The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review
Abstract
:1. Introduction
2. Inflammation in the Acute Coronary Syndrome
2.1. In Coronary Arteries
2.2. In Infarcted Myocardium
3. The Role of NK Cells in Atherosclerosis
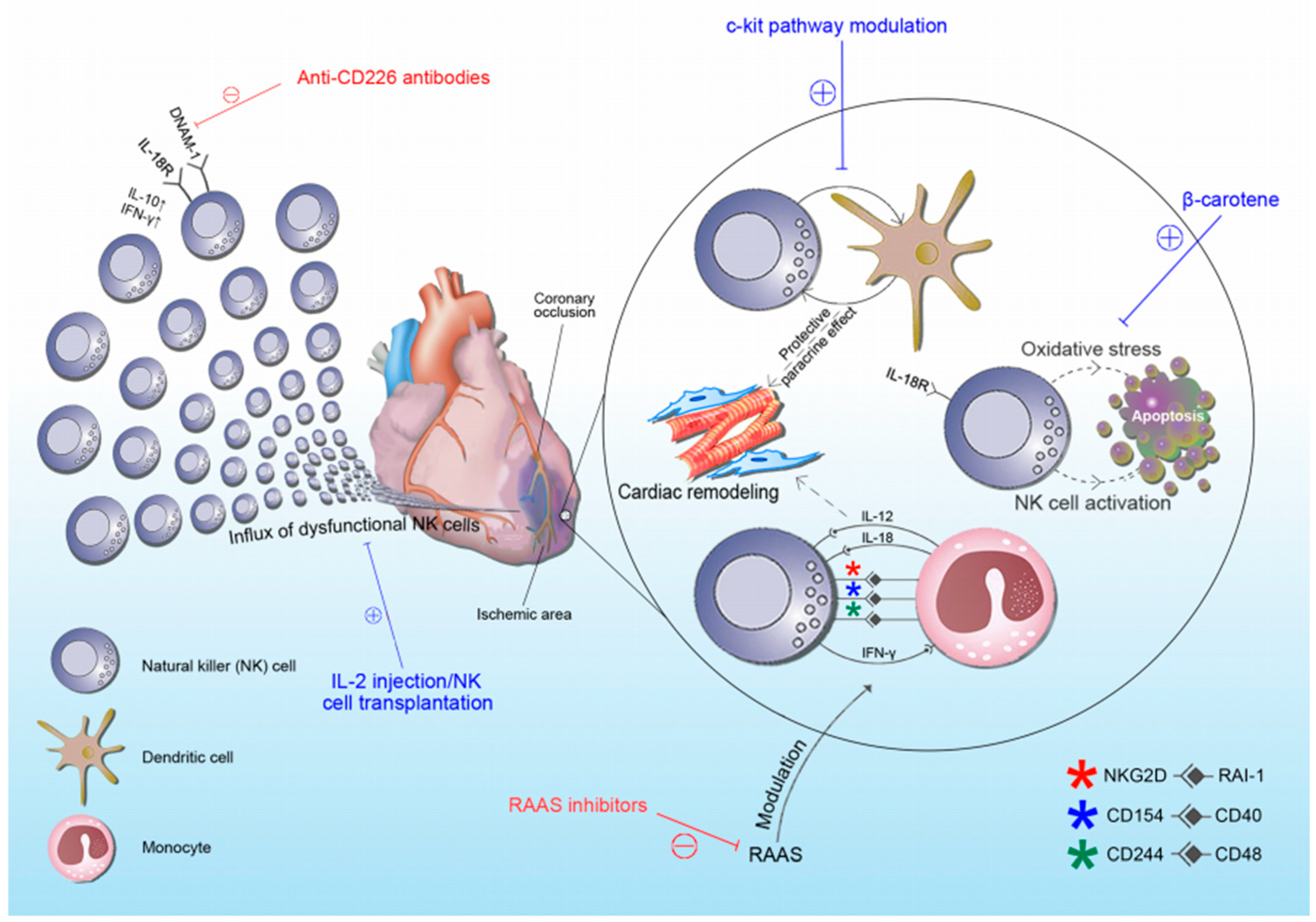
4. NK Cells in Acute Coronary Syndrome (ACS)
5. Therapeutic Scope of NK Cells in ACS
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [Green Version]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowa, M.; Pandya, A.; et al. The Global Economic Burden of Noncommunicable Diseases. In Proceedings of the World Economic Forum, Geneva, Switzerland, 19–20 September 2011. [Google Scholar]
- French, B.A.; Kramer, C.M. Mechanisms of Post-Infarct Left Ventricular Remodeling. Drug Discov. Today Dis. Mech. 2007, 4, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Rodríguez, A.C.; Marín-Jáuregui, L.S.; Martínez-Shio, E.; Hernández Castro, B.; González-Amaro, R.; Escobedo-Uribe, C.D.; Monsiváis-Urenda, A.E. Altered NK cell receptor repertoire and function of natural killer cells in patients with acute myocardial infarction: A three-month follow-up study. Immunobiology 2020, 225, 151909. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.R. Innate lymphoid cell regulation of adaptive immunity. Immunology 2016, 149, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zitti, B.; Bryceson, Y.T. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 2018, 42, 37–46. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [Green Version]
- Paust, S.; von Andrian, U.H. Natural killer cell memory. Nat. Immunol. 2011, 12, 500–508. [Google Scholar] [CrossRef]
- van den Boorn, J.G.; Jakobs, C.; Hagen, C.; Renn, M.; Luiten, R.M.; Melief, C.J.; Tüting, T.; Garbi, N.; Hartmann, G.; Hornung, V. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity 2016, 44, 1406–1421. [Google Scholar] [CrossRef] [Green Version]
- Bassing, C.H.; Swat, W.; Alt, F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 2002, 109, S45–S55. [Google Scholar] [CrossRef] [Green Version]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward from Mechanisms to Precision Treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef]
- Simionescu, M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 266–274. [Google Scholar] [CrossRef]
- Chandran, S.; Watkins, J.; Abdul-Aziz, A.; Shafat, M.; Calvert, P.A.; Bowles, K.M.; Flather, M.D.; Rushworth, S.A.; Ryding, A.D. Inflammatory Differences in Plaque Erosion and Rupture in Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millonig, G.; Malcom, G.T.; Wick, G. Early inflammatory-immunological lesions in juvenile atherosclerosis from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY)-study. Atherosclerosis 2002, 160, 441–448. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Manduteanu, I.; Simionescu, M. Inflammation in atherosclerosis: A cause or a result of vascular disorders? J. Cell. Mol. Med. 2012, 16, 1978–1990. [Google Scholar] [CrossRef] [Green Version]
- Woollard, K.J.; Geissmann, F. Monocytes in atherosclerosis: Subsets and functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef]
