Isoharringtonine Induces Apoptosis of Non-Small Cell Lung Cancer Cells in Tumorspheroids via the Intrinsic Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of IHT
2.2. Characterization Data for IHT
2.3. Cell Lines and Cell Culture
2.4. siRNA Transfection and IHT Treatment
2.5. Cell Viability Assay
2.6. Live/Dead Cell Staining
2.7. Quantitative Real-Time PCR
2.8. Apoptosis Analysis by Enzyme-Linked Immunospecific Assay (ELISA)
2.9. Apoptosis Analysis by Flow Cytometry with Annexin V/7-Amino-Actinomycin D (7-AAD) Staining
2.10. Flow Cytometer Analysis for Mitochondria Potential
2.11. Immunofluorescence Staining
2.12. Western Blot Analysis
2.13. RNA Interference and Drug Treatment in Caenorhabditis Elegans (C. Elegans)
2.14. Mouse Xenograft Study
2.15. Statistical Analysis
3. Results
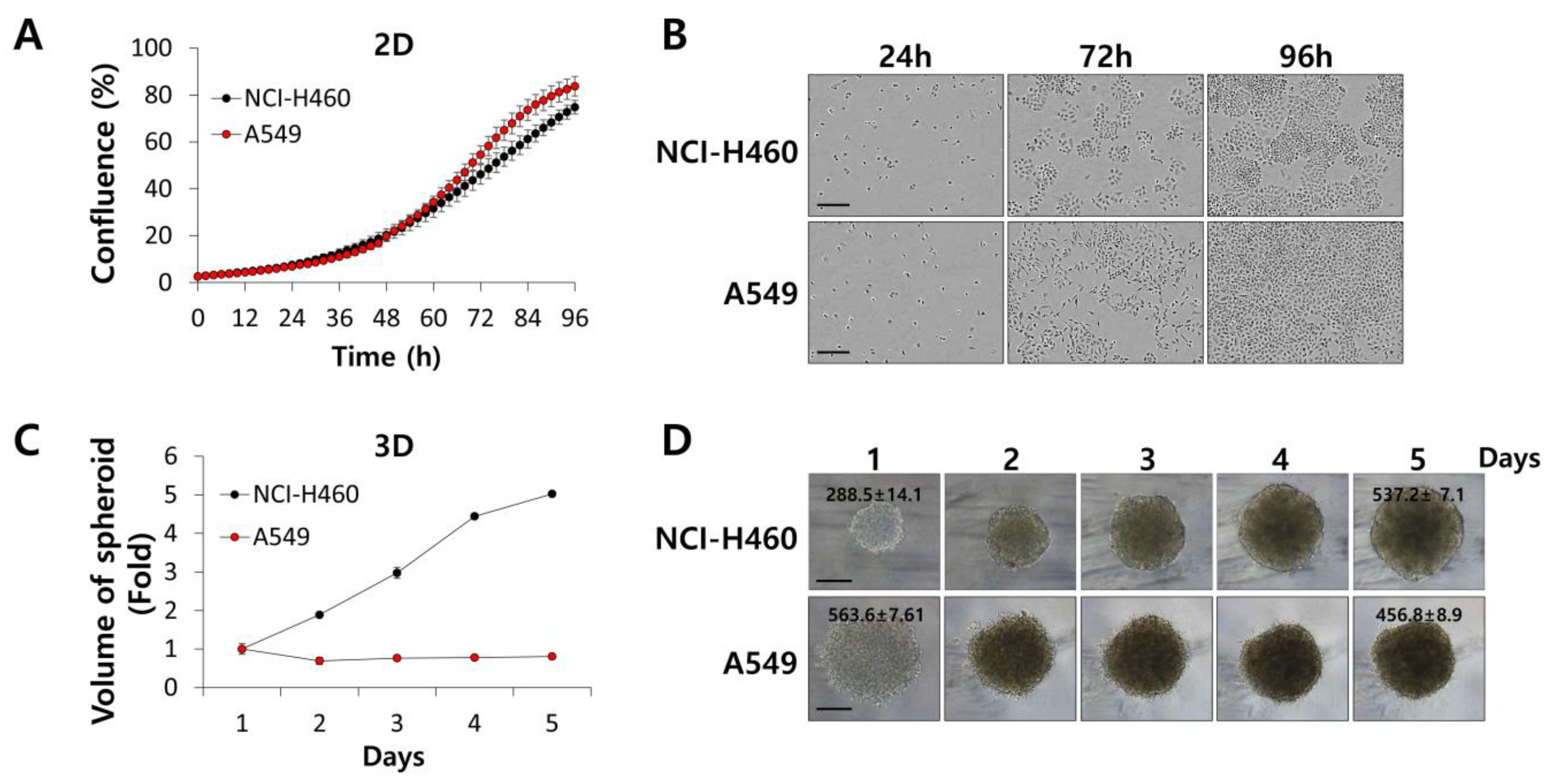
3.1. IHT Inhibits the Growth of NSCLC Tumorspheroids
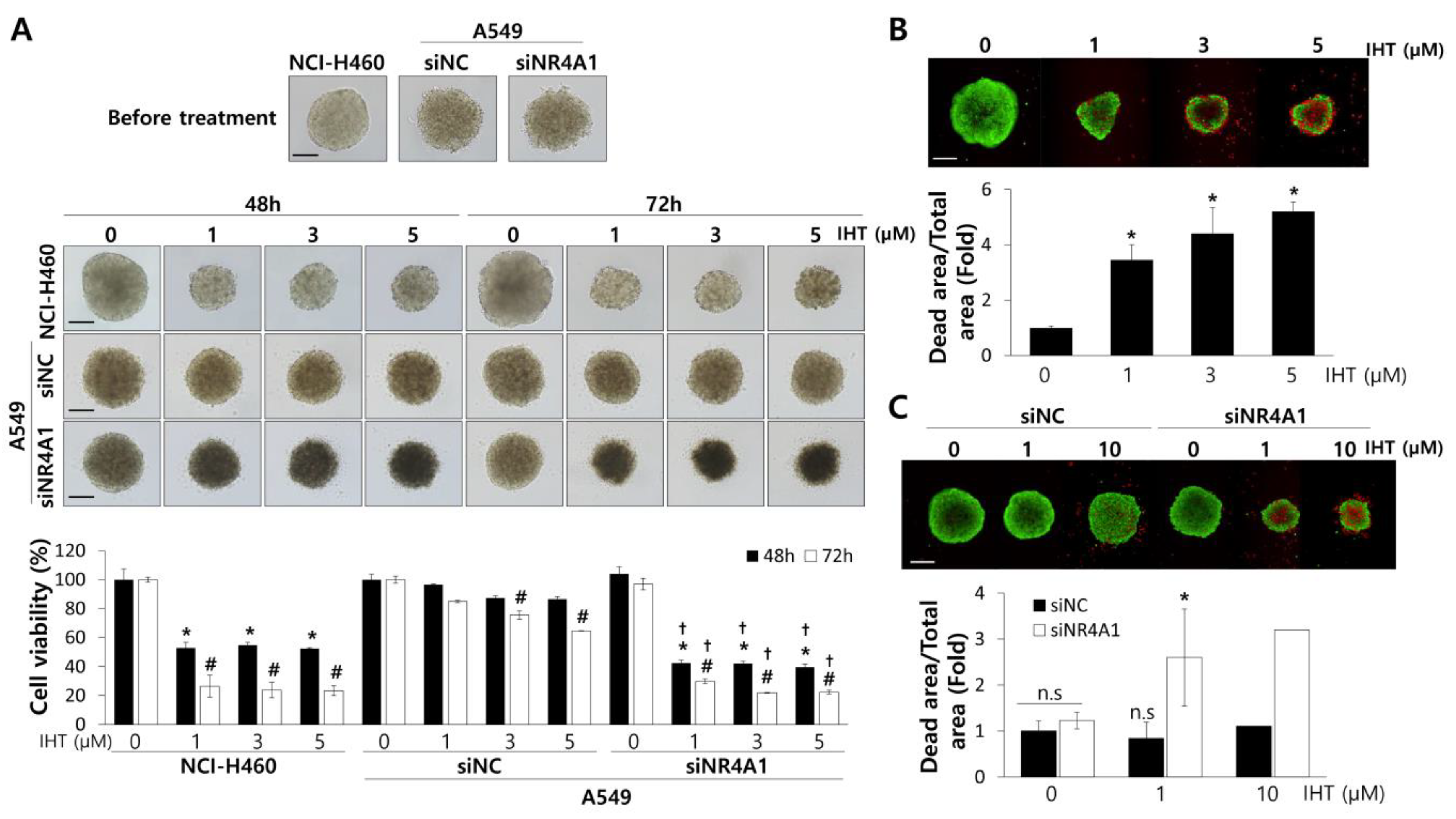
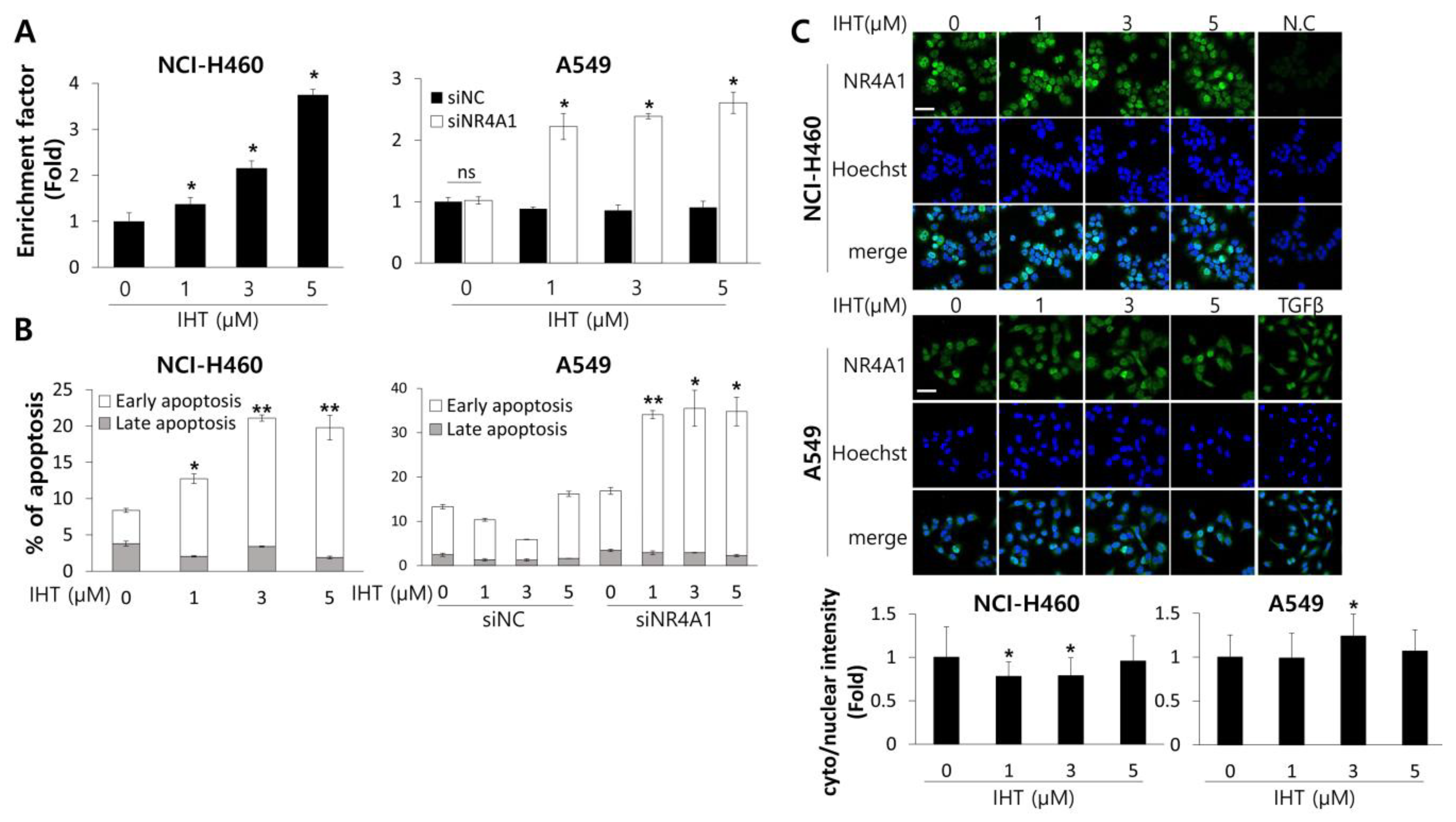
3.2. IHT Induces Apoptotic Cell Death in NCI-H460 Tumorspheroids and iNR4A1 Sensitizes A549 Tumorspheroids to IHT-Induced Apoptosis
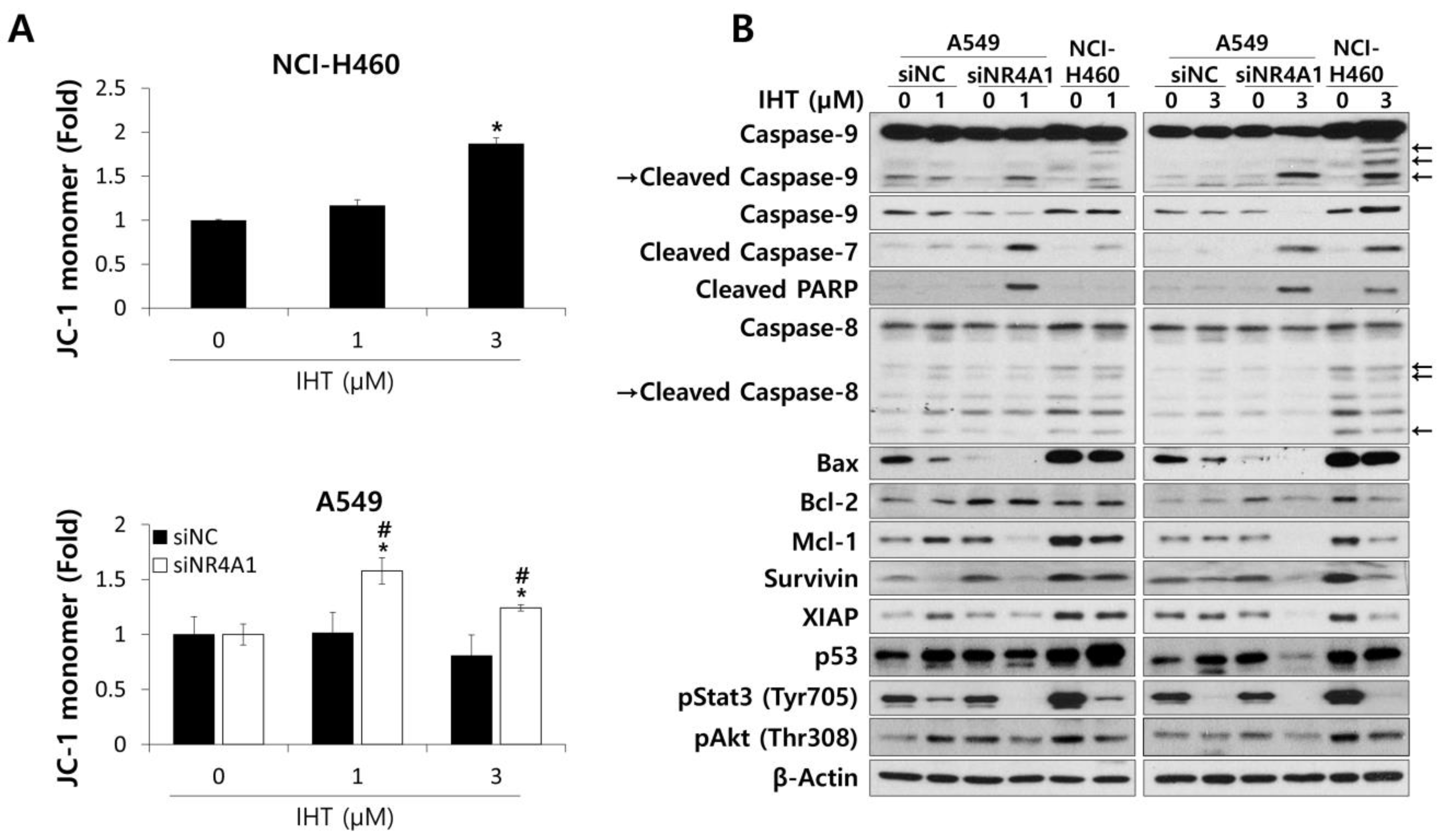
3.3. IHT Induces Apoptosis of NSCLC Tumorspheroids via the Intrinsic Pathway
3.4. IHT with iNR4A1 has Anti-Tumor Effects In Vivo and Expression of NR4A1 in Lung Cancer Tissues Correlates with Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nat. Cell Biol. 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Govindan, R.; Grannis, F.W., Jr.; et al. Non–Small Cell Lung Cancer, Version 2.2013. J. Natl. Compr. Cancer Netw. 2013, 11, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Tartarone, A.; Lerose, R.; Aieta, M. Focus on lung cancer screening. J. Thorac. Dis. 2020, 12, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Buettner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2020, 30164–30168. [Google Scholar] [CrossRef]
