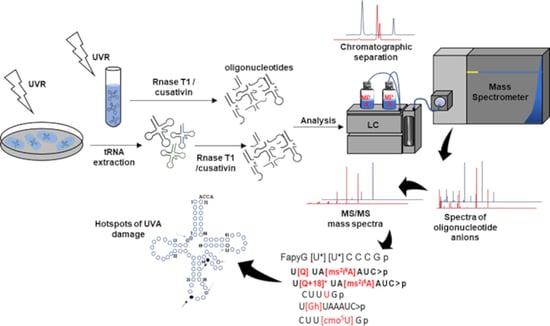
Characterization of UVA-Induced Alterations to Transfer RNA Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. UVR Exposure
2.4. E. coli Cell Culture and UVR Exposure
2.5. Transfer RNATyr Purification
2.6. Oxidized Oligoribonucleotide Characterization
2.7. Data Analysis
3. Results
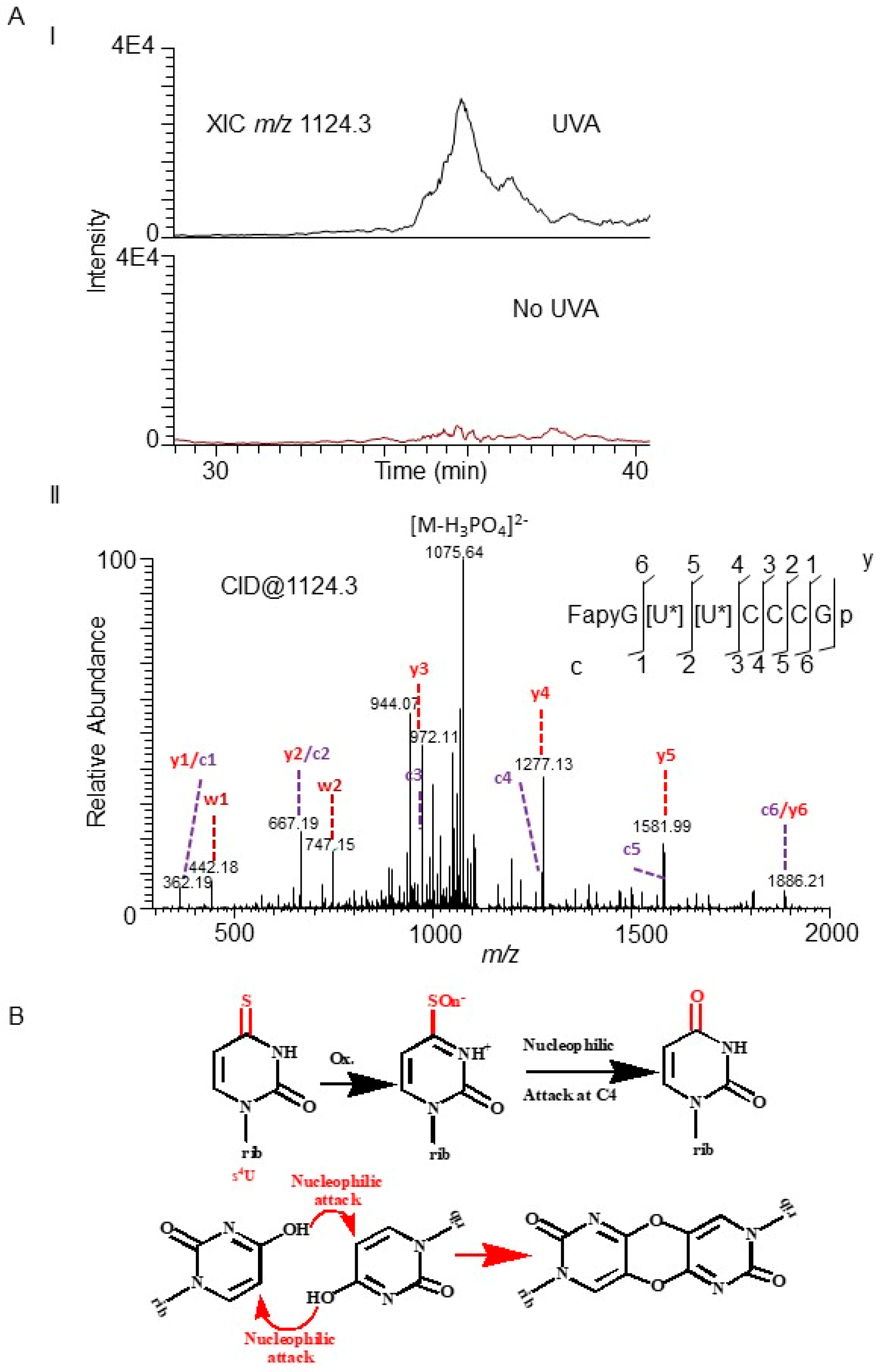
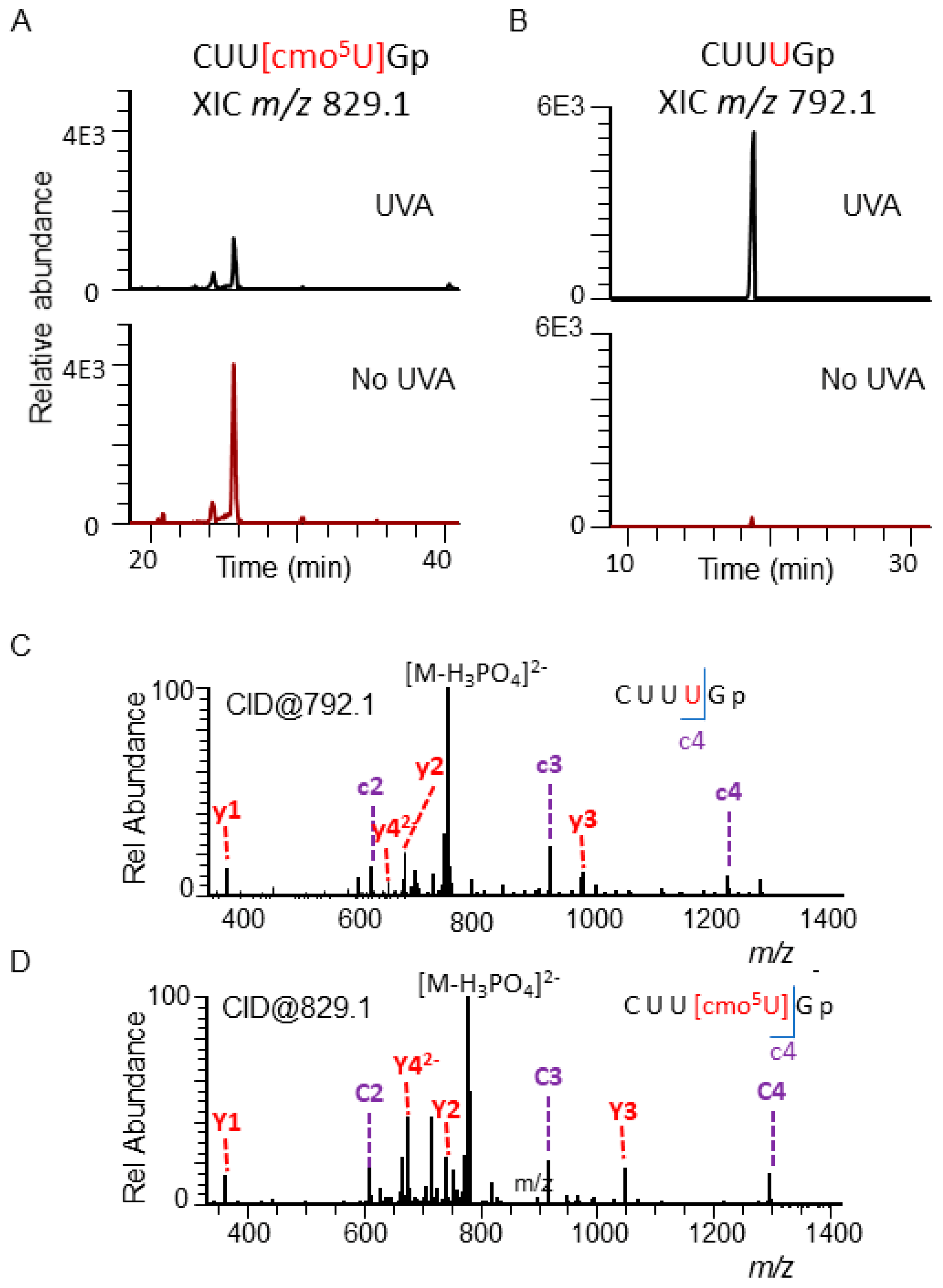
3.1. Identification of the Susceptible Regions of tRNA to Photooxidation
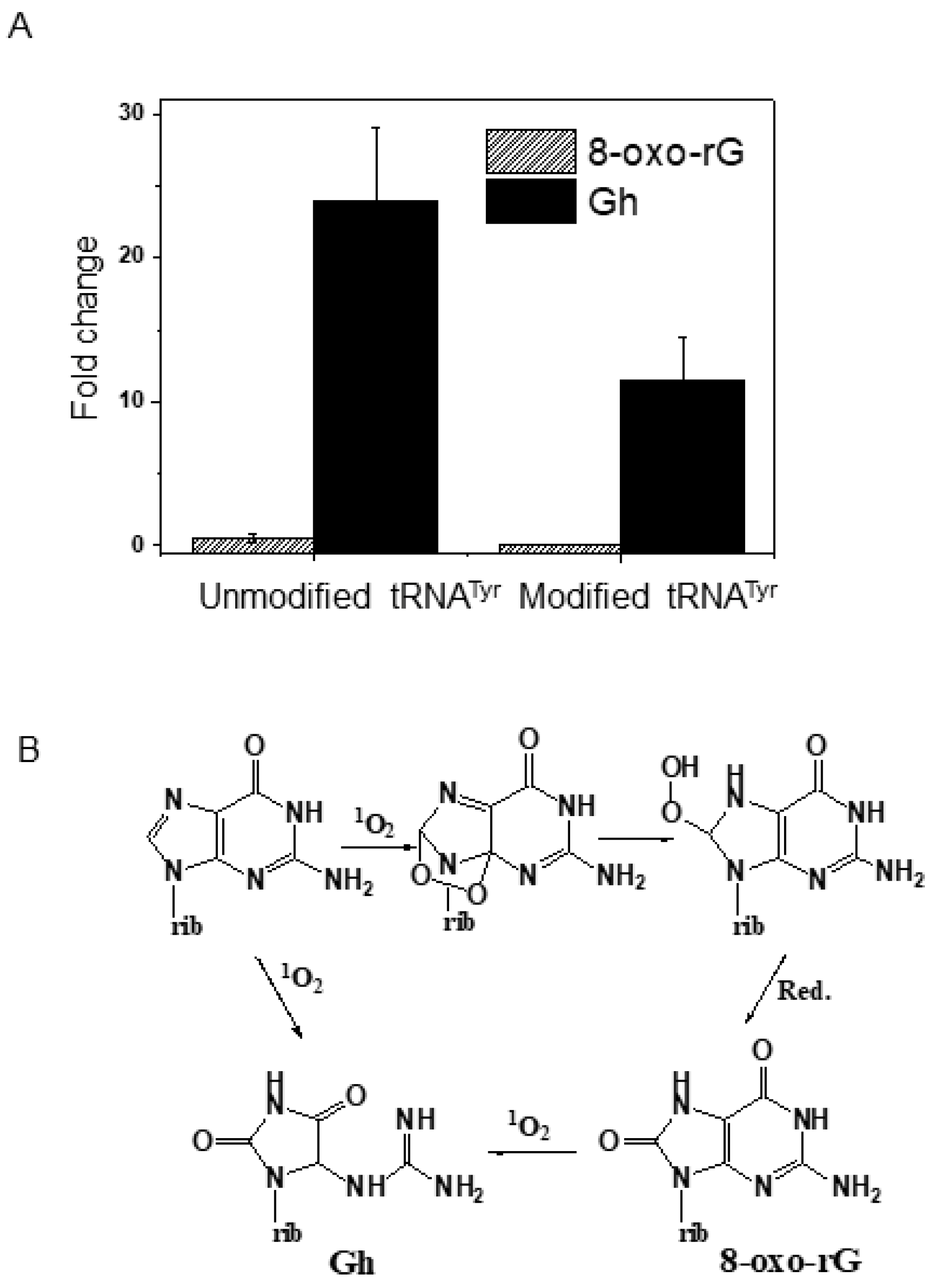
3.2. UVA Exposure Effects on tRNA Under In Vivo (In Cellular) Conditions
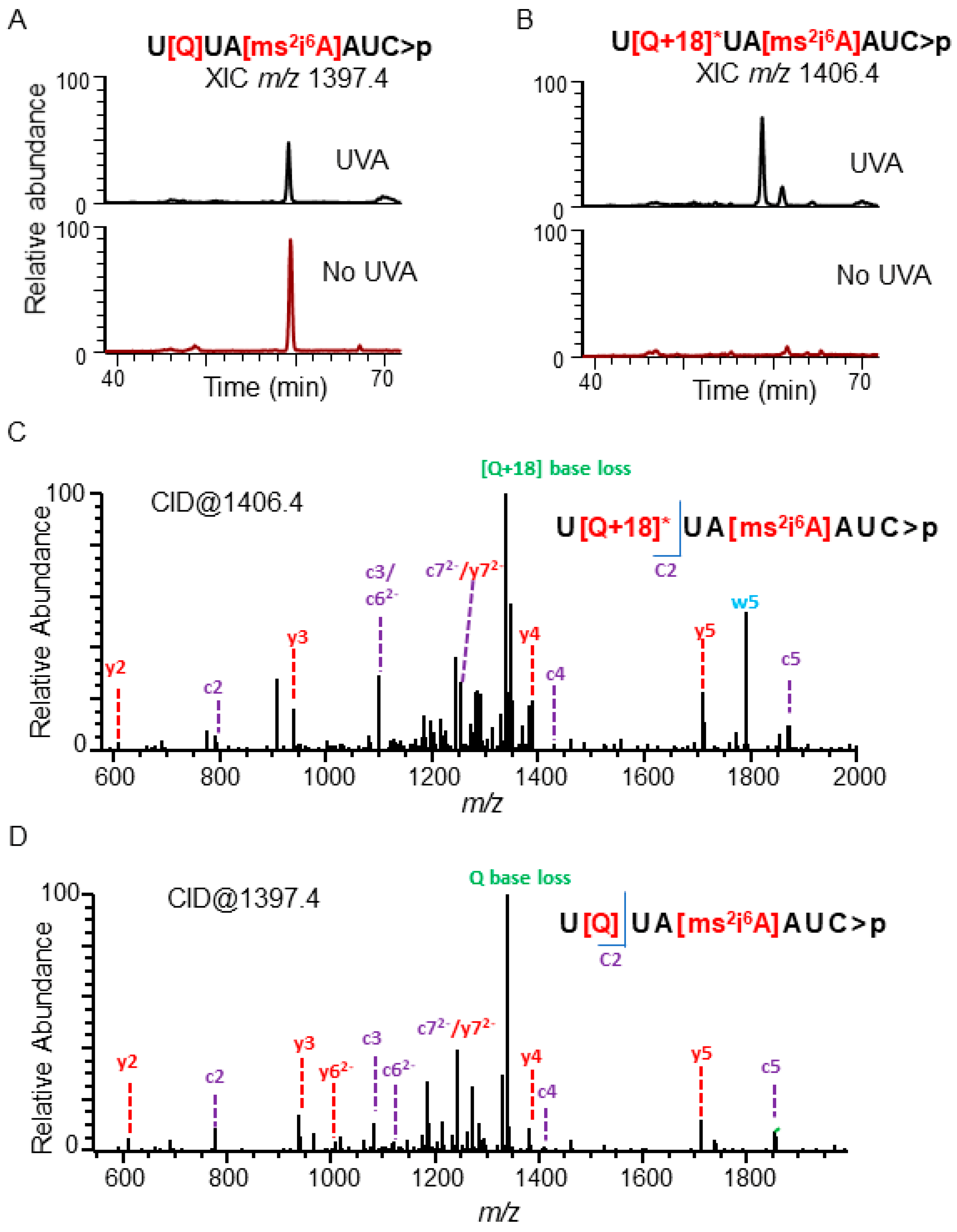
3.3. Mapping UVA-Induced Effects on Other tRNAs
4. Discussion
4.1. Hotspots of Photooxidative Damage in tRNA
4.2. Modifications in tRNA can Influence the Pattern of Photooxidative Damage
4.3. Detection of the Photooxidative Products of Post-Transcriptional Nucleoside Modifications
4.4. Potential Consequences on tRNA due to Photooxidative Degradation of PTMs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Quigley, G.; Rich, A. Structural domains of transfer RNA molecules. Science 1976, 194, 796–806. [Google Scholar] [CrossRef]
- Schmeing, T.M.; Voorhees, R.M.; Kelley, A.C.; Gao, Y.-G.; Murphy, F.V.; Weir, J.R.; Ramakrishnan, V. The Crystal Structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009, 326, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ferre-D’Amare, A.R. The tRNA Elbow in structure, recognition, and evolution. Life 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Ibba, M. Bacterial transfer RNAs. FEMS Microbiol. Rev. 2015, 39, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piatkowski, P.; Baginski, B.; Wirecki, T.K.; de Crecy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018, 46, D303–D307. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Narendran, A.; Sarachan, K.; Väre, V.Y.P.; Eruysal, E. The importance of being modified: The role of RNA modifications in translational fidelity. In The Enzymes; Elsevier: Oxford, UK, 2017; Volume 41, pp. 1–50. [Google Scholar] [CrossRef]
- Jackman, J.E.; Alfonzo, J.D. Transfer RNA modifications: Nature’s combinatorial chemistry playground. Wiley Interdiscip. Rev. RNA 2013, 4, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Helm, M. TRNA stabilization by modified nucleotides. Biochemistry 2010, 49, 4934–4944. [Google Scholar] [CrossRef]
