protective effects of plasma carotenoids on the risk of bladder cancer. J. Urol. 2006, 176, 1192–1197. [Google Scholar] [CrossRef]
- Boccardo, F.; Canobbio, L.; Resasco, M.; Decensi, A.U.; Pastorino, G.; Brema, F. Phase II study of tamoxifen and high-dose retinyl acetate in patients with advanced breast cancer. J. Cancer Res. Clin. Oncol. 1990, 116, 503–506. [Google Scholar] [CrossRef]
- Shi, J.; Sun, J.; Liu, C.; Chai, Z.; Wang, N.; Zhang, H.; Cheng, S. All-trans-retinoic acid (ATRA) plus oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX) versus FOLFOX alone as palliative chemotherapy in patients with advanced hepatocellular carcinoma and extrahepatic metastasis: Study protocol for a randomized controlled trial. Trials 2019, 20. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Stokkel, M.P.; Pereira, A.M.; Corssmit, E.P.; Morreau, H.A.; Romijn, J.A.; Smit, J.W.A. Bexarotene increases uptake of radioiodide in metastases of differentiated thyroid carcinoma. Eur. J. Endocrinol. 2006, 154, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Ravaud, A.; Berton, D.; Chevreau, C.; Douillard, J.Y.; Dietrich, P.Y. Phase II study of interferon-alpha and all-trans retinoic acid in metastatic renal cell carcinoma. J. Immunother. 1998, 21, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Tokura, Y.; Sugaya, M.; Ohtsuka, M.; Tsuboi, R.; Nagatani, T.; Kiyohara, E.; Tani, M.; Setoyama, M.; Matsushita, S.; et al. Long-term efficacy and safety of bexarotene for Japanese patients with cutaneous T-cell lymphoma: The results of a phase 2 study (B-1201). J. Dermatol. 2019, 46, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Modiano, M.R.; Dalton, W.S.; Lippman, S.M.; Joffe, L.; Booth, A.R.; Meyskens, F.L. Phase II study of Fenretinide (N-[4-Hydroxyphenyl]retinamide) in advanced breast cancer and melanoma. Investig. New Drugs 1990, 8, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Worden, F.P.; Gadgeel, S.M.; Parchment, R.E.; Hodges, C.M.; Zwiebel, J.; Dunn, R.L.; Wozniak, A.J.; Kraut, M.J.; Kalemkerian, G.P. Phase II trial of fenretinide (NSC 374551) in patients with recurrent small cell lung cancer. Investig. New Drugs 2009, 27, 571–578. [Google Scholar] [CrossRef]
- Clark, P.E.; Hall, M.C.; Borden, L.S.; Miller, A.A.; Hu, J.J.; Lee, W.R.; Stindt, D.; D’Agostino, R.; Lovato, J.; Harmon, M.; et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006, 67, 1257–1261. [Google Scholar] [CrossRef]
- Datta, M.; Taylor, M.L.; Frizzell, B. Dietary and serum lycopene levels in prostate cancer patients undergoing intensity-modulated radiation therapy. J. Med. Food 2013, 16, 1131–1137. [Google Scholar] [CrossRef]
- Ansari, M.S.; Gupta, N.P. Lycopene: A novel drug therapy in hormone refractory metastatic prostate cancer. Urol. Oncol. 2004, 22, 415–420. [Google Scholar] [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review. Biofactors 2019, 45, 5–23. [Google Scholar] [CrossRef]
- Morifuji, M.; Ichikawa, S.; Kitade, M.; Fukasawa, T.; Asami, Y.; Manabe, Y.; Sugawara, T. Exopolysaccharides from milk fermented by lactic acid bacteria enhance dietary carotenoid bioavailability in humans in a randomized crossover trial and in rats. Am. J. Clin. Nutr. 2020, 111, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A. Cancer chemoprevention by natural carotenoids as an efficient strategy. Anticancer Agents Med. Chem. 2015, 15, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- McEligot, A.J.; Rock, C.L.; Shanks, T.G.; Flatt, S.W.; Newman, V.; Faerber, S.; Pierce, J.P. Comparison of serum carotenoid responses between women consuming vegetable juice and women consuming raw or cooked vegetables. Cancer Epidemiol. Biomark. Prev. 1999, 8, 227–231. [Google Scholar]