- Rosenthal-Allieri, M.A.; Ticchioni, M.; Breittmayer, J.P.; Shimizu, Y.; Bernard, A. Influence of beta 1 integrin intracytoplasmic domains in the regulation of VLA-4-mediated adhesion of human T cells to VCAM-1 under flow conditions. J. Immunol. 2005, 175, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Tall, A.; Accili, D. The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ. Res. 2010, 106, 58–67. [Google Scholar] [CrossRef]
- Le Dall, J.; Ho-Tin-Noé, B.; Louedec, L. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc. Res. 2010, 85, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Lijnen, H.R. Metalloproteinases in development and progression of vascular disease. Haemostasis 2003, 33, 275–281. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Lijnen, R. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 1997, 17, 439–444. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. BioMed Res. Int. 2016, 9582430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.J.; Koplev, S.; Fisher, E.A.; Tabas, I.; Björkegren, J.; Doran, A.C.; Kovacic, J.C. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (Part 2). J. Am. Coll. Cardiol. 2018, 72, 2181–2197. [Google Scholar] [CrossRef]
- Dhume, A.S.; Soundararajan, K.; Hunter, W.J., 3rd; Agrawal, D.K. Comparison of vascular smooth muscle cell apoptosis and fibrous cap morphology in symptomatic and asymptomatic carotid artery disease. Ann. Vasc. Surg. 2003, 17, 1–8. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [Green Version]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [Green Version]
- Panizzi, P.; Swirski, F.K.; Figueiredo, J.L.; Waterman, P.; Sosnovik, D.E.; Aikawa, E. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J. Am. Coll. Cardiol. 2010, 55, 1629–1638. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G.; Youker, K.A.; Rossen, R.D.; Gwechenberger, M.; Lindsey, M.H.; Mendoza, L.H. Cytokines and the microcirculation in ischemia and reperfusion. J. Mol. Cell. Cardiol. 1998, 30, 2567–2576. [Google Scholar] [CrossRef]
- Mentz, R.J.; Bakris, G.L.; Waeber, B.; McMurray, J.J.; Gheorghiade, M.; Ruilope, L.M.; Maggioni, A.P.; Swedberg, K.; Piña, I.L.; Fiuzat, M.; et al. The past, present and future of renin-angiotensin aldosterone system inhibition. Int. J. Cardiol. 2013, 167, 1677–1687. [Google Scholar] [CrossRef] [Green Version]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.B.; Jalil, J.E.; Pick, R.; Janicki, J.S.; Weber, K.T. Cardiac myocyte necrosis induced by angiotensin II. Circ. Res. 1991, 69, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Hutchinson, H.G.; Fujinaga, M.; Hayashida, W.; Morishita, R.; Zhang, L.; Horiuchi, M.; Pratt, R.E.; Dzau, V.J. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: Gain-of-function study using gene transfer. Proc. Natl. Acad. Sci. USA 1995, 92, 10663–10667. [Google Scholar] [CrossRef] [Green Version]
- Wiemer, G.; Schölkens, B.A.; Wagner, A.; Heitsch, H.; Linz, W. The possible role of angiotensin II subtype AT2 receptors in endothelial cells and isolated ischemic rat hearts. J. Hypertens. Suppl. 1993, 11, S234–S235. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Pitt, B.; White, H.; Nicolau, J.; Martinez, F.; Gheorghiade, M.; Aschermann, M.; van Veldhuisen, D.J.; Zannad, F.; Krum, H.; Mukherjee, R.; et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J. Am. Coll. Cardiol. 