Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. cDNA Constructs, Transfections and CRISPR-Cas
2.3. Organotypic Cultures
2.4. Immunocytochemistry and Immunofluorescent Imaging
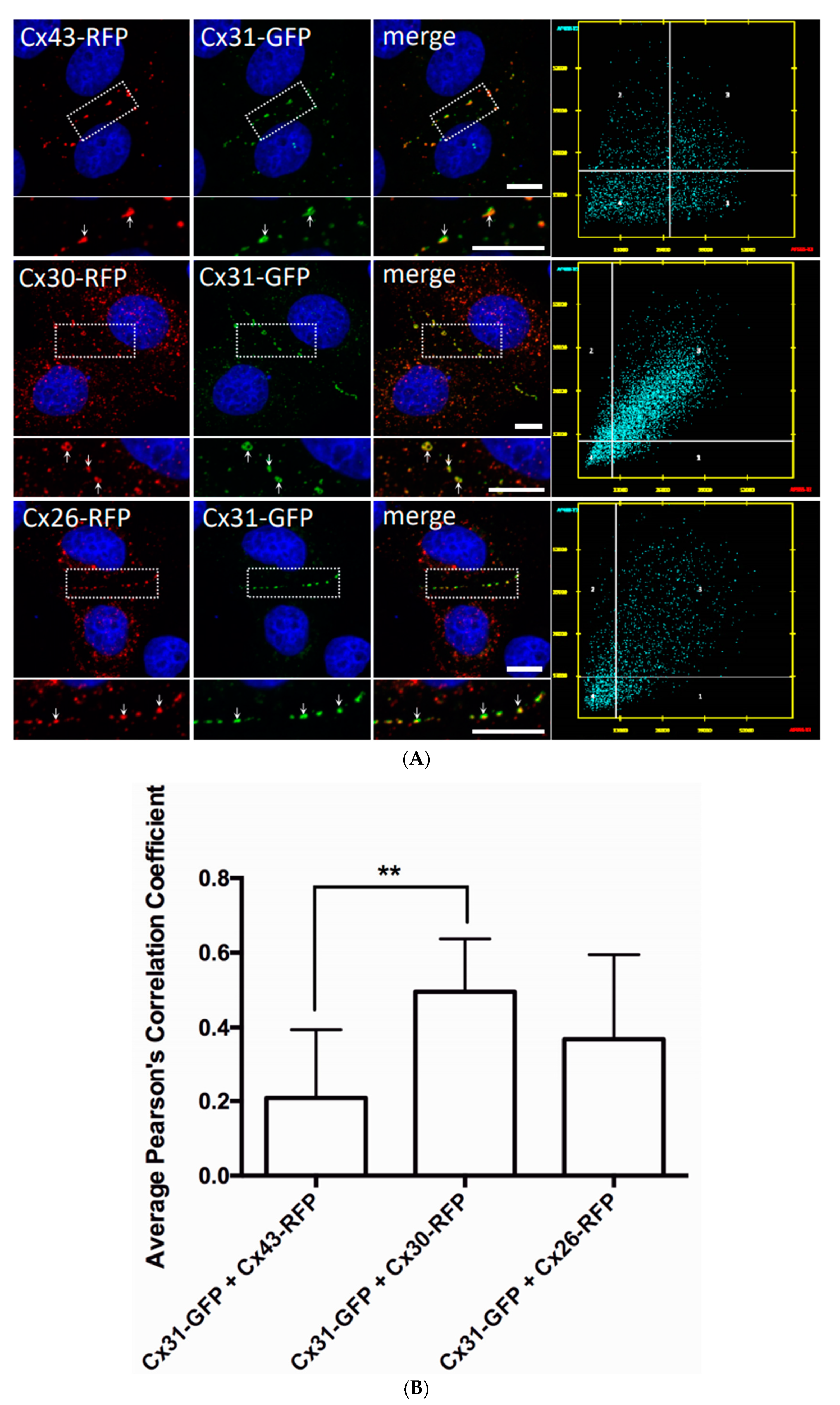
2.5. Connexin Co-Localization Analysis
2.6. Live Cell Time-Lapse Imaging
2.7. Cell Lysates and Immunoblotting
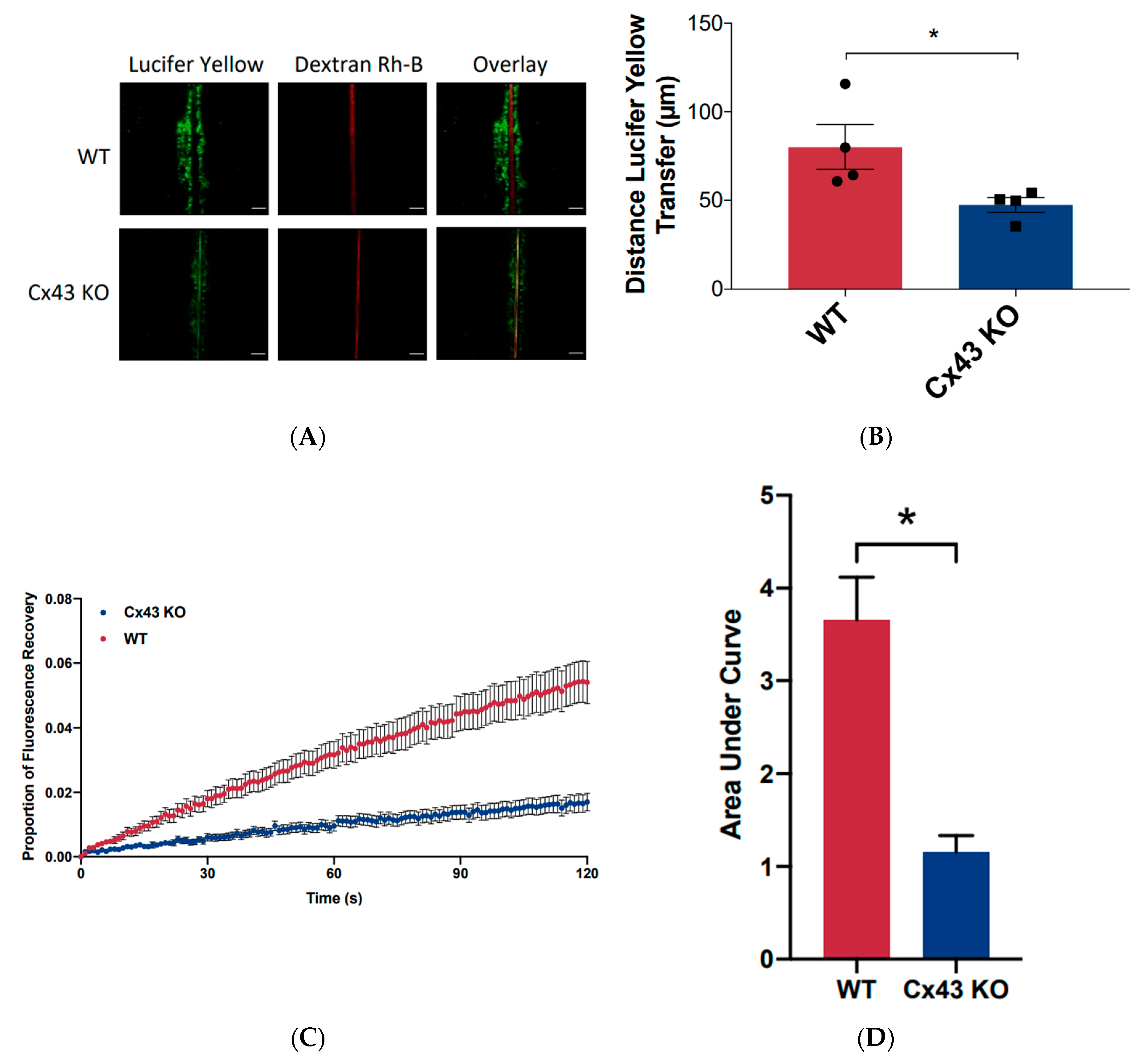
2.8. Scrape Load Assay
2.9. Fluorescence Recovery after Photobleaching
2.10. Drug Treatment
3. Results
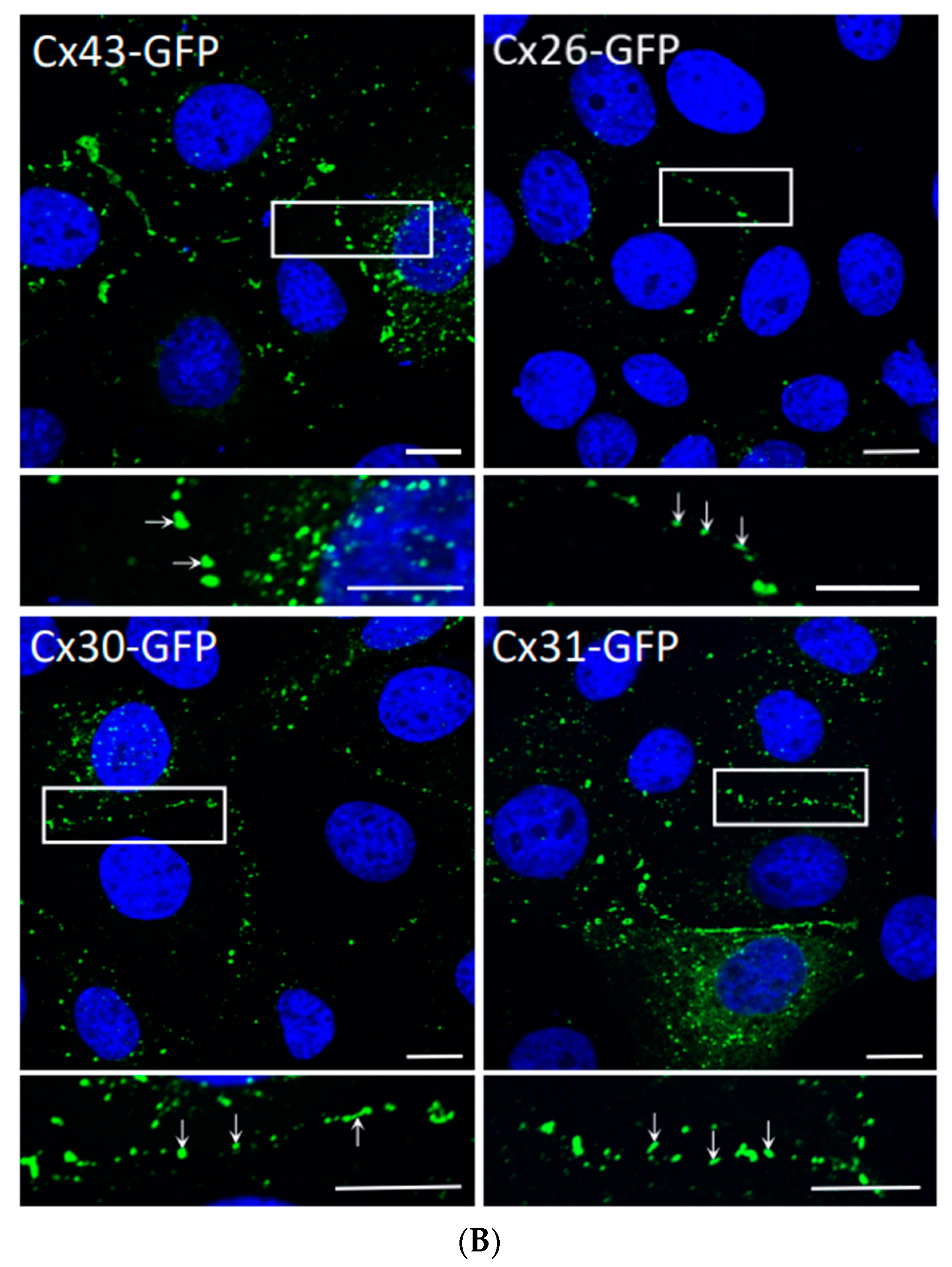
3.1. Monolayer REKs Are Enriched in Cx43 and Make Prototypical Gap Junctions
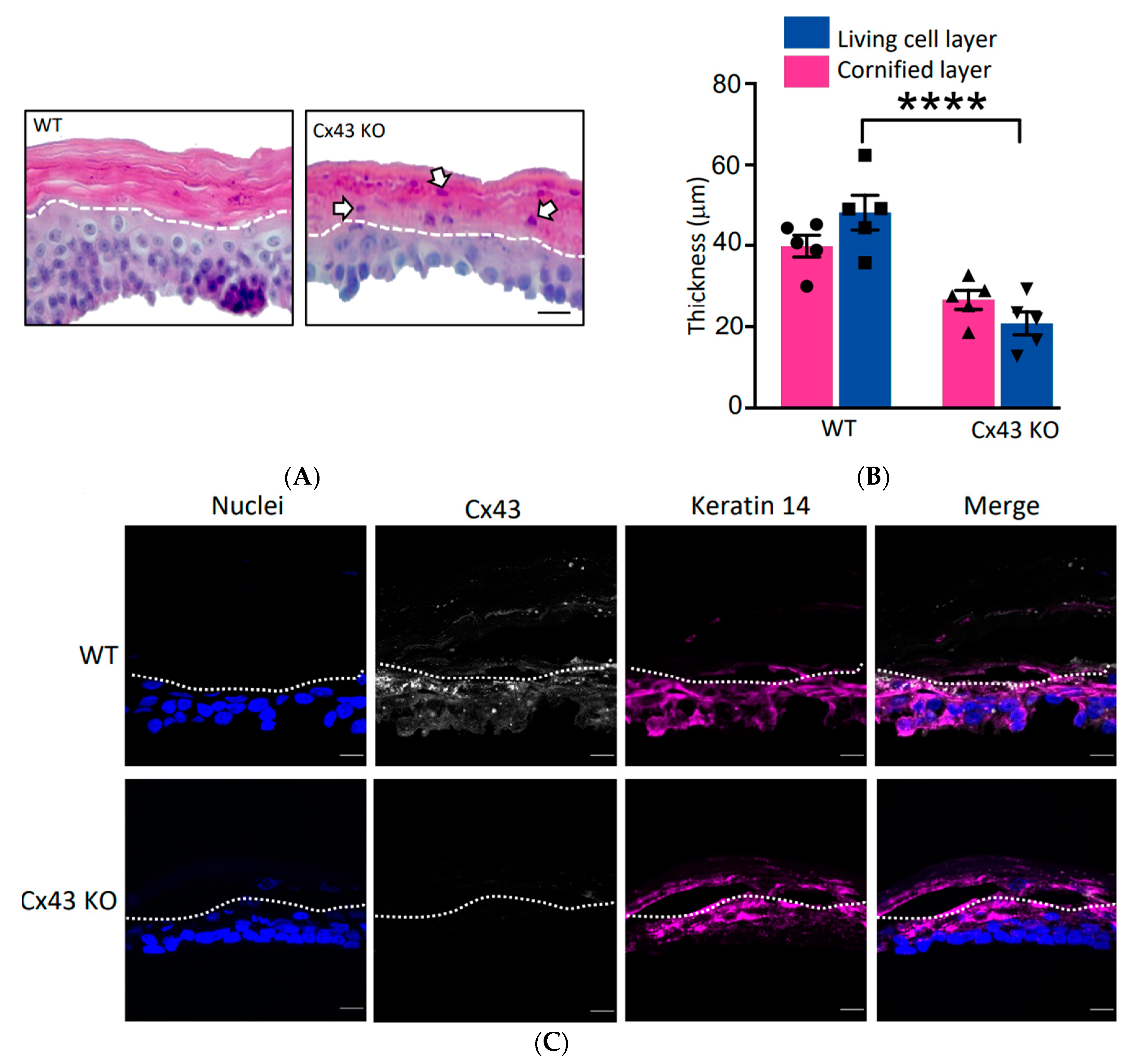
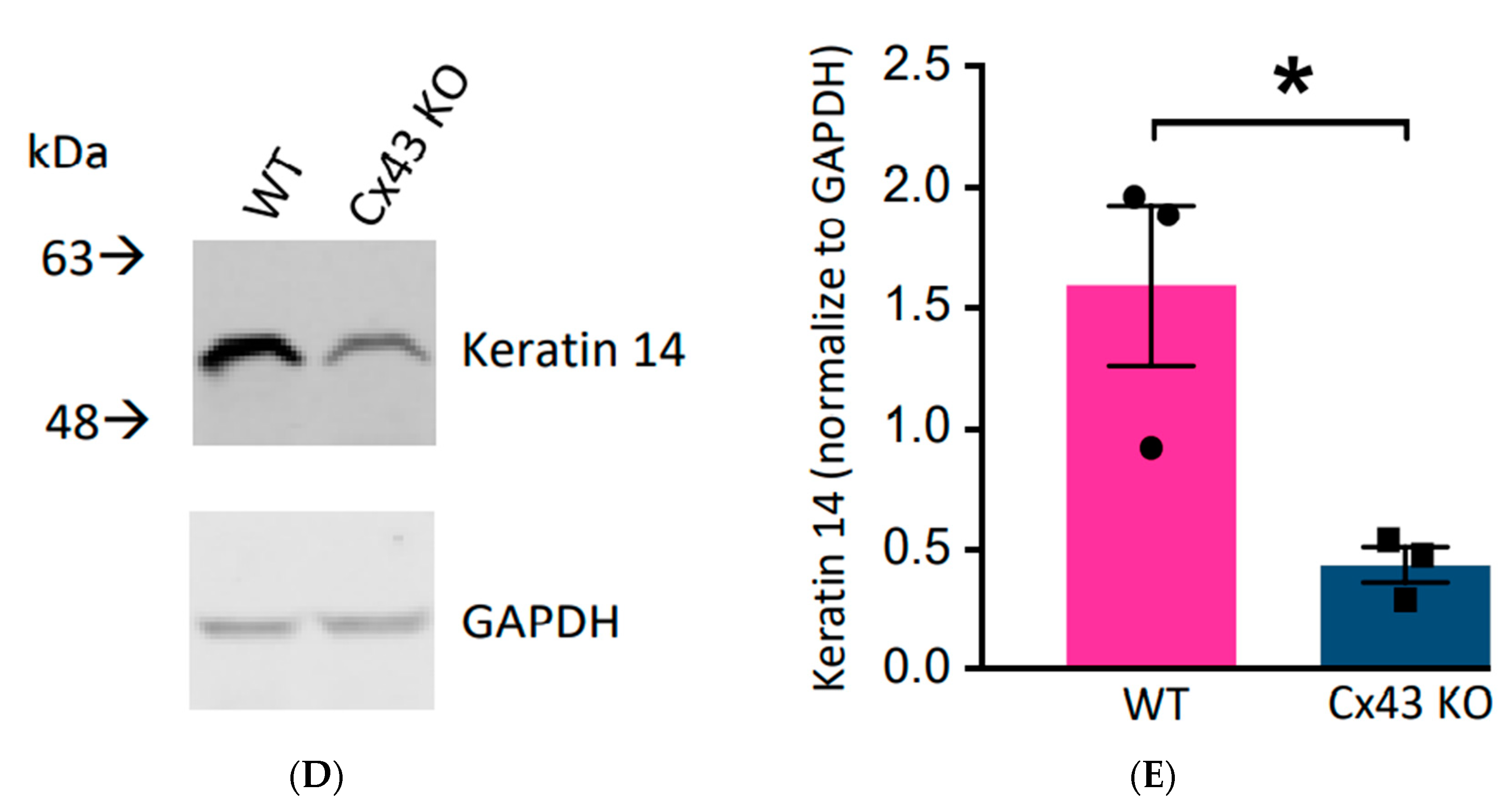
3.2. Cx43 Ablation had NO Effect on REKs in 2D Cultures but Grossly Dysregulated Differentiation in Organotypic Cultures
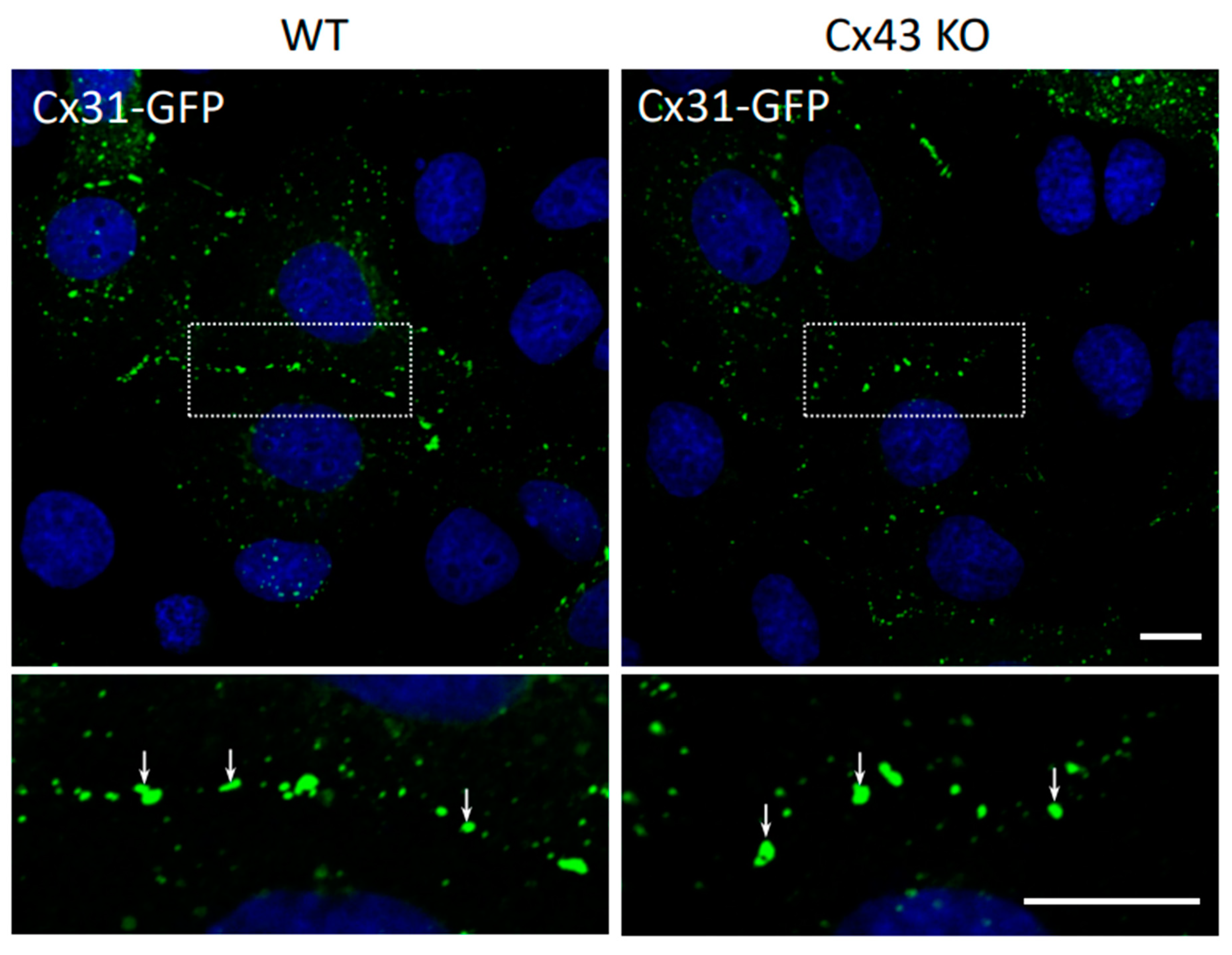
3.3. Cx31 Trafficking and Gap Junction Assembly Occurs Independent of the Cx43 Status
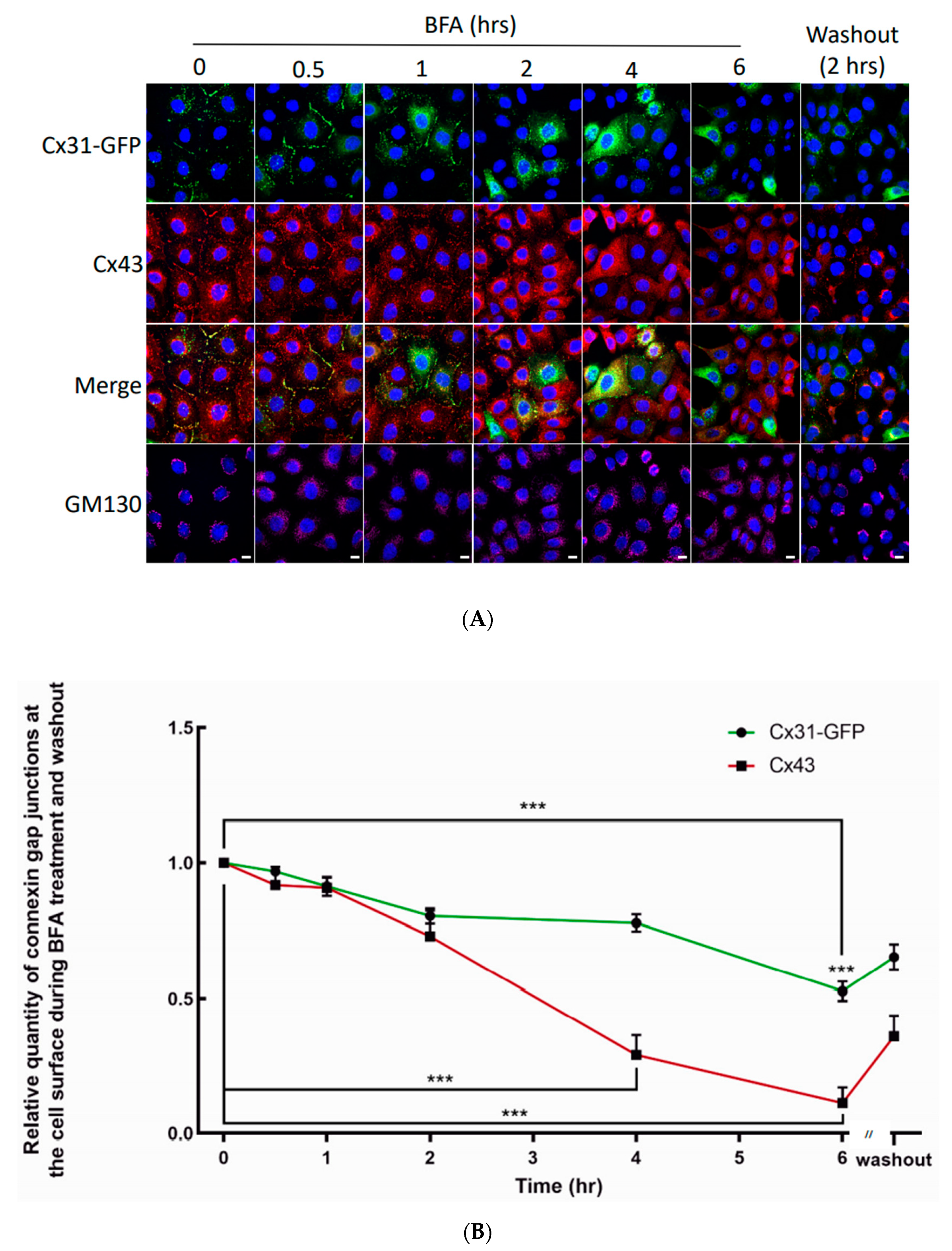
3.4. Cx31 Gap Junctions Are Dynamic and Persist Longer at the Cell Surface Compared to Cx43
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Paul, D.L. Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003, 4, 285–294. [Google Scholar] [CrossRef]
- Harris, A.L. Voltage-sensing and substate rectification: Moving parts of connexin channels. J. Gen. Physiol. 2002, 119, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Di, W.L.; Rugg, E.L.; Leigh, I.M.; Kelsell, D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Investig. Dermatol. 2001, 117, 958–964. [Google Scholar] [CrossRef] [Green Version]