- Whipple, J.M.; Lane, E.A.; Chernyakov, I.; D’Silva, S.; Phizicky, E.M. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011, 25, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Lünse, C.E.; Mörl, M. tRNA modifications: Impact on structure and thermal adaptation. Biomolecules 2017, 7, 35. [Google Scholar] [CrossRef]
- Väre, V.Y.P.; Eruysal, E.R.; Narendran, A.; Sarachan, K.L.; Agris, P.F. Chemical and conformational diversity of modified nucleosides affects tRNA structure and function. Biomolecules 2017, 7, 29. [Google Scholar] [CrossRef]
- Deng, W.; Babu, I.R.; Su, D.; Yin, S.; Begley, T.J.; Dedon, P.C. Trm9-catalyzed tRNA modifications regulate global protein expression by codon-biased translation. PLoS Genet. 2015, 11, e1005706. [Google Scholar] [CrossRef]
- Begley, U.; Dyavaiah, M.; Patil, A.; Rooney, J.P.; DiRenzo, D.; Young, C.M.; Conklin, D.S.; Zitomer, R.S.; Begley, T.J. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell 2007, 28, 860–870. [Google Scholar] [CrossRef]
- Torrent, M.; Chalancon, G.; De Groot, N.S.; Wuster, A.; Madan Babu, M. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Chan, C.T.; Pang, Y.L.; Deng, W.; Babu, I.R.; Dyavaiah, M.; Begley, T.J.; Dedon, P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012, 3, 937. [Google Scholar] [CrossRef]
- Duechler, M.; Leszczynska, G.; Sochacka, E.; Nawrot, B. Nucleoside modifications in the regulation of gene expression: Focus on tRNA. Cell. Mol. Life Sci. 2016, 73, 3075–3095. [Google Scholar] [CrossRef]
- van Delft, P.; Akay, A.; Huber, S.M.; Bueschl, C.; Rudolph, K.L.M.; Di Domenico, T.; Schuhmacher, R.; Miska, E.A.; Balasubramanian, S. The profile and dynamics of RNA modifications in animals. Chembiochem. A Eur. J. Chem. Biol. 2017, 18, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef]
- Popis, M.C.; Blanco, S.; Frye, M. Post-transcriptional methylation of transfer and ribosomal RNA in stress response pathways, cell differentiation, and cancer. Curr. Opin. Oncol. 2016, 28, 65–71. [Google Scholar] [CrossRef]
- Huber, S.M.; Leonardi, A.; Dedon, P.C.; Begley, T.J. The versatile roles of the tRNA epitranscriptome during cellular responses to toxic exposures and environmental Stress. Toxics 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Aucamp, P.J.; Bjorn, L.O.; Lucas, R. Questions and answers about the environmental effects of ozone depletion and its interactions with climate change: 2010 assessment. Photochem. Photobiol. Sci. 2011, 10, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L.; Di Mascio, P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem. Photobiol. Sci. 2009, 8, 903–911. [Google Scholar] [CrossRef]