- Yook, C.H.; Jung, J.H.; Jong, I.L. Studies on Chemical Components of Cephalotaxus koreana Nakai. Korean J. Plant Resour. 2000, 13, 89–94. [Google Scholar]
- Ohnuma, T.; Holland, J.F. Homoharringtonine as a new antileukemic agent. J. Clin. Oncol. 1985, 3, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Cooperative Study Group of Zhe Jiang province for Cephalotaxus fortune Hook. Clinical studies of alkaloids from cephalotaxus fortune Hook. Zhe Jiang Zhong Liu Tong Xun 1976, 2, 14. [Google Scholar]
- Powell, R.G.; Weisleder, D.; Smith, C.R. Antitumor Alkaloids from Cephalotaxus harringtonia: Structure and Activity. J. Pharm. Sci. 1972, 61, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Paudler, W.W.; Kerley, G.I.; McKay, J. The Alkaloids of Cephalotaxus drupacea and Cephalotaxus fortunei. J. Org. Chem. 1963, 28, 2194–2197. [Google Scholar] [CrossRef]
- Tujebajeva, R.; Graifer, D.; Karpova, G.; Ajtkhozhina, N. Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett. 1989, 257, 254–256. [Google Scholar] [CrossRef]
- Tscherne, J.S.; Pestka, S. Inhibition of Protein Synthesis in Intact HeLa Cells. Antimicrob. Agents Chemother. 1975, 8, 479–487. [Google Scholar] [CrossRef]
- Gandhi, V.; Plunkett, W.; Cortes, J.E. Omacetaxine: A protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2014, 20, 1735–1740. [Google Scholar] [CrossRef]
