Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots
Abstract
:1. Introduction
2. Results
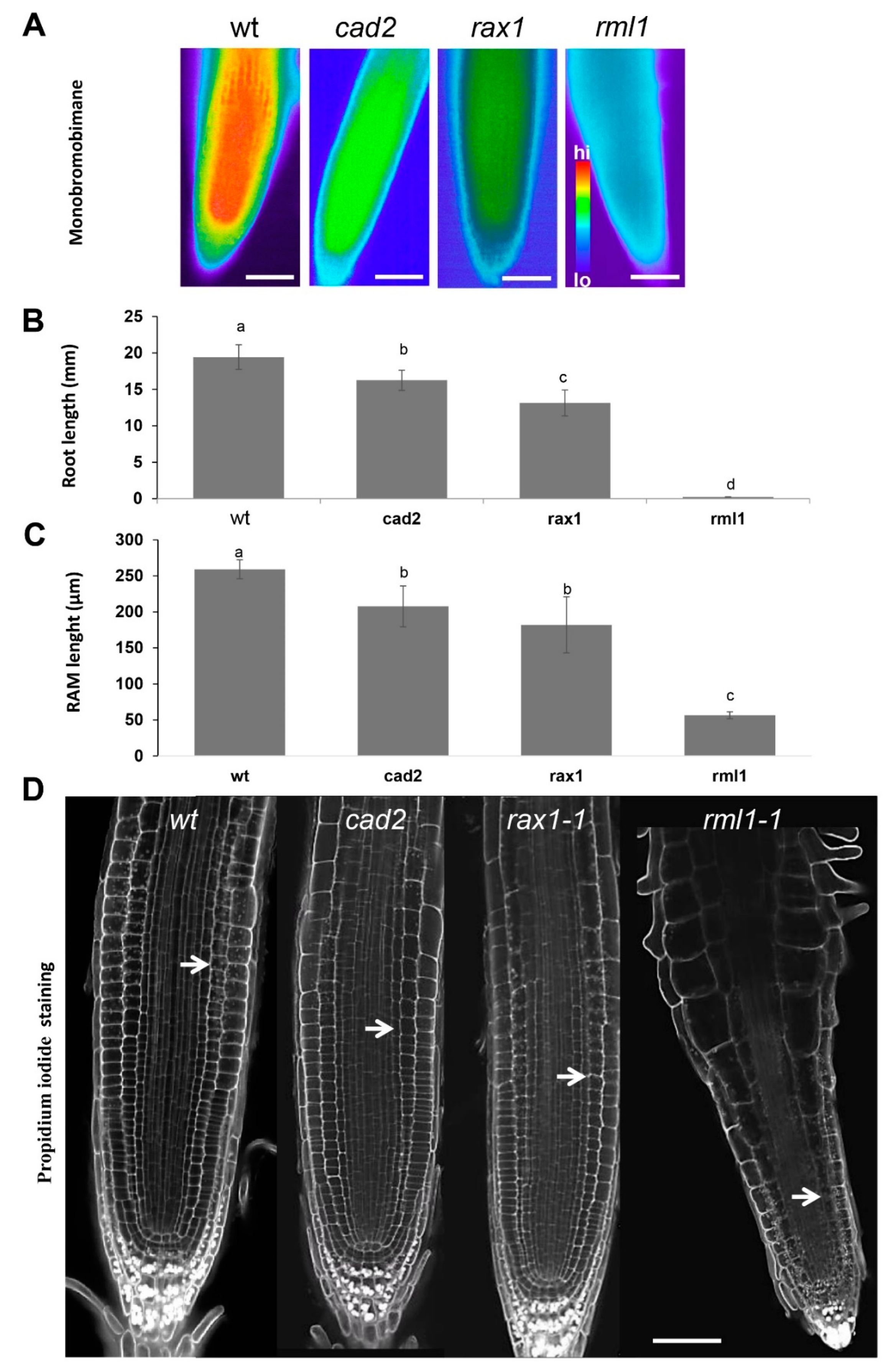
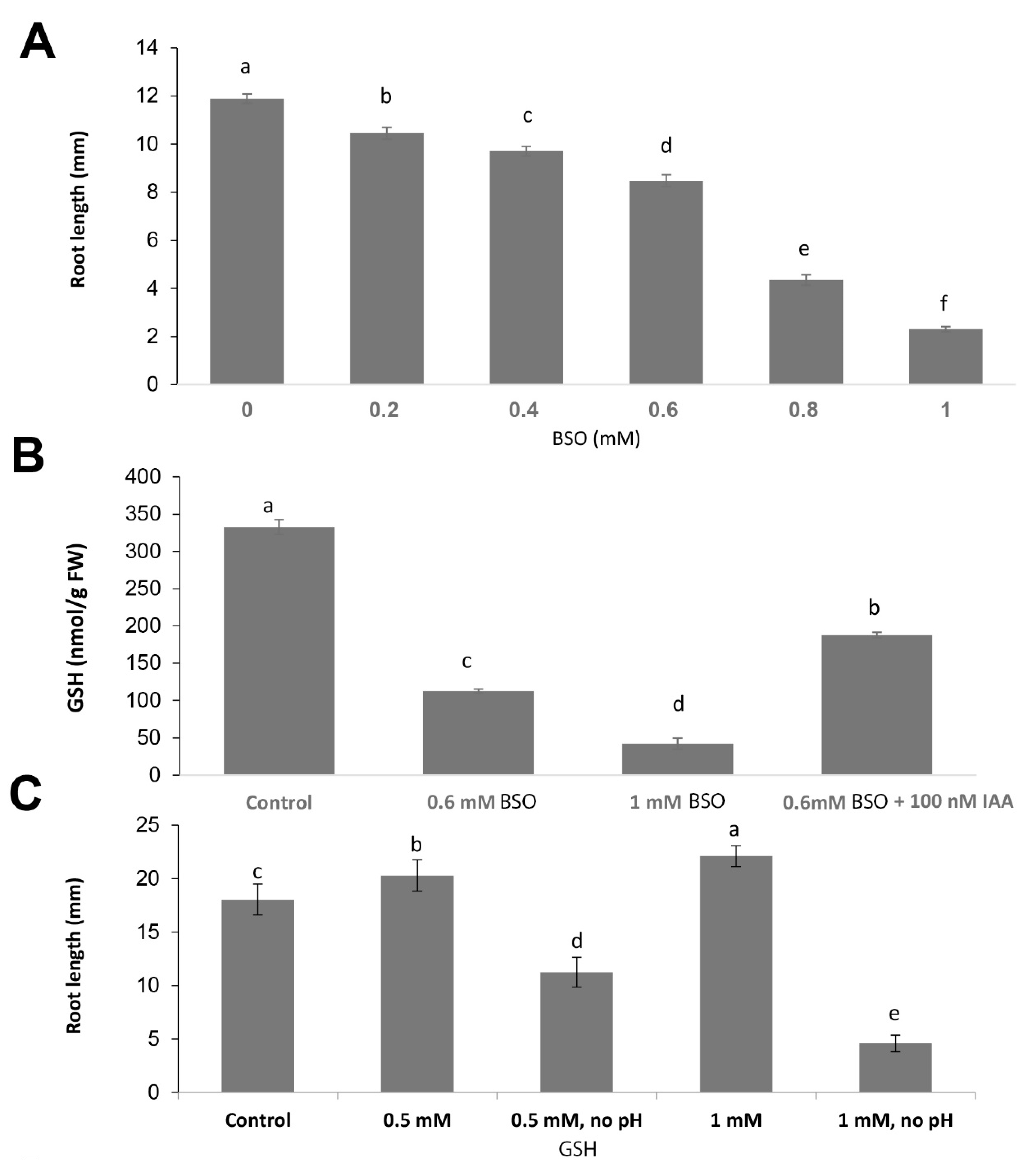
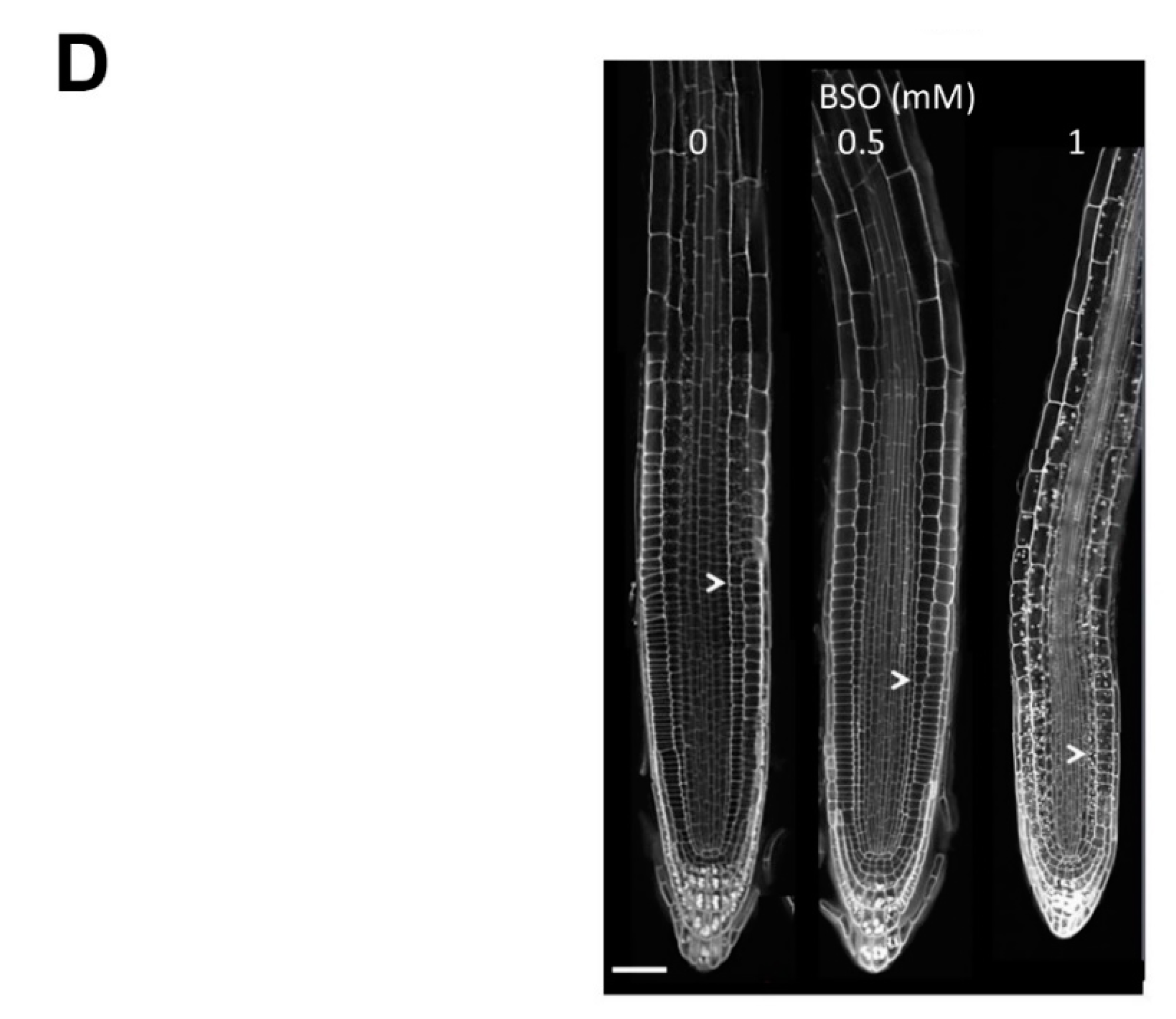
2.1. Gsh Controls Root Apical Meristem and Growth 0f Primary Root
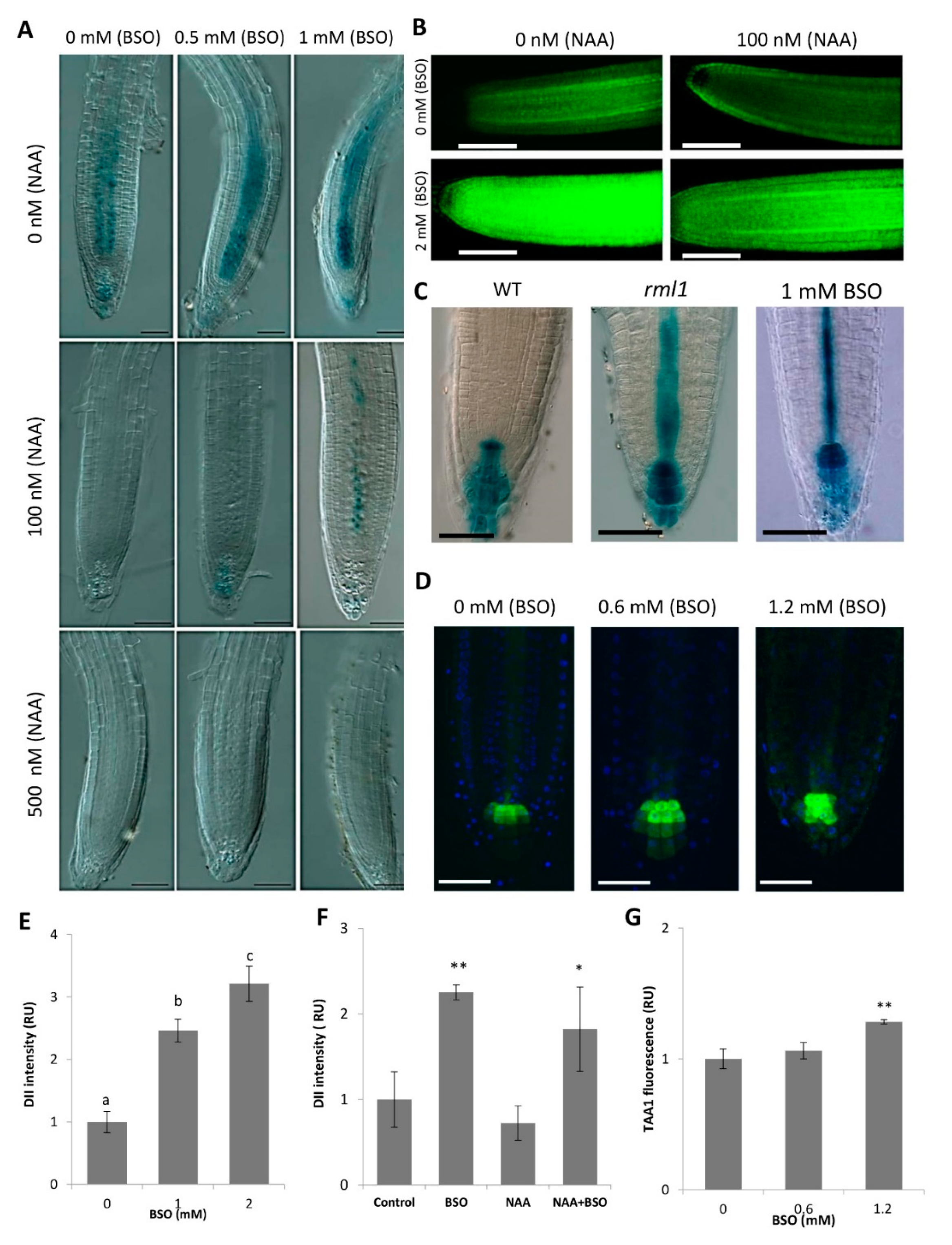
2.2. Gsh Modulates Auxin Signaling and Auxin Biosynthesis in the Ram
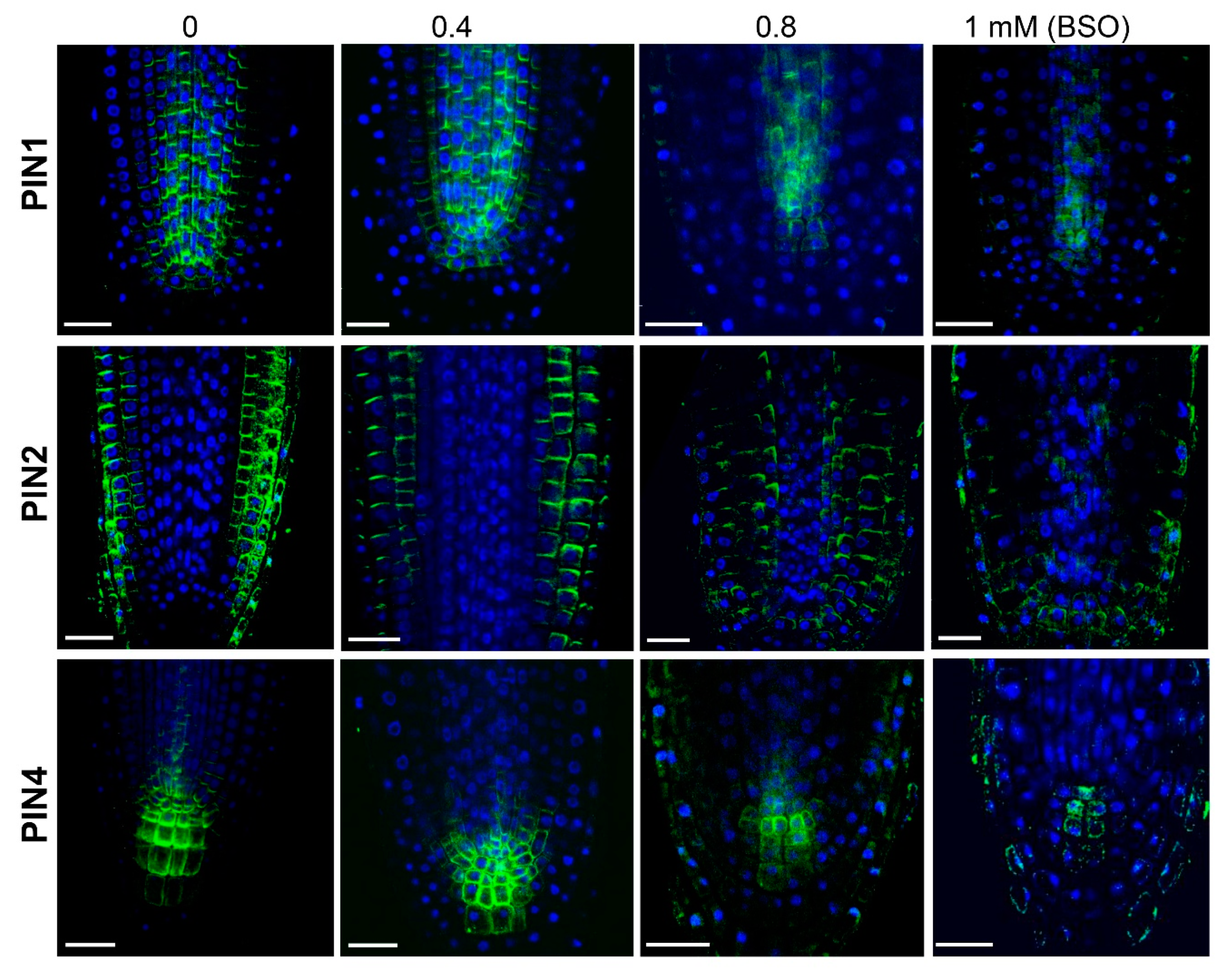
2.3. GSH Level Affects PIN-Modulated Auxin Transport
2.4. GSH Enhances Auxin Signaling in Isolated Root Segments
3. Discussion
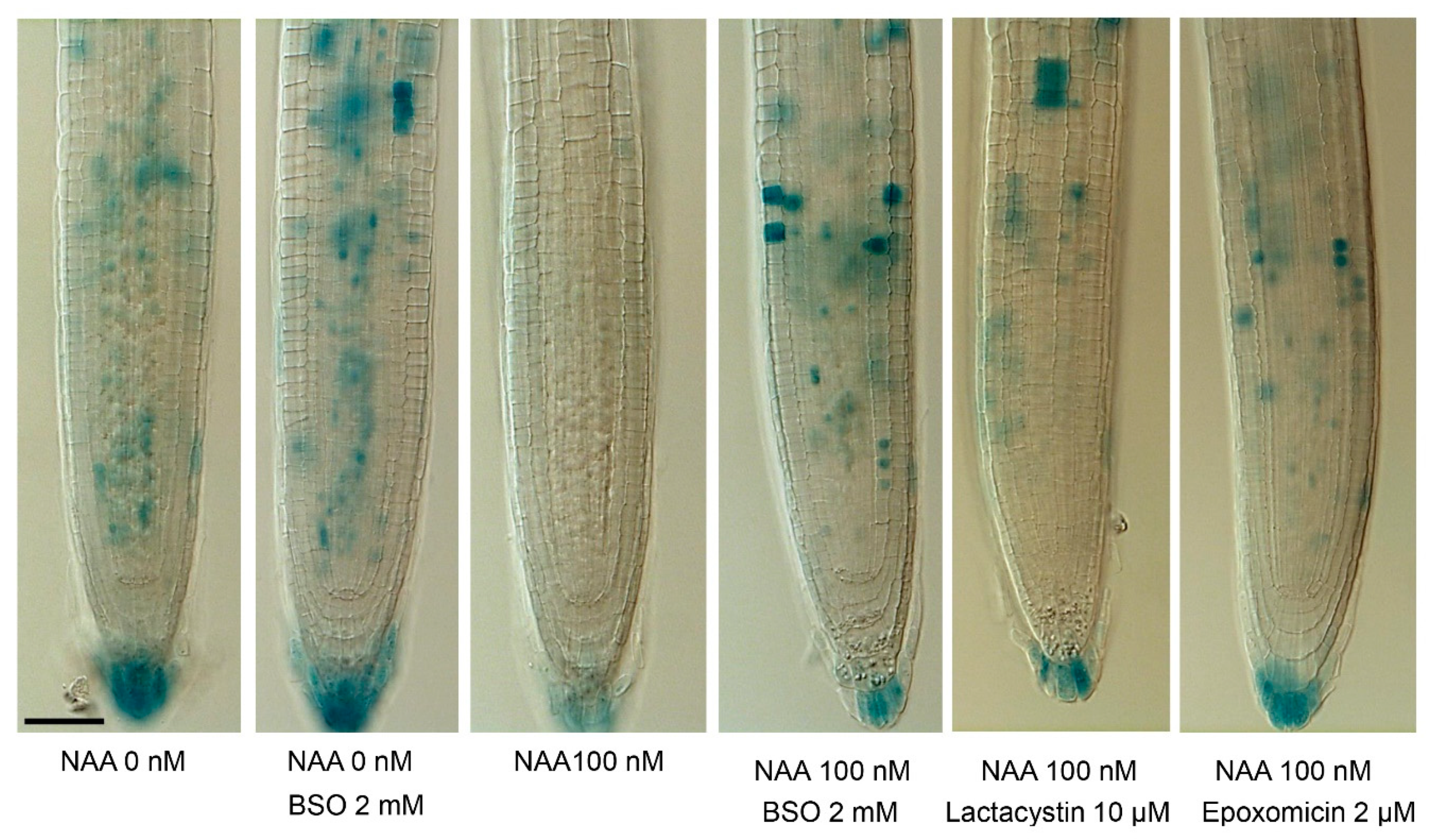
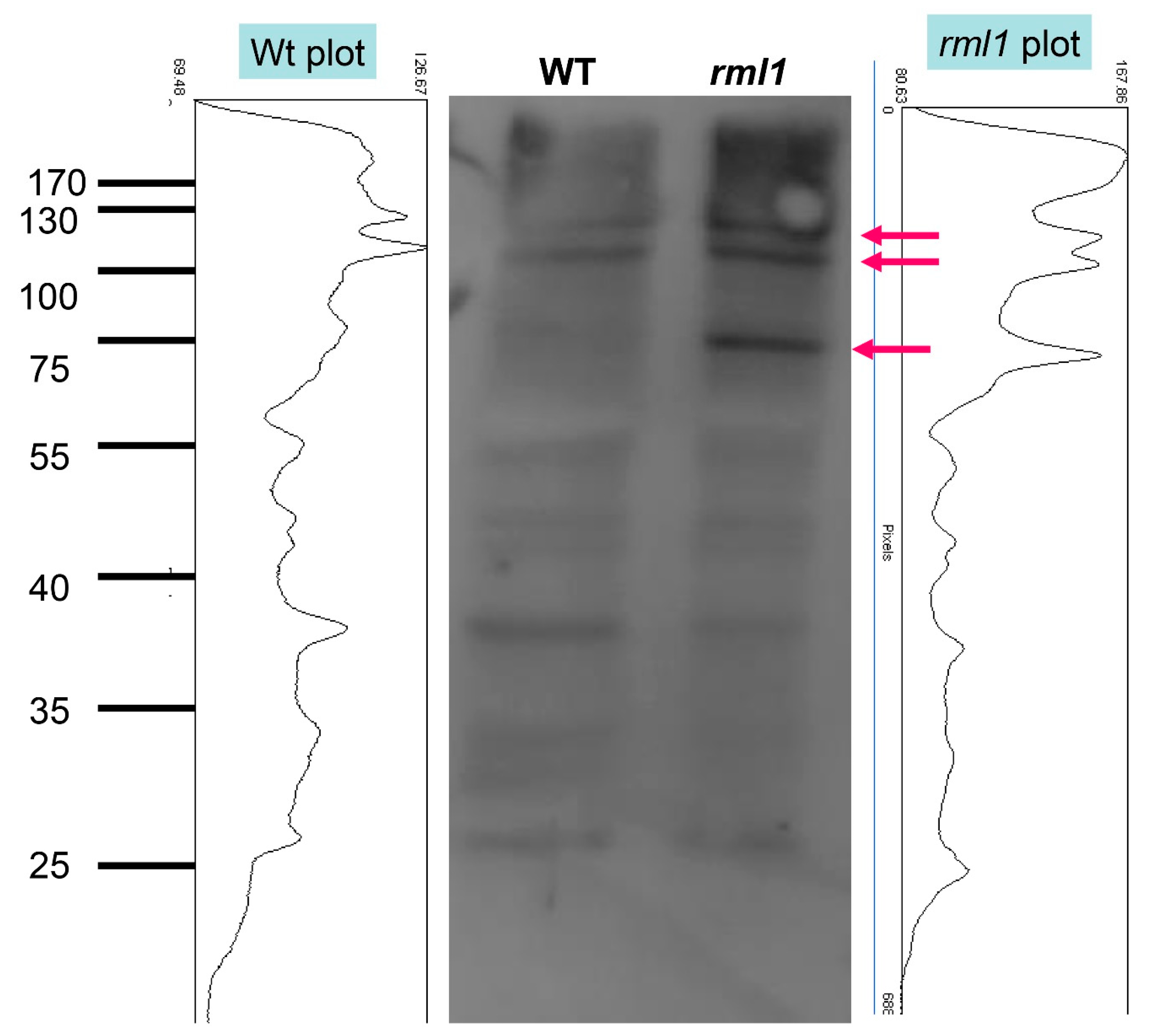
3.1. Glutathione Increases Auxin Sensitivity by Interfering with Ubiquitin-Mediated Proteolysis
3.2. Cross-Talk Between Glutathione and Auxins in the Apical Meristem
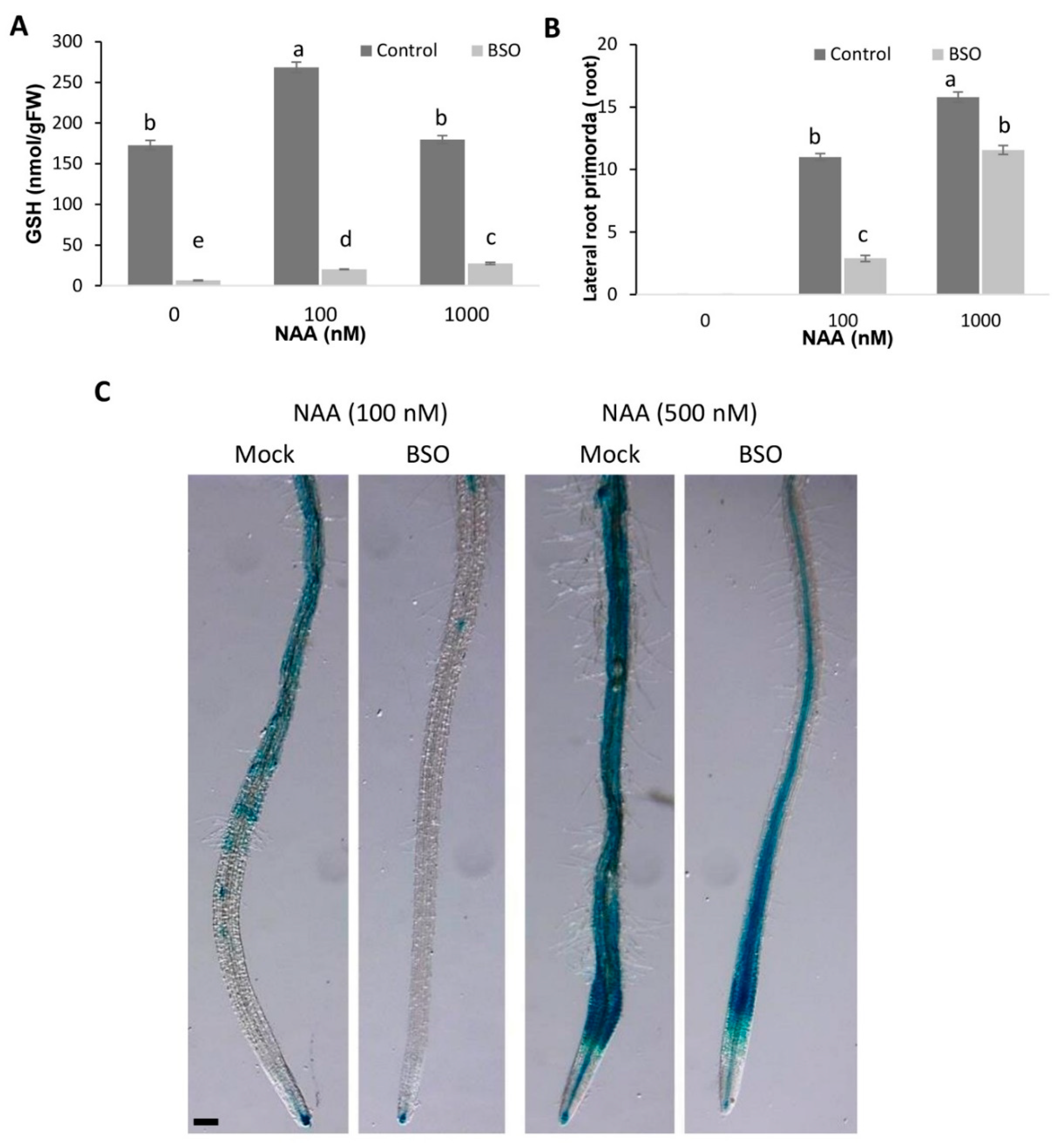
3.3. Cross-Talk between Glutathione and Auxins by the Origination of Lateral Roots
4. Outlook
5. Materials and Methods
5.1. Plant Material and Treatments
5.2. Isolated Root Experiments
5.3. Whole-mount In Situ Immunolocalization
5.4. GUS Assay
5.5. Quantitative Real-Time PCR
5.6. HPLC Determination of GSH
5.7. Root Structure
5.8. Western Blot and Ubiquitination Assay
5.9. Thiol Localizations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potters, G.; Pasternak, T.; Guisez, Y.; Palme, K.; Jansen, M. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Jansen, M.A. Different stresses, similar morphogenic responses: Integrating a plethora of pathways. Plant Cell Environ. 2009, 32, 158–169. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [Green Version]
- Potters, G.; Horemans, N.; Jansen, M. The cellular redox state in plant stress biology—A charging concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Marquez-Garcia, J.; Foyer, C. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995, 252, 26–30. [Google Scholar] [PubMed]