- Sage, E.; Girard, P.M.; Francesconi, S. Unravelling UVA-induced mutagenesis. Photochem. Photobiol. Sci. 2012, 11, 74–80. [Google Scholar] [CrossRef]
- Yagura, T.; Schuch, A.P.; Garcia, C.C.M.; Rocha, C.R.R.; Moreno, N.C.; Angeli, J.P.F.; Mendes, D.; Severino, D.; Bianchini Sanchez, A.; Di Mascio, P.; et al. Direct participation of DNA in the formation of singlet oxygen and base damage under UVA irradiation. Free Radic. Biol. Med. 2017, 108, 86–93. [Google Scholar] [CrossRef]
- Danpure, H.J.; Tyrrell, R.M. Oxygen-dependence of near UV (365 nm) lethality and the interaction of near UV and X-rays in two mammalian cell lines. Photochem. Photobiol. 1976, 23, 171–177. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Misiaszek, R.; Crean, C.; Joffe, A.; Geacintov, N.E.; Shafirovich, V. Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J. Biol. Chem. 2004, 279, 32106–32115. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. One-electron oxidation of DNA and inflammation processes. Nat. Chem. Biol. 2006, 2, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Greer, A. Christopher Foote’s discovery of the role of singlet oxygen [1O2 (1Δg)] in photosensitized oxidation reactions. Acc. Chem. Res. 2006, 39, 797–804. [Google Scholar] [CrossRef]
- Lipscomb, L.A.; Peek, M.E.; Morningstar, M.L.; Verghis, S.M.; Miller, E.M.; Rich, A.; Essigmann, J.M.; Williams, L.D. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc. Natl. Acad. Sci. USA 1995, 92, 719–723. [Google Scholar] [CrossRef]
- McAuley-Hecht, K.E.; Leonard, G.A.; Gibson, N.J.; Thomson, J.B.; Watson, W.P.; Hunter, W.N.; Brown, T. Crystal structure of a DNA duplex containing 8-hydroxydeoxyguanine-adenine base pairs. Biochemistry 1994, 33, 10266–10270. [Google Scholar] [CrossRef]
- Oda, Y.; Uesugi, S.; Ikehara, M.; Nishimura, S.; Kawase, Y.; Ishikawa, H.; Inoue, H.; Ohtsuka, E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991, 19, 1407–1412. [Google Scholar] [CrossRef][Green Version]
