Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. Study Design
2.3. Biochemical Markers in Blood Plasma and Urine
2.4. Protein Content in Tissues
2.5. Amino Acids, Pyruvate, and Branched-Chain Keto Acids (BCKAs) in Blood Plasma and Tissues
2.6. Adenine Nucleotides
2.7. Tricarboxylic Acid (TCA) Cycle Intermediates in Blood Plasma and Tissues
2.8. Statistical Analysis
3. Results
3.1. Biochemical Markers in Blood Plasma and Urine
3.2. Body Weight, Food Intake, and Weights and Protein Contents of Tissues
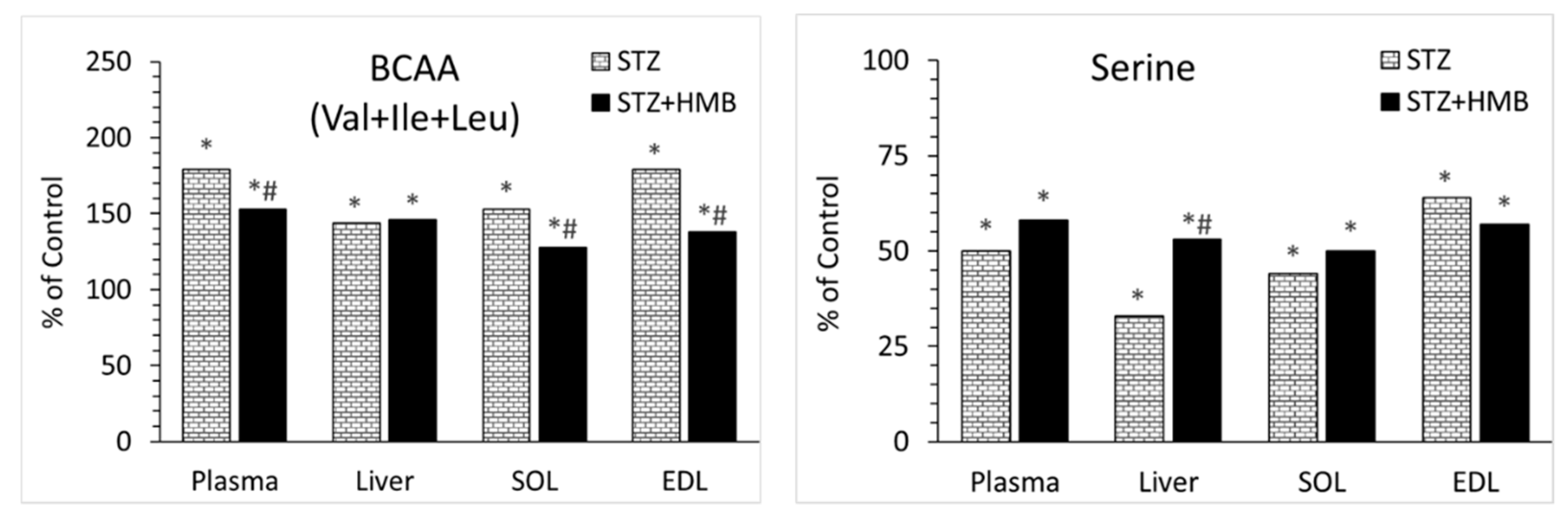
3.3. Amino Acid Concentrations in Blood Plasma
3.4. Amino Acid Concentrations in Liver
3.5. Amino Acid Concentrations in Muscles
3.6. BCKA Concentrations in Blood Plasma and Muscles
3.7. Pyruvate and Intermediates of TCA Cycle
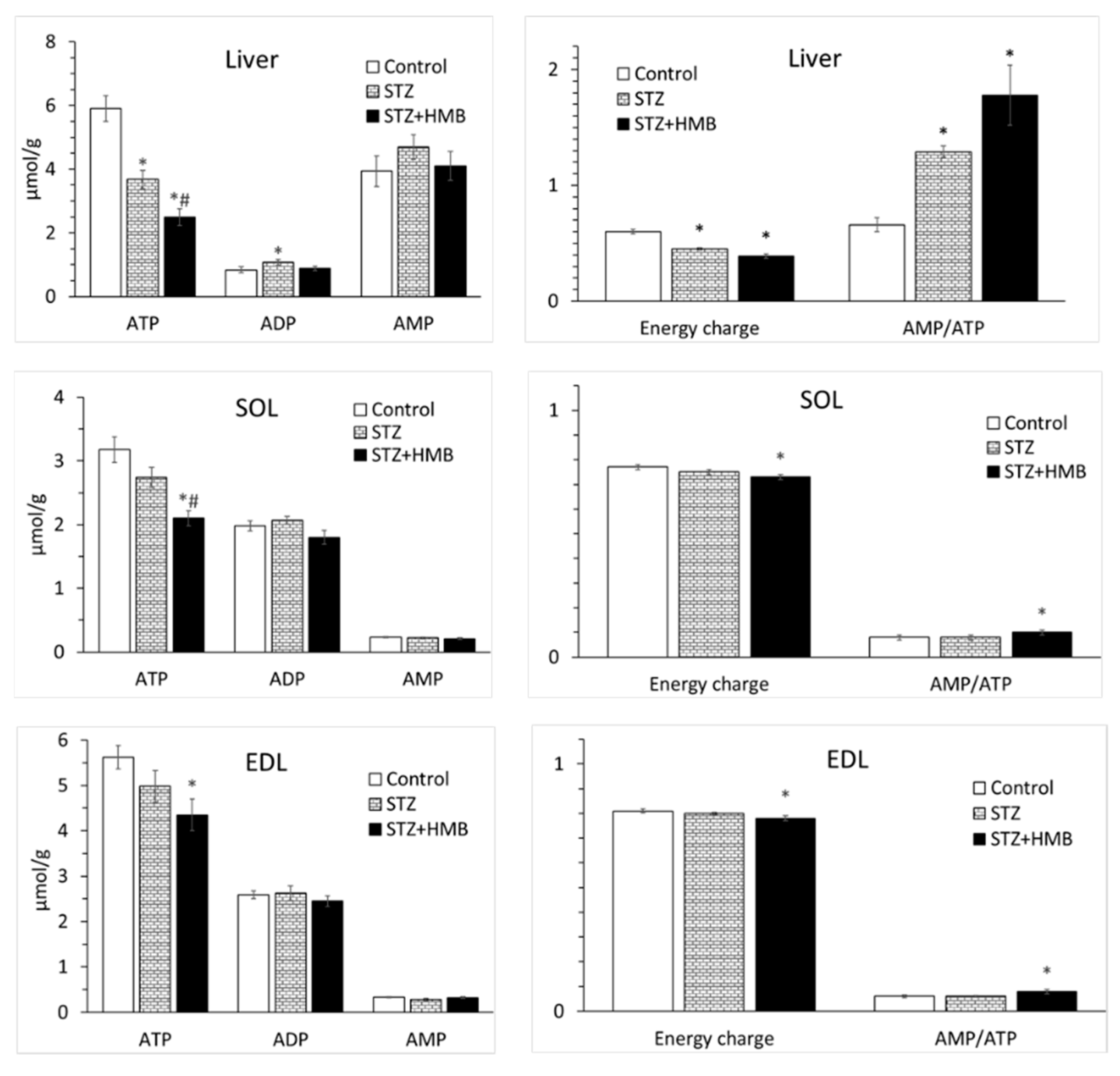
3.8. Adenine Nucleotide Concentrations in the Liver and Muscles
4. Discussion
4.1. Metabolic Alterations Induced by STZ
4.2. Why Are BCAAs Increased in STZ-Treated Animals?
4.3. Decreases in Serine Concentration
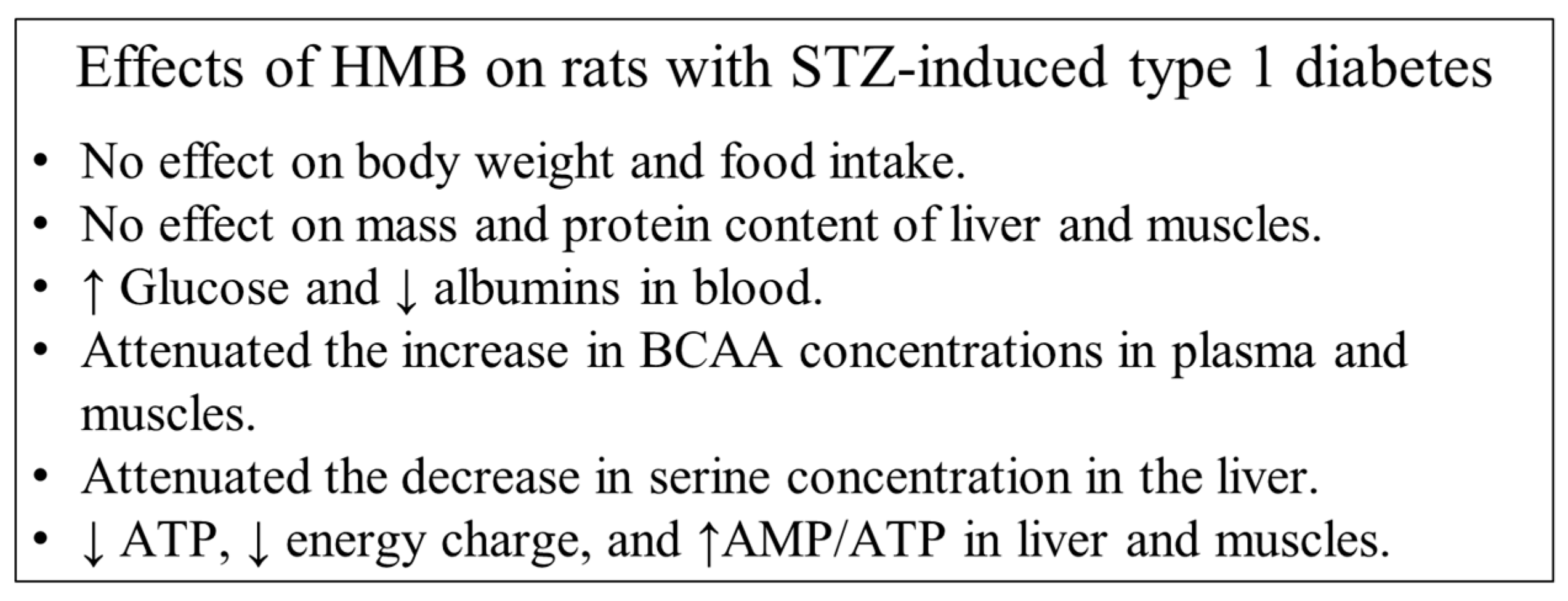
4.4. Effects of HMB
4.5. Differences Between SOL and EDL
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krause, M.P.; Riddell, M.C.; Hawke, T.J. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr. Diabetes 2011, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, G.; Al-Numair, K.S.; Veeramani, C.; Alsaif, M.A.; Almajwal, A.M. Protective effect of kaempferol, a flavonoid compound, on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Prog. Nutr. 2015, 17, 238–244. [Google Scholar]
- Karakelides, H.; Asmann, Y.W.; Bigelow, M.L.; Short, K.R.; Dhatariya, K.; Coenen-Schimke, J.; Kahl, J.; Mukhopadhyay, D.; Nair, K.S. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 2007, 56, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.F.; Hughes, M.C.; Ramos, S.V.; Varah, N.E.; Lamberz, C.; Rahman, F.A.; McGlory, C.; Tarnopolsky, M.A.; Krause, M.P.; Laham, R.; et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 2018, 61, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Nevoret, M.L.; Casellini, C.; Parson, H. Diabetic neuropathy. Endocrinol. Metab. Clin. North Am. 2013, 42, 747–787. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [PubMed]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J. Physiol. Biochem. 2010, 66, 311–319. [Google Scholar] [CrossRef]
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Always, S.E. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am. J. Physiol. 2011, 301, R701–R715. [Google Scholar] [CrossRef]
