Effect of Mild Salinity Stress on the Growth, Fatty Acid and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Cells
2.1.3. Microalgae
2.2. Methods
2.2.1. Culture Conditions
2.2.2. Determination of Microalgae Culture Growth
2.2.3. Pigment Extraction
2.2.4. Dosage of Pigments
2.2.5. Antioxidant Activity
DPPH Scavenging Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
2.2.6. Enzymatic Activities
Tyrosinase Inhibition Assay
Elastase Inhibition Assay
2.2.7. Cytotoxicity Effect
2.2.8. Identification of Pigments
2.2.9. Determination of Fatty Acid Composition
Hydrolyses of Microalgae Oil for FAME Conversion
GC–MS Analyses
2.3. Statistical Analysis
3. Results
3.1. Cultivation under Stress Conditions
3.2. Pigment Content of the Stressed Scenedesmus sp.
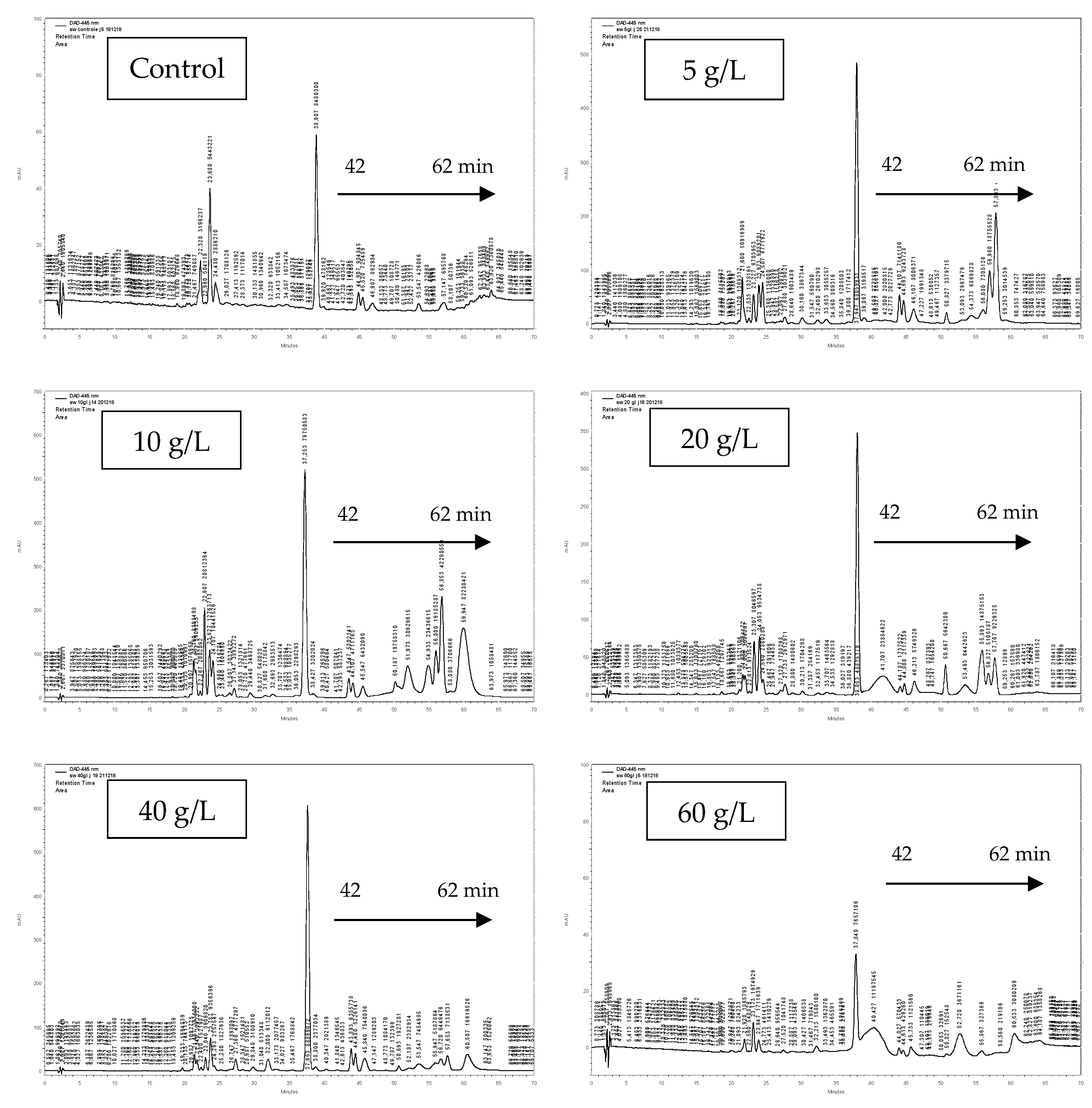
3.3. Pigment Identification
3.4. Antioxidant Activities
3.5. Enzymatic Activities
3.5.1. Tyrosinase Inhibitory Activity
3.5.2. Elastase Inhibitory Activity
3.6. Cytotoxic Activity
3.7. Fatty Acid (FA) Composition of Stressed Scenedesmus sp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Fodorpataki, L.; Bartha, C. Salt stress tolerance of a freshwater green alga under different photon flux densities. Stud. U.B.B. Biol. 2004, 49, 85–93. [Google Scholar]
- Moisnder, P.H.; McClinton, E.; Paer, H.W. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microbiologia 2002, 43, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, J.; Neill, A.; Hayden, B.L.; Pulfer, J.; Cebrian, J. The impact of salinity fluctuations on net oxygen production and inorganic nitrogen uptake by Ulva lactuca (Chlorophyceae). Aquat. Bot. 2003, 75, 339–350. [Google Scholar] [CrossRef]
- Huh, G.H.; Damsz, B.; Matsumoto, T.K.; Reddy, M.P.; Rus, A.M.; Ibeas, J.I.; Narasimhan, M.L.; Bressan, R.A.; Hasegawa, P.M. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002, 29, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Murata, N. Inactivation of photosystems I and II in response to osmotic stress in Synechococcus: Contribution of water channels. Plant Physiol. 2000, 122, 1201–1208. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Plant Sci. J. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef]
- Cavalcanti, F.R.; Lima, J.P.M.S.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. Plant Physiol. 2007, 164, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sekmen, A.H.; Türkan, I.; Takio, S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Plant Physiol. 2007, 131, 399–411. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007, 30, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Barredo, J.L. Microbial Carotenoids from bacteria and microalgae. In Methods and Protocols; Humana Press: New York, NY, USA, 2012; p. 5. [Google Scholar]
- Skjånes, K.; Rebours, C.; Lindblad, P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Biotechnology 2013, 33, 172–215. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; de Brabanter, J.; de Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Jahnke, L.S. Massive carotenoid accumulation in Dunaliella bardawil induced by ultraviolet-A radiation. J. Photochem. Photobiol. 1999, 48, 68–74. [Google Scholar] [CrossRef]