- Pollum, M.; Jockusch, S.; Crespo-Hernández, C.E. Increase in the photoreactivity of uracil derivatives by doubling thionation. Phys. Chem. Chem. Phys. 2015, 17, 27851–27861. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, B.; Pollum, M.; Crespo-Hernández, C.E. Photochemical and photodynamical properties of sulfur-substituted nucleic acid bases. Photochem. Photobiol. 2019, 95, 33–58. [Google Scholar] [CrossRef]
- Saintomé, C.; Clivio, P.; Favre, A.; Fourrey, J.-L.; Riche, C. RNA photolabeling mechanistic studies: X-ray crystal structure of the photoproduct formed between 4-thiothymidine and adenosine upon near UV irradiation. J. Am. Chem. Soc. 1996, 118, 8142–8143. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA under attack: Cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef]
- Hofer, T.; Seo, A.Y.; Prudencio, M.; Leeuwenburgh, C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: Greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol. Chem. 2006, 387, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.E.; Specht, E.; Broedbaek, K.; Henriksen, T.; Ellervik, C.; Mandrup-Poulsen, T.; Tonnesen, M.; Nielsen, P.E.; Andersen, H.U.; Weimann, A. RNA modifications by oxidation: A novel disease mechanism? Free Radic. Biol. Med. 2012, 52, 1353–1361. [Google Scholar] [CrossRef]
- Simms, C.L.; Zaher, H.S. Quality control of chemically damaged RNA. Cell. Mol. Life Sci. 2016, 73, 3639–3653. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Huang, J.Z.; Gao, M.; Guo, G.J.; Zeng, S.S.; Chen, X.; Wang, X.; Gong, Z.C.; Yan, Y.L. Current perspectives on the clinical implications of oxidative RNA damage in aging research: Challenges and opportunities. Geroscience 2020. [Google Scholar] [CrossRef]
- Jora, M.; Burns, A.P.; Ross, R.L.; Lobue, P.A.; Zhao, R.; Palumbo, C.M.; Beal, P.A.; Addepalli, B.; Limbach, P.A. Differentiating positional isomers of nucleoside modifications by higher-energy collisional dissociation mass spectrometry (HCD MS). J. Am. Soc. Mass Spectrom. 2018, 29, 1745–1756. [Google Scholar] [CrossRef]
- Pomerantz, S.C.; McCloskey, J.A. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 1990, 193, 796–824. [Google Scholar] [CrossRef]
- Edmonds, C.G.; Crain, P.F.; Gupta, R.; Hashizume, T.; Hocart, C.H.; Kowalak, J.A.; Pomerantz, S.C.; Stetter, K.O.; McCloskey, J.A. Post-transcriptional modification of tRNA in thermophilic archaea (Archaebacteria). J. Bacteriol. 1991, 173, 3138–3148. [Google Scholar] [CrossRef]