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J. Parenter. Enteral Nutr. 2000, 24, 133–139. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Doña, E.; Palenque, F.J.; Porras, N.; Dorado, A.; Godoy, A.M.; Rubio-Martínez, E.; Rojo-Martínez, G.; Martín-Valero, R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study). Clin. Nutr. 2016, 35, 1015–1022. [Google Scholar] [CrossRef]
- Ekinci, O.; Yanık, S.; Terzioğlu Bebitoğlu, B.; Yılmaz Akyüz, E.; Dokuyucu, A.; Erdem, S. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB), vitamin D, and protein supplementation on postoperative immobilization in malnourished older adult patients with hip fracture: A randomized controlled study. Nutr. Clin. Pract. 2016, 31, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Guo, Q.; Wang, W.; Zhang, L.; Wen, C.; Chen, X.; Yin, Y. β-Hydroxy-β-methyl butyrate is more potent than leucine in inhibiting starvation-induced protein degradation in C2C12 myotubes. J. Agric. Food Chem. 2018, 66, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, S.; Gungor, O.; Gunduz, M.; Cilci, M.; Demirci, M.C.; Tamer, A. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC Nephrol. 2013, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Aftring, R.P.; Miller, W.J.; Buse, M.G. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am. J. Physiol. 1988, 254, E292–E300. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Lugari, R.; Montanari, A.; Dall’Argine, P.; Elia, G.F.; Nicolotti, V.; Simoni, I.; Parmeggiani, A.; Novarini, A.; Gnudi, A. Plasma and skeletal muscle free amino acids in type I, insulin-treated diabetic subjects. Diabetes 1985, 34, 812–815. [Google Scholar] [CrossRef]
- Jensen-Waern, M.; Andersson, M.; Kruse, R.; Nilsson, B.; Larsson, R.; Korsgren, O.; Essén-Gustavsson, B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab. Anim. 2009, 43, 249–254. [Google Scholar] [CrossRef]
- Van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: Role of circulating branched-chain amino acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of branched-chain amino acid levels in diabetes and NAFL and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract. 2015, 9, 293–297. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Ttransl. Med. 2011, 3, 80re2. [Google Scholar] [CrossRef]