- Di, W.L.; Gu, Y.; Common, J.E.; Aasen, T.; O’Toole, E.A.; Kelsell, D.P.; Zicha, D. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J. Cell Sci. 2005, 118, 1505–1514. [Google Scholar] [CrossRef] [Green Version]
- Risek, B.; Klier, F.G.; Gilula, N.B. Multiple gap junction genes are utilized during rat skin and hair development. Development 1992, 116, 639–651. [Google Scholar]
- Churko, J.M.; Laird, D.W. Gap junction remodeling in skin repair following wounding and disease. Physiology 2013, 28, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Chanson, M.; Watanabe, M.; O’Shaughnessy, E.M.; Zoso, A.; Martin, P.E. Connexin Communication Compartments and Wound Repair in Epithelial Tissue. Int. J. Mol. Sci. 2018, 19, 1354. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, G.T.; Burt, J.M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta 2005, 1711, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Falk, M.M. Cell-free synthesis for analyzing the membrane integration, oligomerization, and assembly characteristics of gap junction connexins. Methods 2000, 20, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koval, M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006, 16, 159–166. [Google Scholar] [CrossRef]
- Yum, S.W.; Zhang, J.; Scherer, S.S. Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30. Neurobiol. Dis. 2010, 38, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W.; Castillo, M.; Kasprzak, L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J. Cell Biol. 1995, 131, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W.; Puranam, K.L.; Revel, J.P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 1991, 273, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.J.; Shao, Q.; Jagger, D.J.; Laird, D.W. Cx30 exhibits unique characteristics including a long half-life when assembled into gap junctions. J. Cell Sci. 2015, 128, 3947–3960. [Google Scholar] [CrossRef] [Green Version]
- Hoh, J.H.; John, S.A.; Revel, J.P. Molecular cloning and characterization of a new member of the gap junction gene family, connexin-31. J. Biol. Chem. 1991, 266, 6524–6531. [Google Scholar]
- Plum, A.; Winterhager, E.; Pesch, J.; Lautermann, J.; Hallas, G.; Rosentreter, B.; Traub, O.; Herberhold, C.; Willecke, K. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev. Biol. 2001, 231, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Maass, K.; Ghanem, A.; Kim, J.S.; Saathoff, M.; Urschel, S.; Kirfel, G.; Grummer, R.; Kretz, M.; Lewalter, T.; Tiemann, K.; et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol. Biol. Cell 2004, 15, 4597–4608. [Google Scholar] [CrossRef] [Green Version]
- Langlois, S.; Maher, A.C.; Manias, J.L.; Shao, Q.; Kidder, G.M.; Laird, D.W. Connexin levels regulate keratinocyte differentiation in the epidermis. J. Biol. Chem. 2007, 282, 30171–30180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocozzelli, A.G.; White, T.W. Connexin 43 Mutations Lead to Increased Hemichannel Functionality in Skin Disease. Int. J. Mol. Sci. 2019, 20, 6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, M.; Jannace, T.F.; Cocozzelli, A.G.; Li, L.; Slavi, N.; Sellitto, C.; White, T.W. Connexin43 mutations linked to skin disease have augmented hemichannel activity. Sci. Rep. 