- Kowalak, J.A.; Dalluge, J.J.; McCloskey, J.A.; Stetter, K.O. The role of post-transcriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry 1994, 33, 7869–7876. [Google Scholar] [CrossRef]
- Kowalak, J.A.; Bruenger, E.; McCloskey, J.A. Post-transcriptional modification of the central loop of domain V in Escherichia coli 23 S ribosomal RNA. J. Biol. Chem. 1995, 270, 17758–17764. [Google Scholar] [CrossRef]
- Kowalak, J.A.; Pomerantz, S.C.; Crain, P.F.; McCloskey, J.A. A novel method for the determination of post-transcriptional modification in RNA by mass spectrometry. Nucleic Acids Res. 1993, 21, 4577–4585. [Google Scholar] [CrossRef]
- Sun, C.; Jora, M.; Solivio, B.; Limbach, P.A.; Addepalli, B. The effects of ultraviolet radiation on nucleoside modifications in RNA. Acs Chem. Biol. 2018, 13, 567–572. [Google Scholar] [CrossRef]
- Addepalli, B.; Venus, S.; Thakur, P.; Limbach, P.A. Novel ribonuclease activity of cusativin from Cucumis sativus for mapping nucleoside modifications in RNA. Anal. Bioanal. Chem. 2017, 409, 5645–5654. [Google Scholar] [CrossRef]
- Addepalli, B.; Hunt, A.G. A novel endonuclease activity associated with the Arabidopsis ortholog of the 30-kDa subunit of cleavage and polyadenylation specificity factor. Nucleic Acids Res. 2007, 35, 4453–4463. [Google Scholar] [CrossRef]
- Addepalli, B.; Limbach, P.A. Mass spectrometry-based quantification of pseudouridine in RNA. J. Am. Soc. Mass Spectrom. 2011, 22, 1363–1372. [Google Scholar] [CrossRef]
- Thakur, P.; Estevez, M.; Lobue, P.A.; Limbach, P.A.; Addepalli, B. Improved RNA modification mapping of cellular non-coding RNAs using C- and U-specific RNases. Analyst 2020, 145, 816–827. [Google Scholar] [CrossRef]
- Yu, N.; Lobue, P.A.; Cao, X.; Limbach, P.A. RNAModMapper: RNA modification mapping software for analysis of liquid chromatography tandem mass spectrometry data. Anal. Chem. 2017, 89, 10744–10752. [Google Scholar] [CrossRef]
- Yu, N.; Jora, M.; Solivio, B.; Thakur, P.; Acevedo-Rocha, C.G.; Randau, L.; de Crecy-Lagard, V.; Addepalli, B.; Limbach, P.A. tRNA modification profiles and codon-decoding strategies in Methanocaldococcus jannaschii. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef]
- Delaney, S.; Jarem, D.A.; Volle, C.B.; Yennie, C.J. Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic Res. 2012, 46, 420–441. [Google Scholar] [CrossRef]