- Koksal, B. Effect of streptozotocin on plasma insulin levels of rats and mice: A meta-analysis study. Open Access Maced, J. Med. Sci. 2015, 3, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.B.; Gollnick, P.D.; Ianuzzo, C.D. Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res. 1975, 162, 387–394. [Google Scholar] [CrossRef]
- Medina-Sanchez, M.; Rodriguez-Sanchez, C.; Vega-Alvarez, J.A.; Menedez-Pelaez, A.; Perez-Casas, A. Proximal skeletal muscle alterations in streptozotocin-diabetic rats: a histochemical and morphometric analysis. Am. J. Anat. 1991, 191, 48–56. [Google Scholar] [CrossRef]
- Holeček, M.; Mičuda, S. Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol. Res. 2017, 66, 959–967. [Google Scholar] [CrossRef]
- Holecek, M.; Sispera, L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids 2014, 46, 1377–1384. [Google Scholar] [CrossRef]
- Muthny, T.; Kovarik, M.; Sispera, L.; Tilser, I.; Holecek, M. Protein metabolism in slow- and fast-twitch skeletal muscle during turpentine-induced inflammation. Int. J. Exp. Pathol. 2008, 89, 64–71. [Google Scholar] [CrossRef]
- Rodríguez, T.; Alvarez, B.; Busquets, S.; Carbó, N.; López-Soriano, F.J.; Argilés, J.M. The increased skeletal muscle protein turnover of the streptozotocin diabetic rat is associated with high concentrations of branched-chain amino acids. Biochem. Mol. Med. 1997, 61, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Vodeničarovová, M. Effects of beta-hydroxy-beta-methylbutyrate in partially hepatectomized rats. Physiol. Res. 2018, 67, 741–751. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Holeček, M.; Vodeničarovová, M. Muscle wasting and branched-chain amino acid, alpha-ketoglutarate, and ATP depletion in a rat model of liver cirrhosis. Int. J. Exp. Pathol. 2018, 99, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef]
- Odedra, B.R.; Dalal, S.S.; Millward, D.J. Muscle protein synthesis in the streptozotocin-diabetic rat. A possible role for corticosterone in the insensitivity to insulin infusion in vivo. Biochem. J. 1982, 202, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J.; Garlick, P.J.; Nnanyelugo, D.O.; Waterlow, J.C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem. J. 1976, 156, 185–188. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Ann. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Holeček, M. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef]
- May, M.E.; Mancusi, V.J.; Aftring, R.P.; Buse, M.G. Effects of diabetes on oxidative decarboxylation of branched-chain keto acids. Am. J. Physiol. 1980, 239, E215–E222. [Google Scholar] [CrossRef]