- Visani, G.; Russo, D.; Ottaviani, E.; Tosi, P.; Damiani, D.; Michelutti, A.; Manfroi, S.; Baccarani, M.; Tura, S. Effects of homoharringtonine alone and in combination with alpha interferon and cytosine arabinoside on ‘in vitro’ growth and induction of apoptosis in chronic myeloid leukemia and normal hematopoietic progenitors. Leukemia 1997, 11, 624–628. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Talpaz, M.; Santini, V.; Murgo, A.; Cheson, B.; O’Brien, S.M. Homoharringtonine: History, current research, and future direction. Cancer 2001, 92, 1591–1605. [Google Scholar] [CrossRef]
- Shi, B.; Han, R. Apoptosis inductive effect of homoharringtonine and isoharringtonine on human acute promyelocytic leukemia HL-60 cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1999, 21, 356–2361. [Google Scholar]
- Alvandi, F.; Kwitkowski, V.E.; Ko, C.-W.; Rothmann, M.D.; Ricci, S.; Saber, H.; Ghosh, D.; Brown, J.; Pfeiler, E.; Chikhale, E.; et al. Food and Drug Administration Approval Summary: Omacetaxine Mepesuccinate as Treatment for Chronic Myeloid Leukemia. Oncology 2013, 19, 94–99. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Zhang, R.; Zhang, B.; Wang, T.; Zhu, X.; Mei, L.; Chen, H.; Zhang, H.; Ming, P.; et al. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci. Rep. 2015, 5, 8477. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Cheng, M.; Ni, L.; Zou, L.; Yang, Q.; Cai, X.-H.; Jiao, B. Isoharringtonine inhibits breast cancer stem-like properties and STAT3 signaling. Biomed. Pharmacother. 2018, 103, 435–442. [Google Scholar] [CrossRef]