- Massyuk, N.P.; Abdula, E.G. First experiment of growing carotene-containing algae under semi-industrial conditions. Ukr. Bot. Zh. 1969, 26, 21–27. [Google Scholar]
- Borowitzka, L.J.; Moulton, T.P.; Borowitzka, M.A. The mass culture of Dunaliella salina for fine chemicals: From laboratory to pilot plant. In Proceedings of the International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1984; pp. 115–212. [Google Scholar]
- Romanenko, E.A.; Romanenko, P.A.; Babenko, L.M.; Kosakovskaya, I.V. Salt stress effects on growth and photosynthetic pigments’ content in algoculture of acutodesmus dimorphus (Chlorophyta). Int. J. Algae. 2017, 19, 271–282. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; Gonzalez, M.A.; Parra, O.O. The effect of salinity on the growth and carotenogenesis in two Chilean strains of Dunaliella salina Teodoresco. Biol. Res. 1996, 29, 227–236. [Google Scholar] [PubMed]
- El Baz, F.K.; Aboul-Enein, A.M.; El-Baroty, G.S.; Youssef, A.M.; Abdel-Baky, H.H. Accumulation of Antioxidant Vitamins in Dunaliella Salina; F.A.O.: Rome, Italy, 2002. [Google Scholar]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Chu, F.L.; Pirastru, L.; Popovic, R.; Sleno, L. Carotenogenesis up-regulation in Scenedesmus sp. using a targeted metabolomics approach by liquid chromatography−high-resolution mass spectrometry. J. Agric. Food Chem. 2011, 59, 3004–3013. [Google Scholar] [CrossRef]
- Aburai, N.; Sumida, D.; Abe, K. Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus sp. (Chlorophyceae). Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–4639. [Google Scholar] [CrossRef]
- Hu, H.; Li, H.; Xu, X. Alternative cold response modes in Chlorella (Chlorophyta, Trebouxiophyceae) from Antarctica. Phycologia 2008, 47, 28–34. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Kalacheva, G.S.; Zhila, N.O.; Gladyshev, M.I.; Volova, T.G. A temperature dependence of the intra-and extracellular fatty-acid composition of green algae and cyanobacterium. Russ. J. Plant Physiol. 2003, 50, 374–380. [Google Scholar] [CrossRef]
- Tatsuzawa, H.; Takizawa, E.; Wada, M.; Yamamoto, Y. Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. 1. J. Phycol. 1996, 32, 598–601. [Google Scholar] [CrossRef]
- Azachi, M.; Sadka, A.; Fisher, M.; Goldshlag, P.; Gokhman, I.; Zamir, A. Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol. 2002, 129, 1320–1329. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids 1. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, Y.; Chen, F. salt-induced alterations in lipid composition of diatom nitzschia laevis (bacillariophyceae) under heterotrophic culture condition 1. J. Phycol. 2008, 44, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Polle, J.E.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Bbiotechnol. 2003, 13, 165–174. [Google Scholar]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters1. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Rabbani, S.; Beyer, P.; Lintig, J.V.; Hugueney, P.; Kleinig, H. Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol. 1998, 116, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Acién, F.G.; Jiménez-Ruiz, N.; Gómez, C.; Fernández-Sevilla, J.M.; Mhiri, N.; Molina-Grima, E. Evaluation of native microalgae from Tunisia using the pulse-amplitude-modulation measurement of chlorophyll fluorescence and a performance study in semi-continuous mode for biofuel production. Biotechnol. Biofuels 2019, 12, 119. [Google Scholar] [CrossRef]
- Watanabe, A. List of algal strains in collection at the Institute of Applied Microbiology, University of Tokyo. J. Gen. Appl. Microbiol. 1960, 6, 283–292. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Taori, K.; Paul, V.J.; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Forte, M.; Bouallagui, Z.; Donato, S.; Mita, L.; Mita, D.G.; Sayadi, S. Olive compounds attenuate oxidative damage induced in HEK-293 cells via MAPK signaling pathway. J. Funct. Foods 2017, 39, 18–27. [Google Scholar] [CrossRef]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Ø.; Paglia, G. UPLC-UV-MS E analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.I.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Affenzeller, M.J.; Darehshouri, A.; Andosch, A.; Lütz, C.; Lütz-Meindl, U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J. Exp. Bot. 2009, 60, 939–954. [Google Scholar] [CrossRef]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Biores. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Suzuki, I.; Tasaka, Y.; Murata, N. Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc. Natl. Acad. Sci. USA 1999, 96, 5862–5867. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.R.; Singh, N. Salinity as a factor affecting the physiological and biochemical traits of Scenedesmus quadricauda. J. Algal Biomass Util. 2011, 2, 28–34. [Google Scholar]
- Burczyk, J.; Szkawran, H.; Zontek, I.; Czygan, F.C. Carotenoids in the outer cell-wall layer of Scenedesmus (Chlorophyceae). Planta 1981, 151, 247–250. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Gómez, P.I.; Barriga, A.; Cifuentes, A.S.; Gonzalez, M.A. Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol. Res. 2003, 36, 185–192. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Rai, A.K. Biochemical characteristics of photosynthetic response to various external salinities in halotolerant and fresh-water cyanobacteria. F.E.M.S. Microbiol. Lett. 1990, 69, 177–180. [Google Scholar]
- Rai, A.K.; Abraham, G. Salinity tolerance and growth analysis of the cyanobacterium Anabaena doliolum. Bull. Environ. Contamin. Toxicol. 1993, 51, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A.; Kancharaksa, N.; Bunnag, B.; Tanticharoen, M. Role of light and photosynthesis on the acclimation process of the cyanobacterium Spirulina platensis to salinity stress. J. Appl. Phycol. 1996, 8, 119–124. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Kobayashi, M. Astaxanthin biosynthesis enhanced by reactive oxygen species in the green alga Haematococcus pluvialis. Biotechnol. Bioprocess Eng. 2003, 8, 322. [Google Scholar] [CrossRef]