2005, 46, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Bauersachs, J.; Heck, M.; Fraccarollo, D.; Hildemann, S.K.; Ertl, G.; Wehling, M.; Christ, M. Addition of spironolactone to angiotensin-converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: Role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J. Am. Coll. Cardiol. 2002, 39, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Delyani, J.A.; Robinson, E.L.; Rudolph, A.E. Effect of a selective aldosterone receptor antagonist in myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H647–H654. [Google Scholar] [CrossRef]
- Fraccarollo, D.; Galuppo, P.; Schraut, S.; Kneitz, S.; Van Rooijen, N.; Ertl, G. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension 2008, 51, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nour-Eldine, W.; Joffre, J.; Zibara, K.; Esposito, B.; Giraud, A.; Zeboudj, L.; Vilar, J.; Terada, M.; Bruneval, P.; Vivier, E.; et al. Genetic Depletion or Hyperresponsiveness of Natural Killer Cells Do Not Affect Atherosclerosis development. Circ. Res. 2018, 122, 47–57. [Google Scholar] [CrossRef]
- Winkels, H.; Ley, K. Natural Killer Cells at Ease: Atherosclerosis Is Not Affected by Genetic Depletion or Hyperactivation of Natural Killer Cells. Circ. Res. 2018, 122, 6–7. [Google Scholar] [CrossRef]
- Hak, Ł.; Myśliwska, J.; Więckiewicz, J.; Szyndler, K.; Trzonkowski, P.; Siebert, J.; Myśliwski, A. NK cell compartment in patients with coronary heart disease. Immun. Ageing 2007, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Sansoni, P.; Cossarizza, A.; Brianti, V.; Fagnoni, F.; Snelli, G.; Monti, D.; Marcato, A.; Passeri, G.; Ortolani, C.; Forti, E. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood 1993, 82, 2767–2773. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; Yokose, N.; Tamura, H.; An, E.; Nakamura, K.; Dan, K.; Nomura, T. Natural killer cells in the late decades of human life. J. Clin. Immunol. Immunopathol. 1997, 84, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, J.E.; Munné-Collado, J.; Rasal, R.; Cuadrado, E.; Roig, L.; Ois, A.; Muntasell, A.; Baro, T.; Alameda, F.; Roquer, J.; et al. Expansion of the NKG2C+ natural killer-cell subset is associated with high-risk carotid atherosclerotic plaques in seropositive patients for human cytomegalovirus. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2653–2659. [Google Scholar] [CrossRef] [Green Version]
- Akuffo, H.; Alexis, A.; Eidsmo, L.; Saed, A.; Nylén, S.; Maasho, K. Natural killer cells in cross-regulation of IL-12 by IL-10 in Leishmania antigen-stimulated blood donor cells. Clin. Exp. Immunol. 1999, 117, 529–534. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Yssel, H.; de Vries, J.E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 1993, 150, 4754–4765. [Google Scholar] [PubMed]
- Autran, B.; Leblond, V.; Sadat-Sowti, B.; Lefranc, E.; Got, P.; Sutton, L.; Binet, J.L.; Debre, P. A soluble factor released by CD8+CD57+ lymphocytes from bone marrow transplanted patients inhibits cell-mediated cytolysis. Blood 1991, 77, 2237–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalbeth, N.; Gundle, R.; Davies, R.J.; Lee, Y.C.; McMichael, A.J.; Callan, M.F. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J. Immunol. 2004, 173, 6418–6426. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorsi, I.; Spinelli, D.; Cantoni, C.; Barillà, C.; Pipitò, N.; De Pasquale, C.; Oliveri, D.; Cavaliere, R.; Carrega, P.; Benedetto, F.; et al. Symptomatic Carotid Atherosclerotic Plaques Are Associated with Increased Infiltration of Natural Killer (NK) Cells and Higher Serum Levels of NK Activating Receptor Ligands. Front. Immunol. 2019, 10, 1503. [Google Scholar] [CrossRef] [Green Version]