- Gonda, T.J.; Ramsay, R.G. Directly targeting transcriptional dysregulation in cancer. Nat. Rev. Cancer 2015, 15, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kokontis, J. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem. Biophys. Res. Commun. 1988, 155, 971–977. [Google Scholar] [CrossRef]
- Hazel, T.G.; Nathans, D.; Lau, L.F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc. Natl. Acad. Sci. USA 1988, 85, 8444–8448. [Google Scholar] [CrossRef]
- Milbrandt, J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1988, 1, 183–188. [Google Scholar] [CrossRef]
- Herring, J.A.; Elison, W.S.; Tessem, J.S. Function of Nr4a Orphan Nuclear Receptors in Proliferation, Apoptosis and Fuel Utilization Across Tissues. Cells 2019, 8, 1373. [Google Scholar] [CrossRef]
- Thompson, J.; Winoto, A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J. Exp. Med. 2008, 205, 1029–1036. [Google Scholar] [CrossRef]
- Wilson, A.J.; Arango, D.; Mariadason, J.M.; Heerdt, B.G.; Augenlicht, L.H. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003, 63, 5401–5407. [Google Scholar]
- Safe, S.; Kim, K.; Li, X.; Lee, S.-O. NR4A orphan receptors and cancer. Nucl. Recept. Signal 2011, 9, e002. [Google Scholar]
- Yoon, K.; Lee, S.-O.; Cho, S.-D.; Kim, K.; Khan, S.; Safe, S. Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors. Carcinogenesis 2011, 32, 836–842. [Google Scholar] [CrossRef]
- Lee, S.O.; Abdelrahim, M.; Yoon, K.; Chintharlapalli, S.; Papineni, S.; Kim, K.; Wang, H.; Safe, S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010, 70, 6824–6836. [Google Scholar] [CrossRef]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Hutchinson, L.; Kirk, R. High drug attrition rates—Where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, srep19103. [Google Scholar] [CrossRef] [PubMed]