- Hossain, M.; Limbach, P.A. Mass spectrometry-based detection of transfer RNAs by their signature endonuclease digestion products. Rna 2007, 13, 295–303. [Google Scholar] [CrossRef]
- Hossain, M.; Limbach, P.A. Multiple endonucleases improve MALDI-MS signature digestion product detection of bacterial transfer RNAs. Anal. Bioanal. Chem. 2009, 394, 1125–1135. [Google Scholar] [CrossRef]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of hydantoin products from one-electron oxidation of 8-oxo-7,8-dihydroguanosine in a nucleoside model. Chem. Res. Toxicol. 2001, 14, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Steenken, S.; Jovanovic, S.V.; Bietti, M.; Bernhard, K. The trap Depth (in DNA) of 8-Oxo-7,8-dihydro-2‘deoxyguanosine as derived from electron-transfer equilibria in aqueous solution. J. Am. Chem. Soc. 2000, 122, 2373–2374. [Google Scholar] [CrossRef]
- Favre, A.; Moreno, G.; Salet, C.; Vinzens, F. 4-Thiouridine incorporation into the RNA of monkey kidney cells (CV-1) triggers near-UV light long-term inhibition of DNA, RNA, and protein synthesis. Photochem. Photobiol. 1993, 58, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Hajnsdorf, E.; Dubreuil, Y.L.; Bezerra, R.; Favre, A.; Expert-BezanÇon, A. RNA-protein crosslinks introduced into E. coli ribosomes by use of the intrinsic probe 4-thiouridine. Photochem. Photobiol. 1987, 45, 445–451. [Google Scholar] [CrossRef]
- Matuszewski, M.; Wojciechowski, J.; Miyauchi, K.; Gdaniec, Z.; Wolf, W.M.; Suzuki, T.; Sochacka, E. A hydantoin isoform of cyclic N6-threonylcarbamoyladenosine (ct6A) is present in tRNAs. Nucleic Acids Res. 2017, 45, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Blondel, M.O.; Favre, A. tRNAPhe and tRNAPro are the near-ultraviolet molecular targets triggering the growth delay effect. Biochem. Biophys. Res. Commun. 1988, 150, 979–986. [Google Scholar] [CrossRef]
- Favre, A.; Hajnsdorf, E.; Thiam, K.; Caldeira de Araujo, A. Mutagenesis and growth delay induced in Escherichia coli by near-ultraviolet radiations. Biochimie 1985, 67, 335–342. [Google Scholar] [CrossRef]
- Sochacka, E.; Bartos, P.; Kraszewska, K.; Nawrot, B. Desulfuration of 2-thiouridine with hydrogen peroxide in the physiological pH range 6.6-7.6 is pH-dependent and results in two distinct products. Bioorg. Med. Chem. Lett. 2013, 23, 5803–5805. [Google Scholar] [CrossRef]