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Baden, H.P.; Kubilus, J. The growth and differentiation of cultured newborn rat keratinocytes. J. Invest. Dermatol. 1983, 80, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Maher, A.C.; Thomas, T.; Riley, J.L.; Veitch, G.; Shao, Q.; Laird, D.W. Rat epidermal keratinocytes as an organotypic model for examining the role of Cx43 and Cx26 in skin differentiation. Cell Commun. Adhes. 2005, 12, 219–230. [Google Scholar] [CrossRef]
- Berger, A.C.; Kelly, J.J.; Lajoie, P.; Shao, Q.; Laird, D.W. Mutations in Cx30 that are linked to skin disease and non-syndromic hearing loss exhibit several distinct cellular pathologies. J. Cell Sci. 2014, 127, 1751–1764. [Google Scholar] [CrossRef] [Green Version]
- Simek, J.; Churko, J.; Shao, Q.; Laird, D.W. Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J. Cell Sci. 2009, 122, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; Jordan, K.; Simek, J.; Shao, Q.; Jedeszko, C.; Walton, P.; Laird, D.W. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005, 118, 4451–4462. [Google Scholar] [CrossRef] [Green Version]
- Churko, J.M.; Kelly, J.J.; Macdonald, A.; Lee, J.; Sampson, J.; Bai, D.; Laird, D.W. The G60S Cx43 mutant enhances keratinocyte proliferation and differentiation. Exp. Derm. 2012, 21, 612–618. [Google Scholar] [CrossRef]
- Dagnino, L.; Ho, E.; Chang, W.Y. Expression and analysis of exogenous proteins in epidermal cells. Methods Mol. Biol. 2010, 585, 93–105. [Google Scholar]
- Press, E.; Alaga, K.C.; Barr, K.; Shao, Q.; Bosen, F.; Willecke, K.; Laird, D.W. Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice. Cell Death Dis. 2017, 8, e2845. [Google Scholar] [CrossRef]