- Hao, Z.; Parker, B.; Knapp, M.; Yu, L.L. Simultaneous quantification of α-tocopherol and four major carotenoids in botanical materials by normal phase liquid chromatography–atmospheric pressure chemical ionization-tandem mass spectrometry. J. Chromatogr. A 2005, 1094, 83–90. [Google Scholar] [CrossRef]
- Rivera, S.; Vilaró, F.; Canela, R. Determination of carotenoids by liquid chromatography/mass spectrometry: Effect of several dopants. Anal. Bioanal. Chem. 2011, 400, 1339–1346. [Google Scholar] [CrossRef]
- Van Breemen, R.B. Electrospray liquid chromatography-mass spectrometry of carotenoids. Anal Chem. 1995, 67, 2004–2009. [Google Scholar] [CrossRef]
- Erdoğan, A.; Çağır, A.; Dalay, M.C.; Eroğlu, A.E. Composition of carotenoids in Scenedesmus protuberans: Application of chromatographic and spectroscopic methods. Food Anal. Methods 2015, 8, 1970–1978. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Ianora, A. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Natrah, F.M.; Yusoff, F.M.; Shariff, M.; Abbas, F.; Mariana, N.S. Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J. Appl. Phycol. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Sahin, S.C. Scenedesmus obliquus: A Potential Natural Source for Cosmetic Industry. Int. J. Second. Metab. 2019, 6, 129–136. [Google Scholar] [CrossRef]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Anantharaman, P.; Hemalatha, A.; Girija, K.; Parthiban, C.; Saranya, C. Antioxidant properties and total phenolic content of a marine diatom, Navicula clavata and green microalgae, Chlorella marina and Dunaliella salina. Adv. Appl. Sci. Res. 2013, 4, 151–157. [Google Scholar]
- Chen, F.; Sun, Z.; Sun, P.; Chen, T.; Zhang, J. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–415. [Google Scholar]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Shirzad, M.; Hamedi, J.; Motevaseli, E.; Modarressi, M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells. Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 1051–1061. [Google Scholar] [CrossRef]
- Ou, Z.; Rades, T.; Mcdowell, A. Anti-ageing effects of Sonchus oleraceus L. (pūhā) leaf extracts on H2O2-induced cell senescence. Molecules 2015, 20, 4548–4564. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Hwang, T.L. Neutrophil elastase inhibitors: A patent review and potential applications for inflammatory lung diseases (2010–2014). Exp. Opin. Ther. Pat. 2015, 25, 1145–1158. [Google Scholar] [CrossRef]
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]



| Extracts | Pigments (µg/mL of Biomass Extract Solution) | ||
|---|---|---|---|
| Ca | Cb | Ccarot | |
| Control | 23.78 ± 0.87 | 40.49 ± 1.49 | 2997.45 ± 2.01 |
| 5 g/L | 23.96 ± 0.53 | 40.83 ± 0.59 | 2875.31 ± 25.92 |
| 10 g/L | 25.03 ± 0.03 | 42.55 ± 0.63 | 3295.84 ± 5.750 |
| 20 g/L | 23.36 ± 0.82 | 39.73 ± 0.59 | 3077.67 ± 11.36 |
| 40 g/L | 21.33 ± 0.02 | 36.27 ± 0.04 | 2838.11 ± 26.25 |
| 60 g/L | 6.73 ± 1.18 | 11.47 ± 1.21 | 2096.96 ± 31.95 |