- May, M.; Leaver, C.J. Arabidopsis thaliana c-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast and E. coli homologs. Proc. Natl. Acad. Sci. USA 1994, 91, 10059–10063. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.L.; Oliver, D.J. Cloning of the cDNA and genomic clones for glutathione synthetase from Arabidopsis thaliana and complementation of a gsh2 mutant in fission yeast. Plant Mol. Biol. 1996, 31, 1093–1104. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Marrs, K.A. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Janaky, R.; Ogita, K.; Pasqualotto, B.A.; Bains, J.S.; Oja, S.S.; Yoneda, Y.; Shaw, C.A. Glutathione and signal transduction in the mammalian CNS. J. Neurochem. 1999, 73, 889–893. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- May, M.J.; Vernoux, T.; Leaver, C.; Van Montagu, M.; Inzé, D. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 1998, 49, 649–667. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Arisi, A.C.M.; Jouanin, L.; Kunert, K.J.; Rennenberg, H.; Foyer, C.H. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar] [CrossRef]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potters, G.; Horemans, N.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potters, G.; Horemans, N.; Jansen, M.; Guisez, Y.; Pasternak, T. Dehydroascorbate and glutathione regulate the cellular development of Nicotiana tabacum L. SR-1 protoplasts. In Vitro Cell. Dev. Biol. Plant 2010, 46, 289–297. [Google Scholar] [CrossRef]
- Pasternak, T.; Asard, H.; Potters, G.; Jansen, M.A. The thiol compounds glutathione and homoglutathione differentially affect cell development in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2014, 74, 16–23. [Google Scholar] [CrossRef]