- Jordan, K.; Solan, J.L.; Dominguez, M.; Sia, M.; Hand, A.; Lampe, P.D.; Laird, D.W. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol. Biol. Cell 1999, 10, 2033–2050. [Google Scholar] [CrossRef]
- Thomas, T.; Telford, D.; Laird, D.W. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J. Biol. Chem. 2004, 279, 19157–19168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abitbol, J.; Beach, R.; Barr, K.; Esseltine, J.; Allman, B.; Laird, D. Cisplatin-induced ototoxicity in organotypic cochlear cultures occurs independent of gap junctional intercellular communication. Cell Death Dis. 2020, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Esseltine, J.L.; Brooks, C.R.; Edwards, N.A.; Subasri, M.; Sampson, J.; Seguin, C.; Betts, D.H.; Laird, D.W. Dynamic regulation of connexins in stem cell pluripotency. Stem Cells 2020, 38, 52–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlois, S.; Churko, J.M.; Laird, D.W. Optical and biochemical dissection of connexin and disease-linked connexin mutants in 3D organotypic epidermis. Methods Mol. Biol. 2010, 585, 313–334. [Google Scholar]
- Butterweck, A.; Elfgang, C.; Willecke, K.; Traub, O. Differential expression of the gap junction proteins connexin45, -43, -40, -31, and -26 in mouse skin. Eur. J. Cell Biol. 1994, 65, 152–163. [Google Scholar]
- Reaume, A.G.; de Sousa, P.A.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac malformation in neonatal mice lacking connexin43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef]