- Szymanowski, A.; Li, W.; Lundberg, A. Soluble Fas ligand is associated with natural killer cell dynamics in coronary artery disease. Atherosclerosis 2014, 233, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2013, 4, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedvetzki, S.; Sowinski, S.; Eagle, R.A.; Harris, J.; Vely, F.; Pende, D. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 2007, 109, 3776–3785. [Google Scholar] [CrossRef]
- Atochina, O.; Harn, D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin. Diagn. Lab. Immunol. 2005, 12, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.O.; Kim, N.; Kim, T.J.; Kim, K.; Kim, T.W.; Kumar, V.; Lee, K.M. Unidirectional signaling triggered through 2B4 (CD244), not CD48, in murine NK cells. J. Leukoc. Biol. 2010, 88, 707–714. [Google Scholar] [CrossRef]
- Dong, K.; Ge, J.H.; Gu, S.L.; Li, S.; Zhu, W.G.; Fan, F.Y.; Zhu, J.H. Ox-LDL can enhance the interaction of mice natural killer cells and dendritic cells via the CD48-2B4 pathway. Heart Vessels 2011, 26, 637–645. [Google Scholar] [CrossRef]
- Knorr, M.; Münzel, T.; Wenzel, P. Interplay of NK cells and monocytes in vascular inflammation and myocardial infarction. Front. Physiol. 2014, 5, 295. [Google Scholar] [CrossRef]
- Goldszmid, R.S.; Caspar, P.; Rivollier, A.; White, S.; Dzutsev, A.; Hieny, S.; Kelsall, B.; Trinchieri, G.; Sher, A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 2012, 36, 1047–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backteman, K.; Andersson, C.; Dahlin, L.G.; Ernerudh, J.; Jonasson, L. Lymphocyte subpopulations in lymph nodes and peripheral blood: A comparison between patients with stable angina and acute coronary syndrome. PLoS ONE 2012, 7, e32691. [Google Scholar] [CrossRef] [Green Version]
- Backteman, K.; Ernerudh, J.; Jonasson, L. Natural killer (NK) cell deficit in coronary artery disease: No aberrations in phenotype but sustained reduction of NK cells is associated with low-grade inflammation. Clin. Exp. Immunol. 2014, 175, 104–112. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Jin, J.Y.; Li, G.H.; Guo, Y.Z.; Yi, H.Y.; Zhang, J.R.; Lu, Y.J.; Zhang, J.L.; Li, C.Y.; et al. CD226 deletion improves post-infarction healing via modulating macrophage polarization in mice. Theranostics 2020, 10, 2422–2435. [Google Scholar] [CrossRef]
- Yan, W.; Zhou, L.; Wen, S.; Duan, Q.; Huang, F.; Tang, Y.; Liu, X.; Chai, Y.; Wang, L. Differential loss of natural killer cell activity in patients with acute myocardial infarction and stable angina pectoris. Int. J. Clin. Exp. Pathol. 2015, 8, 14667–14675. [Google Scholar]