- Lombardo, Y.B.; Thamotharan, M.; Bawani, S.Z.; Paul, H.S.; Adibi, S.A. Posttranscriptional alterations in protein masses of hepatic branched-chain keto acid dehydrogenase and its associated kinase in diabetes. Proc. Assoc. Am. Physicians 1998, 110, 40–49. [Google Scholar]
- Holeček, M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Louis, E.; Noben, J.P.; Adriaensens, P. Metabolic profiling of type 1 diabetes mellitus in children and adolescents: a case-control study. Diabetol. Metab. Syndr. 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Drábková, P.; Šanderová, J.; Kovařík, J.; Kanďár, R. An assay of selected serum amino acids in patients with type 2 diabetes mellitus. Adv. Clin. Exp. Med. 2015, 24, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.J.; Buschard, K. L-serine: a neglected amino acid with a potential therapeutic role in diabetes. APMIS 2019, 127, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, M.H.; El-Awady, M.S.; Megahed, N.; Gameil, N.M. The ergogenic supplement β-hydroxy-β-methylbutyrate (HMB) attenuates insulin resistance through suppressing GLUT-2 in rat liver. Can. J. Physiol. Pharmacol. 2016, 94, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, C.Y.; Teixeira, S.S.; Campello, R.S.; Gerlinger-Romero, F.; Rodrigues, C.F.; Guimarães-Ferreira, L.; Machado, U.F.; Nunes, M.T. Beta hydroxy beta methylbutyrate supplementation impairs peripheral insulin sensitivity in healthy sedentary Wistar rats. Acta Physiol. 2014, 212, 62–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Zhou, P.; Yan, H.; Zhang, Z.; Zhang, H.; Qi, R.; Liu, J. β-Hydroxy-β-methylbutyrate-induced upregulation of miR-199a-3p contributes to slow-to-fast muscle fiber type conversion in mice and C2C12 cells. J. Agric. Food Chem. 2020, 68, 530–540. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Desikan, V.; Mileva, I.; Garlick, J.; Lane, A.H.; Wilson, T.A.; McNurlan, M.A. The effect of oral leucine on protein metabolism in adolescents with type 1 diabetes mellitus. Int. J. Pediatr. Endocrinol. 2010, 2010, 493258. [Google Scholar] [CrossRef]
- Hoppeler, H.; Hudlicka, O.; Uhlmann, E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J. Physiol. 1987, 385, 661–675. [Google Scholar] [CrossRef]
- Hamm, R. Transaminases of skeletal muscle. 2. Transaminase activities in white and red muscles of pigs and cows. J. Food Sci. 1969, 34, 449–452. [Google Scholar] [CrossRef]

| Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) | |