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta 2018, 1860, 48–64. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Anjo, S.I.; Pereira, P.; Manadas, B.; Girao, H. Interacting Network of the Gap Junction (GJ) Protein Connexin43 (Cx43) is Modulated by Ischemia and Reperfusion in the Heart. Mol. Cell Proteom. 2015, 14, 3040–3055. [Google Scholar] [CrossRef] [Green Version]
- Paznekas, W.A.; Karczeski, B.; Vermeer, S.; Lowry, R.B.; Delatycki, M.; Laurence, F.; Koivisto, P.A.; Van Maldergem, L.; Boyadjiev, S.A.; Bodurtha, J.N.; et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 2009, 30, 724–733. [Google Scholar] [CrossRef]
- Delmar, M.; Laird, D.W.; Naus, C.C.; Nielsen, M.S.; Verselis, V.K.; White, T.W. Connexins and Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029348. [Google Scholar] [CrossRef]
- Laird, D.W. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014, 588, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J. Biol. Chem. 2008, 283, 2997–3001. [Google Scholar] [CrossRef] [Green Version]
- Esseltine, J.L.; Laird, D.W. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016, 26, 944–955. [Google Scholar] [CrossRef]
- Kelly, J.J.; Simek, J.; Laird, D.W. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 2015, 360, 701–721. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Chen, P.; Zeng, K.; Xue, R.; Tian, X.; Liang, L.; Wang, Q.; Shi, M.; Zhang, X. Two de novo GJA1 mutation in two sporadic patients with erythrokeratodermia variabilis et progressiva. Mol. Genet. Genomic Med. 2019, 7, e670. [Google Scholar] [CrossRef] [Green Version]