- McLarty, J.W.; Holiday, D.B.; Girard, W.M.; Yanagihara, R.H.; Kummet, T.D.; Greenberg, S.D. Beta-Carotene, vitamin A, and lung cancer chemoprevention: Results of an intermediate endpoint study. Am. J. Clin. Nutr. 1995, 62, 1431S–1438S. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Caroteno. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gaziano, J.M.; Norkus, E.P.; Buring, J.E.; Sesso, H.D. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am. J. Clin. Nutr. 2008, 88, 747–754. [Google Scholar] [CrossRef]
- Toma, S.; Bonelli, L.; Sartoris, A.; Mira, E.; Antonelli, A.; Beatrice, F.; Giordano, C.; Benazzo, M.; Caroggio, A.; Cavalot, A.L.; et al. 13-cis retinoic acid in head and neck cancer chemoprevention: Results of a randomized trial from the Italian Head and Neck Chemoprevention Study Group. Oncol. Rep. 2004, 11, 1297–1305. [Google Scholar] [CrossRef]
- Hanusova, V.; Skalova, L.; Kralova, V.; Matouskova, P. Potential anti-cancer drugs commonly used for other indications. Curr. Cancer Drug Targets 2015, 15, 35–52. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Effects of Carotenoids on Health: Are All the Same? Results from Clinical Trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef]
- Kucera, R.; Pecen, L.; Topolcan, O.; Dahal, A.R.; Costigliola, V.; Giordano, F.A.; Golubnitschaja, O. Prostate cancer management: Long-term beliefs, epidemic developments in the early twenty-first century and 3PM dimensional solutions. EPMA J. 2020. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Kunin, A.; Polivka, J.; Moiseeva, N.; Golubnitschaja, O. “Dry mouth” and “Flammer” syndromes—neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018, 9, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, V.; Bubnov, R.; Polivka, J.; Zubor, P.; Biringer, K.; Bielik, T.; Kuhn, W.; Golubnitschaja, O. Vaginal dryness: Individualised patient profiles, risks and mitigating measures. EPMA J. 2019, 10, 73–79. [Google Scholar] [CrossRef]
- Kunin, A.; Sargheini, N.; Birkenbihl, C.; Moiseeva, N.; Fröhlich, H.; Golubnitschaja, O. Voice perturbations under the stress overload in young individuals: Phenotyping and suboptimal health as predictors for cascading pathologies. EPMA J. 2020, 11, 517–527. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Liquid Biopsy is Instrumental for 3PM Dimensional Solutions in Cancer Management. J. Clin. Med. 2020, 9, 2749. [Google Scholar] [CrossRef]
- Crigna, A.T.; Samec, M.; Koklesova, L.; Liskova, A.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020, 1–25. [Google Scholar] [CrossRef]
- di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Aspects Med. 2015, 41, 1–115. [Google Scholar] [CrossRef]
- Singh, V.N.; Gaby, S.K. Premalignant lesions: Role of antioxidant vitamins and beta-carotene in risk reduction and prevention of malignant transformation. Am. J. Clin. Nutr. 1991, 53, 386S–390S. [Google Scholar] [CrossRef]
- Lu, R.; Dan, H.; Wu, R.; Meng, W.; Liu, N.; Jin, X.; Zhou, M.; Zeng, X.; Zhou, G.; Chen, Q. Lycopene: Features and potential significance in the oral cancer and precancerous lesions. J. Oral Pathol. Med. 2011, 40, 361–368. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Lotan, R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 2002, 41, 41–55. [Google Scholar] [CrossRef]