|---|---|---|---|
| Blood plasma | |||
| Glucose (mmol/L) | 4.73 ± 0.22 | 9.37 ± 1.17 * | 17.00 ± 2.84 *# |
| Insulin (µU/mL) | 77.53 ± 22.01 | 3.12 ± 0.46 * | 6.25 ± 0.84 * |
| Urea (mmol/L) | 5.37 ± 0.17 | 18.68 ± 1.00 * | 19.63 ± 0.94 * |
| Ammonia (µmol/L) | 35 ± 5 | 60 ± 6 * | 66 ± 6 * |
| Creatinine (µmol/L) | 18.58 ± 0.50 | 14.67 ± 0.78 * | 14.86 ± 0.49 * |
| Proteins (g/L) | 57.65 ± 0.65 | 51.94 ± 0.85 * | 53.56 ± 1.25 * |
| Albumins (g/L) | 38.32 ± 0.70 | 34.97 ± 0.74 * | 29.79 ± 0.61 *# |
| Urine | |||
| Glucose (mmol/mmol creatinine) | 0.35 ± 0.02 | 590.54 ± 55.80 * | 386.23 ± 59.66 *# |
| Urea (mmol/mmol creatinine) | 93 ± 5 | 319 ± 25 * | 389 ± 28 * |
| Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) | |
|---|---|---|---|
| Body weight | |||
| Initial (g) | 205 ± 1 | 208 ± 1 | 206 ± 1 |
| Final (g) | 268 ± 5 | 189 ± 5 * | 175 ± 6 * |
| Gain (g) | 63 ± 5 | –23 ± 2 * | –36 ± 4 * |
| Food intake | |||
| Average (g/day) | 24.7 ± 0.4 | 34.7 ± 0.6 * | 32.7 ± 0.9 * |
| Cumulative (g) | 148 ± 3 | 208 ± 4 * | 196 ± 6 * |
| Liver | |||
| Weight (g) | 8.22 ± 0.17 | 7.11 ± 0.28 * | 6.59 ± 0.32 * |
| (g/kg b.w.) | 30.73 ± 0.36 | 37.49 ± 0.70 * | 37.63 ± 0.83 * |
| Protein (g) | 1.33 ± 0.06 | 1.20 ± 0.05 | 1.11 ± 0.06 * |
| (g/kg b.w.) | 4.97 ± 0.18 | 6.34 ± 0.12 * | 6.08 ± 0.16 * |
| SOL | |||
| Weight (mg) | 109 ± 3 | 87 ± 2 * | 83 ± 4 * |
| (mg/kg b.w.) | 407 ± 8 | 461 ± 10 * | 475 ± 3 * |
| Protein (mg) | 16.14 ± 0.64 | 14.22 ± 0.54 | 14.60 ± 0.78 |
| (mg/kg b.w.) | 60.45 ± 2.64 | 75.42 ± 3.39 * | 80.46 ± 2.99 * |
| EDL | |||
| Weight (mg) | 125 ± 4 | 80 ± 3 * | 71 ± 3 * |
| (mg/kg b.w.) | 465 ± 9 | 420 ± 6 * | 408 ± 5 * |
| Protein (mg) | 19.4 ± 0.7 | 13.2 ± 0.4 * | 12.8 ± 1.0 * |
| (mg/kg b.w.) | 72.5 ± 1.6 | 69.6 ± 1.0 | 70.0 ± 2.4 |
| Plasma | Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) |
|---|---|---|---|
| EAA | |||
| Histidine | 55 ± 2 | 57 ± 1 | 56 ± 2 |
| Isoleucine | 105 ± 4 | 171 ± 7 * | 141 ± 7 *# |
| Leucine | 151 ± 5 | 269 ± 11 * | 228 ± 15 *# |
| Lysine | 376 ± 14 | 333 ± 9 | 338 ± 26 |
| Methionine | 42 ± 1 | 42 ± 4 | 38 ± 4 |
| Phenylalanine | 68 ± 2 | 89 ± 1 * | 87 ± 4 * |
| Threonine | 228 ± 7 | 209 ± 10 | 188 ± 14 |
| Valine | 191 ± 6 | 360 ± 15 * | 316 ± 19 * |
| BCAA | 447 ± 14 | 800 ± 33 * | 685 ± 39 *# |
| Σ EAA | 1216 ± 28 | 1529 ± 42 | 1394 ± 40 *# |
| NEAA | |||
| Alanine | 368 ± 23 | 288 ± 21 | 335 ± 39 |
| Arginine | 110 ± 3 | 124 ± 4 | 122 ± 6 |