- Sochacka, E.; Kraszewska, K.; Sochacki, M.; Sobczak, M.; Janicka, M.; Nawrot, B. The 2-thiouridine unit in the RNA strand is desulfured predominantly to 4-pyrimidinone nucleoside under in vitro oxidative stress conditions. Chem. Commun. 2011, 47, 4914–4916. [Google Scholar] [CrossRef]
- Bartos, P.; Ebenryter-Olbinska, K.; Sochacka, E.; Nawrot, B. The influence of the C5 substituent on the 2-thiouridine desulfuration pathway and the conformational analysis of the resulting 4-pyrimidinone products. Bioorg. Med. Chem. 2015, 23, 5587–5594. [Google Scholar] [CrossRef]
- Sierant, M.; Kulik, K.; Sochacka, E.; Szewczyk, R.; Sobczak, M.; Nawrot, B. Cytochrome c catalyzes the hydrogen peroxide-assisted oxidative desulfuration of 2-thiouridines in transfer RNAs. Chembiochem A Eur. J. Chem. Biol. 2018, 19, 687–695. [Google Scholar] [CrossRef]
- Kramer, G.F.; Baker, J.C.; Ames, B.N. Near-UV stress in Salmonella typhimurium: 4-thiouridine in tRNA, ppGpp, and ApppGpp as components of an adaptive response. J. Bacteriol. 1988, 170, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Söll, D.; Soll, D.; RajBhandary, U.; RajBhandary, T. tRNA: Structure, Biosynthesis, and Function; ASM press: Washington, DC, USA, 1995; p. 572. [Google Scholar] [CrossRef]
- Reichle, V.F.; Kaiser, S.; Heiss, M.; Hagelskamp, F.; Borland, K.; Kellner, S. Surpassing limits of static RNA modification analysis with dynamic NAIL-MS. Methods 2019, 156, 91–101. [Google Scholar] [CrossRef]
- Kimura, S.; Waldor, M.K. The RNA degradosome promotes tRNA quality control through clearance of hypomodified tRNA. Proc. Natl. Acad. Sci. USA 2019, 116, 1394. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Ohno, S.; Nishikawa, K.; Yokogawa, T. Correlation between the stability of tRNA tertiary structure and the catalytic efficiency of a tRNA-modifying enzyme, archaeal tRNA-guanine transglycosylase. Genes Cells 2016, 21, 41–52. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA modifications in gene expression regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Hayakawa, H.; Kuwano, M.; Sekiguchi, M. Specific binding of 8-oxoguanine-containing RNA to polynucleotide phosphorylase protein. Biochemistry 2001, 40, 9977–9982. [Google Scholar] [CrossRef]
- Malla, S.; Li, Z. Functions of conserved domains of human polynucleotide phosphorylase on RNA oxidation. Insights Biomed. Res. 2019, 3, 62–67. [Google Scholar] [CrossRef]