- Goldstein, E.; Yeghiazaryan, K.; Ahmad, A.; Giordano, F.A.; Fröhlich, H.; Golubnitschaja, O. Optimal multiparametric set-up modelled for best survival outcomes in palliative treatment of liver malignancies: Unsupervised machine learning and 3 PM recommendations. EPMA J. 2020, 11, 505–515. [Google Scholar] [CrossRef] [PubMed]
| Carotenoids Group | Carotenoids | Study Design | Mechanism | Ref. |
|---|---|---|---|---|
| Carotenes | α-carotene | Murine LLC, BCRC 60,050 Lewis lung carcinoma cells; murine C57BL/6 xenografts | ↓ MMP-2, ↓ MMP-9, ↓ uPA, ↑ TIMP-1, ↑ PAI-1, ↓ integrin β1-mediated phosphorylation of FAK, ↓ MAPK | [84] |
| β-carotene | BALB/c mouse smoking model | ↓ Notch pathway, ↑ E-cadherin, ↑ ZO-1, ↑ CK5, ↓ Snail-1, ↓ vimentin, ↓ N-cadherin | [85] | |
| SK-N-BE(2)C neuroblastoma cells; SK-N-BE(2)C nude mice | ↓ MMP-2, ↓ MMP-9, ↓ MT2 MMP, ↓ TIMP-1, ↓ TIMP-2, ↓ HIF-1α, ↓ VEGF, ↓ GLUT1 | [86] | ||
| HCT116 colorectal cancer cells; β-carotene-treated M2 macrophages and activated fibroblasts of azoxymethane/dextran sodium sulfate-induced colitis-associated colorectal cancer of male BALB/c mice | ↓ CSC markers (CD133, CD44, SOX2, and NOTCH1), ↓ invasiveness, ↓ migration, ↑ E-cadherin, ↓ IL-6/STAT3 signaling pathway, ↓ M2 macrophage polarization, ↓ fibroblast activation (α-SMA, FAP, and TGF-β1) | [87] | ||
| β-carotene 15,15′-oxygenase | BE(2)C neuroblastoma cells; murine BE(2)C xenografts | ↓ self-renewal, ↓ CSCs markers (DLK1, NOTCH1, SOX2, CD44, and CD133), ↓ MMP-2, ↓ MMP-9, ↓ MT1-MMP, ↓ MT2-MMP), ↓ TIMP-1, ↓ TIMP-2, ↓ HIF-1α, ↓ VEGF, ↓GLUT1, ↑ E-cadherin, ↓ N-cadherin, ↓ vimentin | [88] | |
| Lycopene | SK-Hep-1 liver adenocarcinoma cells | ↓ NOX4, ↓ ROS, ↓ MMP-9, ↓ MMP-2 | [89] | |
| CAL-27 and SCC-9 oral cancer cells; murine CAL-27 oral cancer xenografts | ↑ E-cadherin, ↓ N-cadherin, ↓ migration | [90] | ||
| OV-MZ-6 ovarian cancer cells | ↓ ITGA5, ↓ ITGB1, ↓ MMP-9, ↓ EMT markers (TWIST, ZEB2, SNAI-1 and -2, FOXC2, FN1, TGFB-1 and -2, TGFBR1, and SMAD4) | [91] | ||
| Lycopene-enriched tomato extract | N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinoma of female BALB/c mice | ↓ HIF-1α, ↓ VEGF, ↓ CD31, ↓ MMP-2, ↓ MMP-9 | [94] | |
| Apo-10′-lycopenoic acid | HuH7 liver and A549 lung cancer cells | ↑ PPARγ, ↓ MMP-2 | [93] | |
| Xanthophylls | Astaxanthin | Human HCT116 and murine CT26 colorectal cancer cells; murine colon cancer model | ↑ miR-29a-3p, ↑ miR-200a, ↓ MMP-2, ↓ ZEB1, ↓ EMT, ↓ MYC | [96] |
| A172 human glioblastoma cells | ↓ MMP-2, ↓ MMP-9 | [97] | ||
| MCF-7 ER+ and MDA-MB-231 breast cancer cells | ↓ migration | [98] | ||
| Astaxanthin with human serum albumin | SKOV3 ovarian cancer cells | ↓ migration | [99] | |
| configurational stereoisomers 3S, 3′S of AST | Mice injected with B16F10-PKH26 mouse melanoma cells | ↓ lung metastasis | [100] | |
| Fucoxanthin | A549, H1299, H446 lung cancer cells; murine PC9 xenografts | ↓ Snail, ↓ Twist, ↓ fibronectin, ↓ N-cadherin, ↓ MMP-2, ↓ PI3K/AKT/NF-κB pathway, ↑ TIMP-2 | [101] | |
| p53 wild-type U2OS osteosarcoma and p53 null SKOV3 ovarian cancer cells | ↓ Wnt-1, ↓ β-catenin., ↓ fibronectin, ↓ MMP-2, ↓ vimentin, ↓ VEGF | [102] | ||
| U87 and U251 human glioblastoma cells | ↓ MMP-2, ↓ MMP-9, ↓ uPA, ↓ phosphorylation of p38 | [103] | ||
| Fucoxanthinol | Colorectal CSCs | ↓ N-cadherin, ↓ vimentin, ↑ integrin signaling, ↓ sphere-formation, ↓ migration, ↓ invasion | [104] | |
| β-cryptoxanthin | AGS and SGC-7901 gastric cancer cells; murine AGS xenografts | ↓ MMP-2, ↓ MMP-9, ↓ VEGF, ↓ AMPK signaling, ↑ apoptosis | [105] | |