- Ishida-Yamamoto, A. Erythrokeratodermia variabilis et progressiva. J. Dermatol. 2016, 43, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Boyden, L.M.; Craiglow, B.G.; Zhou, J.; Hu, R.; Loring, E.C.; Morel, K.D.; Lauren, C.T.; Lifton, R.P.; Bilguvar, K.; Paller, A.S.; et al. Dominant De Novo Mutations in GJA1 Cause Erythrokeratodermia Variabilis Et Progressiva, without Features of Oculodentodigital Dysplasia. J. Investig. Dermatol. 2015, 135, 1540–1547. [Google Scholar] [CrossRef] [Green Version]
- Winterhager, E.; Von Ostau, C.; Gerke, M.; Gruemmer, R.; Traub, O.; Kaufmann, P. Connexin expression patterns in human trophoblast cells during placental development. Placenta 1999, 20, 627–638. [Google Scholar] [CrossRef]
- Kibschull, M.; Nassiry, M.; Dunk, C.; Gellhaus, A.; Quinn, J.A.; Rossant, J.; Lye, S.J.; Winterhager, E. Connexin31-deficient trophoblast stem cells: A model to analyze the role of gap junction communication in mouse placental development. Dev. Biol. 2004, 273, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kibschull, M.; Gellhaus, A.; Winterhager, E. Analogous and unique functions of connexins in mouse and human placental development. Placenta 2008, 29, 848–854. [Google Scholar] [CrossRef]
- Kibschull, M.; Magin, T.M.; Traub, O.; Winterhager, E. Cx31 and Cx43 double-deficient mice reveal independent functions in murine placental and skin development. Dev. Dyn 2005, 233, 853–863. [Google Scholar] [CrossRef]
- He, L.Q.; Cai, F.; Liu, Y.; Liu, M.J.; Tan, Z.P.; Pan, Q.; Fang, F.Y.; Liang, D.S.; Wu, L.Q.; Long, Z.G.; et al. Cx31 is assembled and trafficked to cell surface by ER-Golgi pathway and degraded by proteasomal or lysosomal pathways. Cell Res. 2005, 15, 455–464. [Google Scholar] [CrossRef]