- Sanchez-Fernandez, R.; Fricker, M.; Corben, L.B.; White, N.S.; Sheard, N.; Leaver, C.J.; Van Montagu, M.; Inzé, D.; May, M.J. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA 1997, 94, 2745–2750. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, M.F.; Donald, G.; Reid, D.M.; Yenne, E.; Stasolla, C. Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J. Exp. Bot. 2005, 56, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, M.F.; Ambrose, S.J.; Ross, A.R.S.; Abrams, S.; Stasolla, S. Improved development of microspore-derived embryo cultures of Brassica napus cv. Topaz following changes in glutathione metabolism. Physiol. Plantarum 2006, 127, 690–700. [Google Scholar] [CrossRef]
- Stasolla, C. Glutathione redox regulation of in vitro embryogenesis, Plant Physiol. Biochem. 2010, 48, 319–327. [Google Scholar]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Lijng, K.; Meyer, Y.; Reichheld, J.-P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Koprivova, A.; Mugford, S.; Kopriva, S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep. 2010, 29, 1157–1167. [Google Scholar] [CrossRef]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, K.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Galweiler, L.; Guan, C.H.; Muller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.; Guan, C.H.; Galweiler, L.; Tanzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Benkova, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jurgens, G.; et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 108, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Friml, J.; Wisniewska, J.; Benkova, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jurgens, G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; ten Hove, C.A.; Hogeweg, P.; Maree, A.F.M.; Scheres, B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008, 6, 2721–2735. [Google Scholar] [CrossRef] [Green Version]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; Frey, N.F.D.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D.; et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rybel, B.; Vassileva, V.; Parizot, B.; Demeulenaere, M.; Grunewald, W.; Audenaert, D.; Van Campenhout, J.; Overvoorde, P.; Jansen, L.; Vanneste, S.; et al. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 2010, 20, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benkova, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.J.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Eklof, J.; Ljung, K.; Marchant, A.; Bennett, M.; Sandberg, G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002, 29, 325–332. [Google Scholar] [CrossRef]
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 2009, 151, 1855–1866. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Doležal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Simonini, S.; Mas, P.J.; Mas, C.M.; Østergaard, L.; Hart, D.J. Auxin sensing is a property of an unstructured domain in the Auxin Response Factor ETTIN of Arabidopsis thaliana. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.; Friml, J. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 2018, 4, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Paponov, I.; Teale, W.; Lang, D.; Paponov, M.; Reski, R.; Rensing, S.; Palme, K. The evolution of nuclear auxin signaling. BMC Evol. Biol. 2009, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobbett, C.S.; May, M.J.; Howdenn, R.; Rolls, B. The gluthatione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in y-glutamil-cysteine synthetase. Plant J. 1998, 16, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Accotto, G.P.; Bechtold, U.; Creissen, D.; Funck, D.; Jimenez, A.; Kular, B.; Leyland, N.; Mejia-Carranza, J.; Reynolds, H.; et al. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 2004, 16, 2448–2462. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Seeley, K.; Sung, Z. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 1995, 107, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. CSH Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [Green Version]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef]
- Brunoud, G.; Wells, D.M.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103–106. [Google Scholar] [CrossRef]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechold, N.; Weisbeek, P.; et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Zwiewka, M.; Nodzynski, T.; Robert, S.; Vanneste, S.; Friml, J. Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol. Plant 2015, 8, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Paponov, I.A.; Budnyk, V.; Paponov, M.; Teale, W.; Palme, K. Butylated hydroxytoluene (BHT) inhibits PIN1 exocytosis from BFA compartments in Arabidopsis roots. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Vieten, A.; Vanneste, S.; Wiśniewska, J.; Benková, E.; Benjamins, R.; Beeckman, T.; Luschnig, C.; Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005, 132, 4521–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, R.C.; Brady, S.R.; Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, W.M.; Estelle, M. Function of the ubiquitin–proteasome pathway in auxin response. Trends Biochem. Sci. 2000, 25, 133–138. [Google Scholar] [CrossRef]
- Marrè, E.; Arrigoni, O. Metabolic reactions to auxin I. The effects of auxin on glutathione and the effects of glutathione on growth of isolated plant parts. Physiol. Plantarum 1957, 10, 289–301. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010, 61, 1029–1040. [Google Scholar] [CrossRef]
- Furniss, J.J.; Spoel, S.H. Cullin-RING ubiquitin ligases in salicylic acid-mediated plant immune signaling. Front. Plant Sci. 2015, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Boro, P.; Sultana, A.; Mandal, K.; Chattopadhyay, S. Transcriptomic changes under stress conditions with special reference to glutathione contents. Nucleus 2018, 61, 241–252. [Google Scholar] [CrossRef]