- Szodoray, P.; Timar, O.; Veres, K.; Der, H.; Szomjak, E.; Lakos, G.; Aleksza, M.; Nakken, B.; Szegedi, G.; Soltesz, P. TH1/TH2 imbalance, measured by circulating and intracytoplasmic inflammatory cytokines--immunological alterations in acute coronary syndrome and stable coronary artery disease. Scand. J. Immunol. 2006, 64, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Laskarin, G.; Persic, V.; Ruzic, A.; Miletic, B.; Rakic, M.; Samsa, D.; Raljevic, D.; Pehar, V. Perforin-mediated cytotoxicity in non-ST elevation myocardial infarction. Scand. J. Immunol. 2011, 74, 195–204. [Google Scholar] [CrossRef]
- Lynch, L.A.; O’Connell, J.M.; Kwasnik, A.K.; Cawood, T.J.; O’Farrelly, C.; O’Shea, D.B. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 2009, 17, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.T.; Karapetyan, A.; Al-Attar, A. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. J. Immunol. 2011, 186, 4590–4598. [Google Scholar] [CrossRef]
- Jonasson, L.; Backteman, K.; Ernerudh, J. Loss of natural killer cell activity in patients with coronary artery disease. Atherosclerosis 2005, 183, 316–321. [Google Scholar] [CrossRef]
- Klarlund, K.; Pedersen, B.K.; Theander, T.; Andersen, V. Depressed natural killer cell activity in acute myocardial infarction. Clin. Exp. Immunol. 1987, 70, 209–216. [Google Scholar]
- Fehniger, T.A.; Carson, W.E.; Caligiuri, M.A. Costimulation of human natural killer cells is required for interferon gamma production. Transplant. Proc. 1999, 31, 1476–1478. [Google Scholar] [CrossRef]
- Kunikata, T.; Torigoe, K.; Ushio, S.; Okura, T.; Ushio, C.; Yamauchi, H.; Ikeda, M.; Ikegami, H.; Kurimoto, M. Constitutive and induced IL-18 receptor expression by various peripheral blood cell subsets as determined by anti-hIL-18R monoclonal antibody. Cell. Immunol. 1998, 189, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lidebjer, C.; Yuan, X.M.; Szymanowski, A.; Backteman, K.; Ernerudh, J.; Leanderson, P.; Nilsson, L.; Swahn, E.; Jonasson, L. NK cell apoptosis in coronary artery disease: Relation to oxidative stress. Atherosclerosis 2008, 199, 65–72. [Google Scholar] [CrossRef]
- Jonasson, L.; Wikby, A.; Olsson, A.G. Low serum beta-carotene reflects immune activation in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 120–125. [Google Scholar] [CrossRef]
- Hong, Y.J.; Cho, Y.N.; Kim, T.J.; Jin, H.M.; Kim, M.J.; Jung, H.J.; Kang, J.H.; Lee, S.J.; Park, K.J.; Kim, N.; et al. Functional deficiency of natural killer cells in acute coronary syndrome is related to ineffective degranulation. Int. J. Cardiol. 2014, 172, 613–615. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Aranha, F.C.; Ribeiro, U., Jr.; Basse, P.; Corbett, C.E.; Laurenti, M.D. Interleukin-2-activated natural killer cells may have a direct role in the control of leishmania (Leishmania) amazonensis promastigote and macrophage infection. Scand. J. Immunol. 2005, 62, 334–341. [Google Scholar] [CrossRef]
- Taherzadeh, Z.; Vanbavel, E.; De Vos, J.; Matlung, H.L.; Van Montfrans, G.; Brewster, L.M. Strain-dependent susceptibility for hypertension in mice resides in the natural killer gene complex. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1273–H1282. [Google Scholar] [CrossRef]