- Weisleder, D.; Powell, R.G.; Smith, C.R. Carbon-13 nuclear magnetic resonance spectroscopy of cephalotaxus alkaloids. Magn. Reson. Chem. 1980, 13, 114–115. [Google Scholar] [CrossRef]
- Győrffy, B.; Surowiak, P.; Budczies, J.; Lánczky, A. Online Survival Analysis Software to Assess the Prognostic Value of Biomarkers Using Transcriptomic Data in Non-Small-Cell Lung Cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Yoshida, T.; Sopko, N.A.; Kates, M.; Liu, X.; Joice, G.; McConkey, D.J.; Bivalacqua, T.J. Impact of spheroid culture on molecular and functional characteristics of bladder cancer cell lines. Oncol. Lett. 2019, 18, 4923–4929. [Google Scholar] [CrossRef]
- Karlsson, H.; Fryknäs, M.; Larsson, R.; Nygren, P. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp. Cell Res. 2012, 318, 1577–1585. [Google Scholar] [CrossRef]
- To, S.K.Y.; Zeng, J.-Z.; Wong, A.S.T. Nur77: A potential therapeutic target in cancer. Expert Opin. Ther. Targets 2012, 16, 573–585. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, J.-R.; Jia, Y.; Zhang, P.; Shen, L.; Li, X.-L.; Li, J.; Wang, B. Overexpression of NR4A1 is associated with tumor recurrence and poor survival in non-small-cell lung carcinoma. Oncotarget 2017, 8, 113977–113986. [Google Scholar] [CrossRef] [PubMed]
- Reers, M.; Smith, T.W.; Chen, L.B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef]
- Lopez, J.E.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Huang, Y. Structural Basis of Caspase Inhibition by XIAP Differential Roles of the Linker versus the BIR Domain. Cell 2001, 104, 781–790. [Google Scholar] [CrossRef]