| Asparagine | 55 ± 3 | 51 ± 3 | 52 ± 3 |
| Aspartate | 13 ± 1 | 1 ± 1* | 2 ± 2* |
| Citrulline | 70 ± 3 | 156 ± 8 * | 133 ± 9 * |
| Glutamate | 125 ± 9 | 62 ± 4 * | 56 ± 4 * |
| Glutamine | 688 ± 18 | 335 ± 13 * | 425 ± 24 *# |
| Glycine | 394 ± 12 | 240 ± 17 * | 233 ± 22 * |
| Ornithine | 39 ± 1 | 48 ± 3 * | 60 ± 3 * |
| Proline | 129 ± 6 | 114 ± 9 | 143 ± 23 |
| Serine | 259 ± 6 | 129 ± 8 * | 149 ± 18 * |
| Taurine | 212 ± 12 | 429 ± 24 * | 310 ± 44 *# |
| Tyrosine | 83 ± 5 | 70 ± 3 | 75 ± 9 |
| Σ NEAA | 2655 ± 73 | 2142 ± 86 * | 2172 ± 104 * |
| Σ Amino acids | 3882 ± 97 | 3671 ± 108 | 3566 ± 125 |
| Liver | Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) |
|---|---|---|---|
| EAA | |||
| Histidine | 637 ± 17 | 505 ± 55 * | 570 ± 29 |
| Isoleucine | 180 ± 8 | 231 ± 21 | 214 ± 25 |
| Leucine | 288 ± 11 | 389 ± 33 | 387 ± 47 |
| Lysine | 786 ± 56 | 1051 ± 212 | 994 ± 114 |
| Methionine | 58 ± 2 | 54 ± 2 | 67 ± 5 # |
| Phenylalanine | 105 ± 4 | 108 ± 4 | 99 ± 5 |
| Threonine | 756 ± 68 | 613 ± 155 | 1,481 ± 508 |
| Valine | 291 ± 12 | 477 ± 45 * | 512 ± 86 * |
| BCAA | 760 ± 31 | 1098 ± 98 * | 1113 ± 157 * |
| Σ EAA | 3025 ± 183 | 3293 ± 256 | 4323 ± 722 |
| NEAA | |||
| Alanine | 1199 ± 112 | 827 ± 41 | 1199 ± 218 |
| Asparagine | 249 ± 12 | 141 ± 27 * | 160 ± 20* |
| Aspartate | 1300 ± 56 | 685 ± 50 * | 1014 ± 186 |
| Glutamate | 1436 ± 57 | 1783 ± 434 | 1763 ± 104 |
| Glutamine | 7334 ± 251 | 3220 ± 428 * | 3818 ± 541 * |
| Glycine | 2887 ± 122 | 2274 ± 241 * | 2486 ± 147 |
| Ornithine | 338 ± 18 | 608 ± 89 | 975 ± 223 * |
| Proline | 256 ± 23 | 217 ± 15 | 321 ± 51 |
| Serine | 1104 ± 63 | 365 ± 15 * | 581 ± 83 *# |
| Taurine | 3065 ± 273 | 2808 ± 339 | 4800 ± 1075 |
| Tyrosine | 103 ± 5 | 84 ± 3 * | 92 ± 7 |
| Σ NEAA | 19,927 ± 483 | 13,180 ± 405* | 18,398 ± 1243 # |
| Σ Amino acids | 22,953 ± 650 | 16,474 ± 603* | 22,721 ± 1828 # |
| SOL | Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) |
|---|---|---|---|
| EAA | |||
| Histidine | 426 ± 24 | 504 ± 46 | 458 ± 31 |
| Isoleucine | 96 ± 4 | 127 ± 12 * | 101 ± 6 |
| Leucine | 138 ± 5 | 209 ± 15 * | 172 ± 6 *# |
| Lysine | 941 ± 99 | 1236 ± 104 | 1167 ± 158 |
| Methionine | 47 ± 2 | 58 ± 6 | 48 ± 4 |
| Phenylalanine | 71 ± 2 | 110 ± 4 * | 102 ± 6 * |
| Threonine | 708 ± 73 | 515 ± 86 | 405 ± 84 * |
| Valine | 175 ± 7 | 290 ± 22 * | 249 ± 11 *# |
| BCAA | 409 ± 15 | 626 ± 48 * | 522 ± 20 * |
| Σ EAA | 2602 ± 161 | 3048 ± 127 | 2703 ± 197 |
| NEAA | |||
| Alanine | 2698 ± 78 | 1596 ± 166 * | 1454 ± 233 * |