- Laird, D.W.; Jordan, K.; Thomas, T.; Qin, H.; Fistouris, P.; Shao, Q. Comparative analysis and application of fluorescent protein-tagged connexins. Microsc. Res. Tech. 2001, 52, 263–272. [Google Scholar] [CrossRef]
- Falk, M.M. Connexin-specific distribution within gap junctions revealed in living cells. J. Cell Sci. 2000, 113, 4109–4120. [Google Scholar]
- Stout, R.F., Jr.; Snapp, E.L.; Spray, D.C. Connexin Type and Fluorescent Protein Fusion Tag Determine Structural Stability of Gap Junction Plaques. J. Biol. Chem. 2015, 290, 23497–23514. [Google Scholar] [CrossRef] [Green Version]
- Hoh, J.H.; Lal, R.; John, S.A.; Revel, J.P.; Arnsdorf, M.F. Atomic force microscopy and dissection of gap junctions. Science 1991, 253, 1405–1408. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Au, A.; Shao, Q.; White, K.K.; Lucaciu, S.A.; Esseltine, J.L.; Barr, K.; Laird, D.W. Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes. Biomolecules 2020, 10, 1443. https://doi.org/10.3390/biom10101443
Au A, Shao Q, White KK, Lucaciu SA, Esseltine JL, Barr K, Laird DW. Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes. Biomolecules. 2020; 10(10):1443. https://doi.org/10.3390/biom10101443
Chicago/Turabian StyleAu, Akina, Qing Shao, Kyra K. White, Sergiu A. Lucaciu, Jessica L. Esseltine, Kevin Barr, and Dale W. Laird. 2020. "Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes" Biomolecules 10, no. 10: 1443. https://doi.org/10.3390/biom10101443
APA StyleAu, A., Shao, Q., White, K. K., Lucaciu, S. A., Esseltine, J. L., Barr, K., & Laird, D. W. (2020). Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes. Biomolecules, 10(10), 1443. https://doi.org/10.3390/biom10101443