- Hoffman, W.H.; Biade, S.; Zilfou, J.T.; Chen, J.; Murphy, M.E. Transcriptional Repression of the Anti-apoptoticsurvivinGene by Wild Type p53. J. Biol. Chem. 2001, 277, 3247–3257. [Google Scholar] [CrossRef]
- Brognard, J.; Clark, A.S.; Ni, Y.; Dennis, P.A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001, 61, 3986–3997. [Google Scholar]
- Westover, D.; Zugazagoitia, J.; Cho, B.; Lovly, C.; Paz-Ares, L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018, 29, i10–i19. [Google Scholar] [CrossRef]
- Bae, Y.-K.; Sung, J.Y.; Kim, Y.-N.; Kim, S.; Hong, K.M.; Kim, H.T.; Choi, M.S.; Kwon, J.Y.; Shim, J. An In Vivo C. elegans Model System for Screening EGFR-Inhibiting Anti-Cancer Drugs. PLoS ONE 2012, 7, e42441. [Google Scholar] [CrossRef]
- Hanna, N.; Johnson, D.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; Leighl, N.B.; et al. Systemic Therapy for Stage IV Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 3484–3515. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Thomas, A.; Liu, S.V.; Subramaniam, D.S.; Giaccone, G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat. Rev. Clin. Oncol. 2015, 12, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2002, 2, 740–749. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting transcription factors in cancer—From undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Deutsch, A.J.; Rinner, B.; Wenzl, K.; Pichler, M.; Troppan, K.; Steinbauer, E.; Schwarzenbacher, D.; Reitter, S.; Feichtinger, J.; Tierling, S.; et al. NR4A1-mediated apoptosis suppresses lymphomagenesis and is associated with a favorable cancer-specific survival in patients with aggressive B-cell lymphomas. Blood 2014, 123, 2367–2377. [Google Scholar] [CrossRef]