- Kossmann, S.; Schwenk, M.; Hausding, M.; Karbach, S.H.; Schmidgen, M.I.; Brandt, M. Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Ayach, B.B.; Yoshimitsu, M.; Dawood, F.; Sun, M.; Arab, S.; Chen, M.; Higuchi, K.; Siatskas, C.; Lee, P.; Lim, H.; et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 2304–2309. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour Babaei, M.; Kamalidehghan, B.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Devel. Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, C.; Terme, M.; Taïeb, J.; Ménard, C.; Flament, C.; Robert, C.; Maruyama, K.; Wakasugi, H.; Angevin, E.; Thielemans, K.; et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Investig. 2004, 114, 379–388. [Google Scholar] [CrossRef]
- Strassheim, D.; Dempsey, E.C.; Gerasimovskaya, E.; Stenmark, K.; Karoor, V. Role of Inflammatory Cell Subtypes in Heart Failure. J. Immunol. Res. 2019, 2164017. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.; Rose, N.R.; Čiháková, D. Natural killer cells in inflammatory heart disease. Clin. Immunol. 2017, 175, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Ormiston, M.L.; Deng, Y.; Stewart, D.J.; Courtman, D.W. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2010, 43, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Tamosiuniene, R.; Tian, W.; Dhillon, G.; Wang, L.; Sung, Y.K.; Gera, L.; Patterson, A.J.; Agrawal, R.; Rabinovitch, M.; Ambler, K.; et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ. Res. 2011, 109, 867–879. [Google Scholar] [CrossRef]
- Edwards, A.L.; Gunningham, S.P.; Clare, G.C.; Hayman, M.W.; Smith, M.; Frampton, C.M.; Robinson, B.A.; Troughton, R.W.; Beckert, L.E. Professional killer cell deficiencies and decreased survival in pulmonary arterial hypertension. Respirology 2013, 18, 1271–1277. [Google Scholar] [CrossRef]
- Eguizabal, C.; Zenarruzabeitia, O.; Monge, J.; Santos, S.; Vesga, M.A.; Maruri, N.; Arrieta, A.; Riñón, M.; Tamayo-Orbegozo, E.; Amo, L.; et al. Natural killer cells for cancer immunotherapy: Pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front. Immunol. 2014, 5, 439. [Google Scholar] [CrossRef]
- Porgador, A.; Mandelboim, O.; Restifo, N.P.; Strominger, J.L. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: Implications for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13140–13145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Magalhaes-Silverman, M.; Donnenberg, A.; Lembersky, B.; Elder, E.; Lister, J.; Rybka, W.; Whiteside, T.; Ball, E. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. Immunotherapy 2000, 23, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Farace, F.; Angevin, E.; Charpentier, F.; Nitenberg, G.; Triebel, F.; Hercend, T. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: Improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur. J. Cancer 1994, 30A, 1078–1083. [Google Scholar] [CrossRef]
- Spanholtz, J.; Preijers, F.; Tordoir, M.; Trilsbeek, C.; Paardekooper, J.; de Witte, T.; Schaap, N.; Dolstra, H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE 2011, 6, e20740. [Google Scholar] [CrossRef]
- Su, M.; Huang, C.X.; Dai, A.P. Immune checkpoint inhibitors: Therapeutic tools for breast cancer. Asian Pac. J. Cancer Prev. 2016, 17, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Deng, W.; Li, J.; Tang, Y.; Zhang, L.; Cui, Y.; Liang, X.Q. Peripheral blood lymphocyte subset levels differ in patients with hepatocellular carcinoma. Oncotarget 2016, 7, 77558–77564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.W.; Zhu, X.; Fu, X.; Yang, J.S.; Cao, Z.G.; Pu, C. Expression of Tim-3 on peripheral CD56(+) NK cells and its correlation with liver fibrosis in patients with advanced schistosomiasis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2015, 33, 346–350. [Google Scholar]