| Asparagine | 515 ± 27 | 526 ± 38 | 484 ± 28 |
| Aspartate | 2526 ± 114 | 466 ± 81 * | 728 ± 252 * |
| Glutamate | 3686 ± 183 | 2101 ± 170 * | 2075 ± 274 * |
| Glutamine | 8832 ± 405 | 7019 ± 726 * | 7523 ± 442 |
| Glycine | 2479 ± 135 | 2107 ± 122 | 1685 ± 203 * |
| Ornithine | 67 ± 3 | 66 ± 5 | 84 ± 7 *# |
| Proline | 288 ± 9 | 314 ± 31 | 273 ± 27 |
| Serine | 2699 ± 151 | 1179 ± 92 * | 1353 ± 122 * |
| Taurine | 22,948 ± 712 | 27,506 ± 1356 * | 27,586 ± 1262 * |
| Tyrosine | 102 ± 5 | 100 ± 5 | 98 ± 13 |
| Σ NEAA | 48,969 ± 1577 | 45,492 ± 1806 | 45,501 ± 1328 |
| Σ Amino acids | 51,571 ± 1692 | 48,540 ± 1833 | 48,204 ± 1409 |
| EDL | Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) |
|---|---|---|---|
| EAA | |||
| Histidine | 168 ± 4 | 213 ± 24 | 163 ± 11 |
| Isoleucine | 113 ± 3 | 184 ± 13 * | 147 ± 6 |
| Leucine | 151 ± 4 | 274 ± 17 * | 215 ± 14 *# |
| Lysine | 514 ± 43 | 593 ± 28 | 462 ± 51 |
| Methionine | 52 ± 2 | 81 ± 5 * | 69 ± 5 * |
| Phenylalanine | 80 ± 2 | 132 ± 5 * | 121 ± 8 * |
| Threonine | 415 ± 31 | 327 ± 19 * | 297 ± 19 * |
| Valine | 203 ± 6 | 378 ± 25 * | 300 ± 13 *# |
| BCAA | 467 ± 13 | 836 ± 54 * | 647 ± 27 *# |
| Σ EAA | 1695 ± 74 | 2181 ± 66 * | 1760 ± 82 |
| NEAA | |||
| Alanine | 2192 ± 76 | 2451 ± 101 | 2186 ± 140 |
| Asparagine | 213 ± 10 | 292 ± 28 * | 238 ± 17 |
| Aspartate | 490 ± 22 | 527 ± 42 | 383 ± 26 # |
| Glutamate | 989 ± 65 | 886 ± 157 | 749 ± 90 |
| Glutamine | 3744 ± 205 | 2443 ± 344 * | 2632 ± 200 * |
| Glycine | 3693 ± 221 | 2193 ± 113 * | 2022 ± 150 * |
| Ornithine | 37 ± 2 | 45 ± 3 | 42 ± 4 |
| Proline | 233 ± 10 | 412 ± 24 * | 375 ± 28 * |
| Serine | 956 ± 49 | 612 ± 85 * | 540 ± 44 * |
| Taurine | 16,188 ± 416 | 20,297 ± 552 * | 19,947 ± 793 * |
| Tyrosine | 117 ± 5 | 126 ± 5 | 124 ± 6 |
| Σ NEAA | 33,491 ± 685 | 34,842 ± 746 | 34,352 ± 980 |
| Σ Amino acids | 35,186 ± 737 | 37,024 ± 766 | 36,111 ± 1047 |
| Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) | |
|---|---|---|---|
| Blood plasma | |||
| KIV | 14.4 ± 0.9 | 10.2 ± 0.8 * | 9.5 ± 0.8 * |
| KIC | 42.1 ± 3.0 | 30.3 ± 2.1 * | 27.4 ± 2.9 * |
| KMV | 34.2 ± 2.4 | 26.1 ± 1.8 * | 28.9 ± 2.6 |
| ∑ BCKA | 90.7 ± 6.1 | 66.6 ± 4.4 * | 65.7 ± 5.0 * |
| SOL | |||
| KIV | 10.5 ± 0.8 | 7.5 ± 0.6 * | 7.7 ± 0.8 * |
| KIC | 1.1 ± 0.2 | 3.5 ± 0.4 * | 2.2 ± 0.3 # |
| KMV | 33.4 ± 3.6 | 33.6 ± 2.2 | 26.9 ± 3.2 |
| ∑ BCKA | 44.7 ± 4.1 | 44.2± 2.6 | 36.8 ± 3.4 |
| EDL | |||
| KIV | 24.1 ± 0.8 | 29.6 ± 0.9 * | 32.5 ± 2.3 * |