- Han, Y.; Mhamdi, A.; Chaouch, S.; Noctor, G. Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ. 2013, 36, 1135–1146. [Google Scholar] [CrossRef]
- Lee, J.G.; Baek, K.; Soetandyo, N.; Ye, Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Meng, Y.L.; Feldman, L.J. Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 2003, 130, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, W.A.; Blakeslee, J.J.; Yang, H.B.; Murphy, A.S. Seven things we think we know about auxin transport. Mol. Plant 2011, 4, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-M.; Xu, H.-H.; Liu, W.-C.; Lu, Y.-T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 2013, 54, 766–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamowski, M.; Friml, J. PIN-Dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [Green Version]
- De Tullio, M.C.; Jiang, K.; Feldman, L.J. Redox regulation of root apical meristem organization: Connecting root development to its environment. Plant Physiol. Biochem. 2010, 48, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Chen, R.; Li, P.; Yu, Y.; Zheng, R.; Ge, D.; Zheng, W.; Wang, X.; Gu, Y.; Gelova, Z.; et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 2019, 568, 240–243. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Meng, X. MAPK signaling: Emerging roles in lateral root formation. Trends Plant Sci. 2020, 25, 126–129. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, R.; He, J.; Zhou, Z.; Wang, J.; Xiong, Y.; Xu, T. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc. Natl. Acad. Sci. USA 2019, 116, 21285–21290. [Google Scholar] [CrossRef] [Green Version]
- Lv, B.; Yu, Q.; Liu, J.; Wen, X.; Yan, Z.; Hu, K.; Li, H.; Kong, X.; Li, C.; Tian, H.; et al. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signalling. EMBO J. 2020, 39, e101515. [Google Scholar] [CrossRef]
- Kerk, N.M.; Feldman, L.J. A biochemical model for the initiation and maintenance of the quiescent centre: Implications for organization of root meristem. Development 1995, 121, 2825–2833. [Google Scholar]