- Bouchentouf, M.; Williams, P.; Forner, K.A.; Cuerquis, J.; Michaud, V.; Paradis, P.; Schiffrin, E.L.; Galipeau, J. Interleukin-2 enhances angiogenesis and preserves cardiac function following myocardial infarction. Cytokine 2011, 56, 732–738. [Google Scholar] [CrossRef]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.K.; Kadri, N.; Snäll, J.; Brodin, P.; Gilfillan, S.; Colonna, M.; Bernhardt, G.; Höglund, P.; Kärre, K.; Chambers, B.J. Expression of CD226 is associated to but not required for NK cell education. Nat. Commun. 2017, 8, 15627. [Google Scholar] [CrossRef] [Green Version]
- Guillerey, C.; de Andrade, L.F.; Vuckovic, S.; Miles, K.; Ngiow, S.F.; Yong, M.C.; Teng, M.W.; Colonna, M.; Ritchie, D.S.; Chesi, M.; et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J. Clin. Investig. 2015, 125, 2077–2089. [Google Scholar] [CrossRef] [Green Version]
- Iguchi-Manaka, A.; Kai, H.; Yamashita, Y.; Shibata, K.; Tahara-Hanaoka, S.; Honda, S.; Yasui, T.; Kikutani, H.; Shibuya, K.; Shibuya, A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J. Exp. Med. 2008, 205, 2959–2964. [Google Scholar] [CrossRef] [Green Version]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef]
- Santos, M.S.; Gaziano, J.M.; Leka, L.S.; Beharka, A.A.; Hennekens, C.H.; Meydani, S.N. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: An investigation of the role of cytokines. Am. J. Clin. Nutr. 1998, 68, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Tosello-Trampont, A.; Surette, F.A.; Ewald, S.E.; Hahn, Y.S. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Front. Immunol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cell Type | Associated Molecules | |
|---|---|---|
| Monocyte |  | TNF-α, tissue factor, tPA, uPA, PAI-1,2, CSF1, MIF, MCP-1, IL-1,6,15,20, PDGF, VEGF, resistin, NO, TIMP-1,2,3 |
| Dysfunctional EC |  | P-selectin, CD106, CD54, TNF-α, IL-1, 6,8,11,14,15,18, fibronectin, collagen type IV, von Willebrand factor, PAI-1, PAF, thrombomodulin, tPA, urokinase, TFPI, MCP-1, CCL5, MIF, CSF1, CSF2, NO, endothelin, MMP-1, 2, TIMP-2, HMGB1, HSP 60, laminin, versican, perlecan |
| NK cell |  | IFN-γ, NKG2C, NKG2D, TRAIL, LILRB1, CD244, IL-18 |
| Dendritic cell |  | TNF-α, CCL19, IFN-α, IL-6,12, MMP-9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumrić, M.; Tičinović Kurir, T.; Borovac, J.A.; Božić, J. The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review. Biomolecules 2020, 10, 1514. https://doi.org/10.3390/biom10111514
Kumrić M, Tičinović Kurir T, Borovac JA, Božić J. The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review. Biomolecules. 2020; 10(11):1514. https://doi.org/10.3390/biom10111514
Chicago/Turabian StyleKumrić, Marko, Tina Tičinović Kurir, Josip A. Borovac, and Joško Božić. 2020. "The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review" Biomolecules 10, no. 11: 1514. https://doi.org/10.3390/biom10111514
APA StyleKumrić, M., Tičinović Kurir, T., Borovac, J. A., & Božić, J. (2020). The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review. Biomolecules, 10(11), 1514. https://doi.org/10.3390/biom10111514