| KIC | 2.2 ± 0.3 | 6.1 ± 1.2 * | 3.8 ± 0.5 |
| KMV | 29.0 ± 6.9 | 50.1 ± 3.7 * | 50.0 ± 2.1 * |
| ∑ BCKA | 55.3 ± 7.2 | 85.7 ± 5.2 * | 86.3 ± 3.9 * |
| Control (n = 8) | STZ (n = 9) | STZ + HMB (n = 8) | |
|---|---|---|---|
| Blood plasma | |||
| pyruvate | 539 ± 59 | 379 ± 26 * | 464 ± 24 |
| malate | 821 ± 112 | 1179 ± 224 | 1297 ± 200 |
| α-ketoglutarate | 72 ± 10 | 104 ± 20 | 115 ± 18 |
| fumarate | 10 ± 1 | 9 ± 1 | 10 ± 1 |
| cis-aconitate | 44 ± 4 | 27 ± 1 * | 33 ± 2 * |
| Liver | |||
| pyruvate | 18.7 ± 1.6 | 19.8 ± 1.3 | 22.1 ± 1.8 |
| malate | 16.96 ± 1.49 | 12.13 ± 1.17 * | 11.27 ± 1.46 * |
| α-ketoglutarate | 1.81 ± 0.19 | 2.08 ± 0.13 | 2.06 ± 0.18 |
| fumarate | 0.17 ± 0.01 | 0.20 ± 0.02 | 0.22 ± 0.02 * |
| oxaloacetate | 3.90 ± 0.39 | 3.64 ± 0.75 | 2.94 ± 0.22 |
| cis-aconitate | 0.25 ± 0.01 | 0.17 ± 0.01 * | 0.19 ± 0.01 * |
| SOL | |||
| pyruvate | 56.1 ± 3.9 | 42.7 ± 5.1 | 43.6 ± 7.4 |
| malate | 3.49 ± 0.17 | 3.28 ± 0.30 | 2.95 ± 0.11 |
| α-ketoglutarate | 2.42 ± 0.16 | 3.00 ± 0.15 * | 2.48 ± 0.10 |
| fumarate | 0.26 ± 0.01 | 0.11 ± 0.01 * | 0.12 ± 0.01 * |
| oxaloacetate | 139 ± 9 | 110 ± 4 * | 109 ± 3 * |
| cis-aconitate | 0.07 ± 0.03 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| EDL | |||
| pyruvate | 207 ± 16 | 263 ± 14 | 156 ± 23# |
| malate | 5.99 ± 1.08 | 3.41 ± 0.95 | 2.06 ± 0.46 * |
| α-ketoglutarate | 1.64 ± 0.32 | 2.85 ± 0.11 * | 3.15 ± 0.49 * |
| fumarate | 0.14 ± 0.00 | 0.13 ± 0.01 | 0.09 ± 0.01 *# |
| oxaloacetate | 226 ± 7 | 217 ± 20 | 219 ± 10 |
| cis-aconitate | 0.012 ± 0.002 | 0.012 ± 0.001 | 0.007 ± 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holeček, M.; Vodeničarovová, M.; Fingrová, R. Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes. Biomolecules 2020, 10, 1475. https://doi.org/10.3390/biom10111475
Holeček M, Vodeničarovová M, Fingrová R. Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes. Biomolecules. 2020; 10(11):1475. https://doi.org/10.3390/biom10111475
Chicago/Turabian StyleHoleček, Milan, Melita Vodeničarovová, and Radana Fingrová. 2020. "Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes" Biomolecules 10, no. 11: 1475. https://doi.org/10.3390/biom10111475
APA StyleHoleček, M., Vodeničarovová, M., & Fingrová, R. (2020). Dual Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Amino Acid, Energy, and Protein Metabolism in the Liver and Muscles of Rats with Streptozotocin-Induced Type 1 Diabetes. Biomolecules, 10(11), 1475. https://doi.org/10.3390/biom10111475