- Shaw, J.P.; Chou, I.N. Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J. Cell. Physiol. 1986, 129, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Espunya, M.C.; Díaz, M.; Moreno-Romero, J.; Martínez, M.C. Modification of intracellular levels of glutathione-dependent formaldehyde dehydrogenase alters glutathione homeostasis and root development. Plant Cell Environ. 2006, 29, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Nongmaithem, S.; Ponukumatla, R.; Sreelakshmi, Y.; Frasse, P.; Bouzayen, M.; Sharma, R. Enhanced polar auxin transport in tomato polycotyledon mutant seems to be related to glutathione levels. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Vivancos, P.D.; Dong, Y.; Ziegler, K.; Markovic, J.; Pallardó, F.V.; Pellny, T.K.; Verrier, P.J.; Foyer, C.H. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010, 64, 825–838. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Doerner, P.W.; Colón-Carmona, A.; Rost, T.L. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol. 2000, 124, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, V.B.; Mühlenbock, P.; van Breusegem, F. Stress homeostasis—The redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef]
- Ruegsegger, A.; Schmutz, D.; Brunold, C. Regulation of glutathione synthesis by cadmium in Pisum sativum. Plant Physiol. 1990, 93, 1579–1584. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, L.; Rennenberg, H. Glutathione metabolism in plants. In Sulfur Nutrition and Assimilation in Higher Plants; De Kok, L.J., Stulen, I., Rennenberg, H., Brunold, C., Rauser, W., Eds.; SPB Academic: The Hague, The Netherlands, 1993; pp. 109–123. [Google Scholar]
- Kocsy, G.; Szalai, G.; Galiba, G. Induction of glutathione synthesis and glutathione reductase activity by abiotic stresses in maize and wheat. Sci. World J. 2002, 2, 1726–1732. [Google Scholar] [CrossRef] [Green Version]