| Rt (min) | [M + H]+ (m/z) | [M-H]-(m/z) | λ max (nm) | Identification |
|---|---|---|---|---|
| 22.78 | 601 | 599 | 406/430/457 | (di-Z)-violaxanthin |
| 23.75 | 601 | 599 | 202/440/467 | (9-Z)-neoxanthin |
| 24.3 | 601 | 599 | 402/423/449 | zeaxanthin 5,6:5’,8’-diepoxide-a |
| 24.87 | 585 | 583 | 432/435/461 | β-cryptoxanthin 5,6-5’,6’-diepoxide |
| 25.78 | 537 | 535 | 269/445/472 | β-cryptoxanthin 5’,6’-epoxide |
| 26.83 | 553 | 551 | 413/436/465 | (all-E)-zeinoxanthine |
| 27.85 | 601 | 599 | 416/440/469 | (all-E)-violaxanthin |
| 33.29 | 601 | 599 | 398/422/448 | (all-E)-luteoxanthin |
| 36.47 | 585 | 583 | 439/441/469 | (9-Z)-antheroxanthine |
| 42.07 | 569 | 567 | 230/445/473 | lutein |
| 43.22 | 568 | 566 | 224/450 | (all-E)-Zeaxanthin |
| 48.41 | 553 | 551 | 419/442/469 | 5,6 epoxy-α-carotene suspected |
| 53.5 | 893 | 891 | 385/430/664 | chlorophyll a |
| Samples | Antioxidant Activities (IC50, mg/mL) | Enzymatic Activities (IC50, mg/mL) | ||
|---|---|---|---|---|
| Activities | DPPH | FRAP | Tyrosinase | Elastase |
| Control | 1.550 | 1.163 | 1.405 | 0.917 |
| Ss10 | 0.727 | 0.269 | 0.698 | 0.715 |
| Ascorbic acid | 0.013 | 0.369 | - | - |
| Kojic acid | - | - | 1.989 | - |
| Quercetin | - | - | - | 62.964 |
| Peaks | Rt (min) | Fatty Acid | Empirical Formula | Identification |
|---|---|---|---|---|
| 1 | 7.040 | C14:0 | C15H30O2 | Myristic acid methyl ester |
| 3 | 9.333 | C16:0 | C16H32O2 | Palmitic acid |
| 7 | 10.012 | C17H26O2 | Methyl 4,7,10,13-hexaoctatetraenoate | |
| 10 | 11.342 | C19H40O | n-Nonadecanol-1 | |
| 11 | 11.771 | C18:0 | C19H38O2 | Stearic acid methyl ester |
| 12 | 11.832 | C18:1(trans-9) | C18H34O2 | Elaidic acid |
| 14 | 12.071 | C18:2 (all cis-9,12) | C18H32O2 | Linoleic acid |
| 15 | 12.446 | C18:3 (all cis-9,12,15) | C18H30O2 | alpha-Linolenic acid |
| 18 | 15.535 | C16:1 (cis-9) | C16H30O2 | Palmitoleic acid |
| 20 | 16.847 | C18:1 (cis-9) | C18H34O2 | Oleic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elloumi, W.; Jebali, A.; Maalej, A.; Chamkha, M.; Sayadi, S. Effect of Mild Salinity Stress on the Growth, Fatty Acid and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp. Biomolecules 2020, 10, 1515. https://doi.org/10.3390/biom10111515
Elloumi W, Jebali A, Maalej A, Chamkha M, Sayadi S. Effect of Mild Salinity Stress on the Growth, Fatty Acid and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp. Biomolecules. 2020; 10(11):1515. https://doi.org/10.3390/biom10111515
Chicago/Turabian StyleElloumi, Wiem, Ahlem Jebali, Amina Maalej, Mohamed Chamkha, and Sami Sayadi. 2020. "Effect of Mild Salinity Stress on the Growth, Fatty Acid and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp." Biomolecules 10, no. 11: 1515. https://doi.org/10.3390/biom10111515
APA StyleElloumi, W., Jebali, A., Maalej, A., Chamkha, M., & Sayadi, S. (2020). Effect of Mild Salinity Stress on the Growth, Fatty Acid and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp. Biomolecules, 10(11), 1515. https://doi.org/10.3390/biom10111515