- Wu, H.; Bi, J.; Peng, Y.; Huo, L.; Yu, X.; Yang, Z.; Zhou, Y.; Qin, L.; Xu, Y.; Liao, L.; et al. Nuclear receptor NR4A1 is a tumor suppressor down-regulated in triple-negative breast cancer. Oncotarget 2017, 8, 54364–54377. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.D.; Lee, S.-O.; Chintharlapalli, S.; Abdelrahim, M.; Khan, S.; Yoon, K.; Kamat, A.M.; Safe, S. Activation of Nerve Growth Factor-Induced Bα by Methylene-Substituted Diindolylmethanes in Bladder Cancer Cells Induces Apoptosis and Inhibits Tumor Growth. Mol. Pharmacol. 2009, 77, 396–404. [Google Scholar] [CrossRef]
- Cho, S.D.; Yoon, K.; Chintharlapalli, S.; Abdelrahim, M.; Lei, P.; Hamilton, S.; Khan, S.; Ramaiah, S.K.; Safe, S. Nur77 Agonists Induce Proapoptotic Genes and Responses in Colon Cancer Cells through Nuclear Receptor-Dependent and Nuclear Receptor-Independent Pathways. Cancer Res. 2007, 67, 674–683. [Google Scholar] [CrossRef]
- Wu, H.; Lin, Y.; Li, W.; Sun, Z.; Gao, W.; Zhang, H.; Xie, L.; Jiang, F.; Qin, B.; Yan, T.; et al. Regulation of Nur77 expression by β-catenin and its mitogenic effect in colon cancer cells. FASEB J. 2010, 25, 192–205. [Google Scholar] [CrossRef]
- Hedrick, E.; Lee, S.-O.; Doddapaneni, R.; Singh, M.; Safe, S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr. Relat. Cancer 2015, 22, 831–840. [Google Scholar] [CrossRef]
- Heavey, S.; Cuffe, S.; Finn, S.; Young, V.; Ryan, R.; Nicholson, S.; Leonard, N.; McVeigh, N.; Barr, M.; O’Byrne, K.; et al. In pursuit of synergy: An investigation of the PI3K/mTOR/MEK co-targeted inhibition strategy in NSCLC. Oncotarget 2016, 7, 79526–79543. [Google Scholar] [CrossRef]