- Xiang, C.; Oliver, D.J. Glutathione and its central role in mitigating plant stress. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: Basel, Switzerland, 1999; pp. 697–708. [Google Scholar]
- Ruiz, J.M.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar] [CrossRef]
- Colon-Carmona, A.; You, R.; Haimovitch-Gal, T.; Doerner, P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999, 20, 503–508. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pasternak, T.; Tietz, O.; Rapp, K.; Begheldo, M.; Nitschke, R.; Ruperti, B.; Palme, K. Protocol: An improved and universal procedure for whole-mount immunolocalization in plants. Plant Methods 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Truernit, E.; Bauby, H.; Dubreucq, B.; Grandjean, O.; Runions, J.; Barthélémy, J.; Palauqui, J.C. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 2008, 20, 1494–1503. [Google Scholar] [CrossRef] [Green Version]
- Makowski, G.S.; Ramsby, M.L. pH modification to enhance the molecular sieving properties of sodium dodecyl sulfate-10% polyacrylamide gels. Anal. Biochem. 1993, 212, 283–285. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasternak, T.; Palme, K.; Paponov, I.A. Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots. Biomolecules 2020, 10, 1550. https://doi.org/10.3390/biom10111550
Pasternak T, Palme K, Paponov IA. Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots. Biomolecules. 2020; 10(11):1550. https://doi.org/10.3390/biom10111550
Chicago/Turabian StylePasternak, Taras, Klaus Palme, and Ivan A. Paponov. 2020. "Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots" Biomolecules 10, no. 11: 1550. https://doi.org/10.3390/biom10111550
APA StylePasternak, T., Palme, K., & Paponov, I. A. (2020). Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots. Biomolecules, 10(11), 1550. https://doi.org/10.3390/biom10111550