- Hayakawa, H.; Uchiumi, T.; Fukuda, T.; Ashizuka, M.; Kohno, K.; Kuwano, M.; Sekiguchi, M. Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry 2002, 41, 12739–12744. [Google Scholar] [CrossRef]
- Carpousis, A.J. The Escherichia coli RNA degradosome: Structure, function, and relationship in other ribonucleolytic multienzyme complexes. Biochem. Soc. Trans. 2002, 30, 150–155. [Google Scholar] [CrossRef]
- Hossain, S.T.; Malhotra, A.; Deutscher, M.P. How RNase R degrades structured RNA: Role of the helicase activity and the S1 domain. J. Biol. Chem. 2016, 291, 7877–7887. [Google Scholar] [CrossRef]
- Wasmuth, E.V.; Lima, C.D. Structure and activities of the eukaryotic RNA exosome. Enzymes 2012, 31, 53–75. [Google Scholar] [CrossRef]

| tRNA Region | Unmodified RNA | Modified | |
|---|---|---|---|
| In vitro Exposure | In vivo Exposure | ||
| Acceptor and D-arm junction | __ | UUCCCGp FapyG[U]*[U]*CCCGp | pGGUGGGGUUCCC>p |
| Anticodon | U[Gh]UAAAUC>p | U[Q-99]ψA[ms2A]Aψ C>p | U[Q+18]ψA[ms2i6A]AψC>p |
| Variable loop | UGCC[Gh]UCAUC>p AUC[Gh]AC>p UUCGAAG[Gh]UUC>p | UGCC[Gh]UCAUCGAC>p | __ |
| tRNA | Modified Oligonucleotide | Potential UVA Alteration |
|---|---|---|
| Cys | U[s4U]AACAAAGp | UUAACAAAGp |
| Asn | ACU[Q]UU[t6A]AψCCGp | ACU[Q+18]UU[t6A]AψCCGp |
| Ala | CUU[cmo5U]Gp | CUUUGp |
| Glu | CCCU[mnm5s2U]UC[m2A]CGp | CCCUUUCACGp |
| Arg (ICG) | [m2A]ACCGp | AACCGp |
| Gln(UUG) | [m2A]ψACCGp | AψACCGp |
| Gln(CUG) | [m2A]ψψCCGp | - |
| His(GUG) | [m2A]ψψCCAGp | AψψCCAGp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Limbach, P.A.; Addepalli, B. Characterization of UVA-Induced Alterations to Transfer RNA Sequences. Biomolecules 2020, 10, 1527. https://doi.org/10.3390/biom10111527
Sun C, Limbach PA, Addepalli B. Characterization of UVA-Induced Alterations to Transfer RNA Sequences. Biomolecules. 2020; 10(11):1527. https://doi.org/10.3390/biom10111527
Chicago/Turabian StyleSun, Congliang, Patrick A. Limbach, and Balasubrahmanyam Addepalli. 2020. "Characterization of UVA-Induced Alterations to Transfer RNA Sequences" Biomolecules 10, no. 11: 1527. https://doi.org/10.3390/biom10111527
APA StyleSun, C., Limbach, P. A., & Addepalli, B. (2020). Characterization of UVA-Induced Alterations to Transfer RNA Sequences. Biomolecules, 10(11), 1527. https://doi.org/10.3390/biom10111527