| Lutein | MDA-MB-157 and MCF-7 breast cancer cells | ↑ E-cadherin, ↓ vimentin, ↓ N-cadherin, ↓ NOTCH signaling, ↓ invasion, ↓ migration, ↓ HES1, ↓ ROS, ↑ hydrogen peroxide | [106] | |
| Zeaxanthin | C918 cultured uveal melanoma cells | ↓ MMP-2, ↓ NF-κB, ↓ migration, ↓ invasion | [107] | |
| Apocarotenoids | All-trans retinoic acids | Mice injected with K7M2 WT osteosarcoma cells | ↓ M2 polarization of TAMs, ↓ pulmonary metastatic nodes of osteosarcoma, ↓ MMP-12 | [108] |
| C57BL/6 mice injected with B16F10 murine melanoma cells | ↓ tumor nodules in lungs and liver | [109] | ||
| Paclitaxel-resistant HCT116, LoVo and CT26 colorectal cancer cells; BALB/c mice injected with CT26 murine colon cancer cells | ↓ NF-κΒ, ↑ gap junctions, ↓ fibronectin, ↓ MMP-9, ↓ N-cadherin, ↓ Snail, ↓ vimentin, ↓ β-catenin, ↑ E-cadherin | [110] | ||
| RKO human colon adenocarcinoma cells | ↓ cell movement, ↑ cell adhesion, ↓ MLCK, ↑ occludin, ↑ ZO-1, ↓ ERK1/MAPK signaling pathway | [111] | ||
| Murine hepa1-6 hepatocarcinoma cells | ↓ colony formation, ↓ migration, ↓ invasion, ↓ N-cadherin, ↓ vimentin, ↓ Snail, ↓ Twist, ↑ E-cadherin | [112] | ||
| Crocin and crocetin | 4T1 mammary carcinoma cells | ↓ migration, ↓ cell mobility, ↓ invasion, ↓ adhesion to extracellular matrix, ↓ Wnt/β-catenin, ↓ FZD7, ↓ NEDD9, ↓ VEGF-ɑ, ↓ vimentin, ↑ E-cadherin | [113] | |
| Murine triple negative breast cancer model | ↓ metastatic foci in livers and lungs | [114] | ||
| Crocin | BALB/c mice injected with 4T1 mammary carcinoma cells | ↓ Wnt/β-catenin target genes (NEDD9, VEGF-ɑ, MMP-9, FZD7 and VIM) | [115] | |
| AGS and HGC-27 gastric cancer cells | ↓ KLF5, ↓ HIF-1α, ↑ miR-320, ↓ migration, ↓ invasion, ↑ E-cadherin, ↓ Snail, ↓ N-cadherin | [116] | ||
| IL-6-stimulated Hep3B and HepG2 liver cancer cells | ↓ STAT3, ↓ JAK1, JAK2, ↓ Src kinase, ↓ CXCR4, ↓ VEGF, ↓ invasion | [118] | ||
| Retinamide (VNLG-152) | 22Rv1 prostate cancer cells | ↑ E-cadherin, ↓ N-cadherin, ↓ β-catenin, ↓ MMP-2, ↓ MMP-9, ↓ claudin, ↓ vimentin, ↓ Snail, ↓ Slug, ↓ Twist | [119] | |
| Alkylamide derivatives of bexarotene DK-1–150 and DK-1–166 | BT549, and MDA-MB-231 triple-negative breast cancer cell lines | ↓ migration, modulated CSC markers (c-Myc, KLF4, Nanog, Oct4A, and SOX2), ↑ E-cadherin | [120] | |
| Fenretinide | HepG2 liver cancer cells | ↓ migration, ↑ E-cadherin, ↑ phosphorylation of p38-MAPK, ↓ MLCK | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in Cancer Metastasis—Status Quo and Outlook. Biomolecules 2020, 10, 1653. https://doi.org/10.3390/biom10121653
Koklesova L, Liskova A, Samec M, Zhai K, Abotaleb M, Ashrafizadeh M, Brockmueller A, Shakibaei M, Biringer K, Bugos O, et al. Carotenoids in Cancer Metastasis—Status Quo and Outlook. Biomolecules. 2020; 10(12):1653. https://doi.org/10.3390/biom10121653
Chicago/Turabian StyleKoklesova, Lenka, Alena Liskova, Marek Samec, Kevin Zhai, Mariam Abotaleb, Milad Ashrafizadeh, Aranka Brockmueller, Mehdi Shakibaei, Kamil Biringer, Ondrej Bugos, and et al. 2020. "Carotenoids in Cancer Metastasis—Status Quo and Outlook" Biomolecules 10, no. 12: 1653. https://doi.org/10.3390/biom10121653
APA StyleKoklesova, L., Liskova, A., Samec, M., Zhai, K., Abotaleb, M., Ashrafizadeh, M., Brockmueller, A., Shakibaei, M., Biringer, K., Bugos, O., Najafi, M., Golubnitschaja, O., Büsselberg, D., & Kubatka, P. (2020). Carotenoids in Cancer Metastasis—Status Quo and Outlook. Biomolecules, 10(12), 1653. https://doi.org/10.3390/biom10121653