- Emartin, S.; Ejanouskova, H.; Edontenwill, M. Integrins and p53 pathways in glioblastoma resistance to temozolomide. Front. Oncol. 2012, 2, 157. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, R.; Lin, J.; Liu, B. Integrin β4 reduces DNA damage‑induced p53 activation in colorectal cancer. Oncol. Rep. 2018, 40, 2183–2192. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.-M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Park, S.-Y.; Hwang, W.; Sung, J.Y.; Cho, M.-L.; Shim, J.; Kim, Y.-N.; Yoon, K. Isoharringtonine Induces Apoptosis of Non-Small Cell Lung Cancer Cells in Tumorspheroids via the Intrinsic Pathway. Biomolecules 2020, 10, 1521. https://doi.org/10.3390/biom10111521
Lee JH, Park S-Y, Hwang W, Sung JY, Cho M-L, Shim J, Kim Y-N, Yoon K. Isoharringtonine Induces Apoptosis of Non-Small Cell Lung Cancer Cells in Tumorspheroids via the Intrinsic Pathway. Biomolecules. 2020; 10(11):1521. https://doi.org/10.3390/biom10111521
Chicago/Turabian StyleLee, Ji Hae, So-Young Park, Wonbin Hwang, Jee Young Sung, Myoung-Lae Cho, Jaegal Shim, Yong-Nyun Kim, and Kyungsil Yoon. 2020. "Isoharringtonine Induces Apoptosis of Non-Small Cell Lung Cancer Cells in Tumorspheroids via the Intrinsic Pathway" Biomolecules 10, no. 11: 1521. https://doi.org/10.3390/biom10111521
APA StyleLee, J. H., Park, S.-Y., Hwang, W., Sung, J. Y., Cho, M.-L., Shim, J., Kim, Y.-N., & Yoon, K. (2020). Isoharringtonine Induces Apoptosis of Non-Small Cell Lung Cancer Cells in Tumorspheroids via the Intrinsic Pathway. Biomolecules, 10(11), 1521. https://doi.org/10.3390/biom10111521



