Photodynamic Activation of Cholecystokinin 1 Receptor with Different Genetically Encoded Protein Photosensitizers and from Varied Subcellular Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture (AR4-2J, Escherichia coli)
2.3. Vector Constructs
2.4. Transduction of AR4-2J Cells
2.5. RT-PCR to Detect DsFbFP mRNA Expression
2.6. Photodynamic Action
2.7. Calcium Measurements
2.8. Data Presentation and Statistical Analysis
3. Results
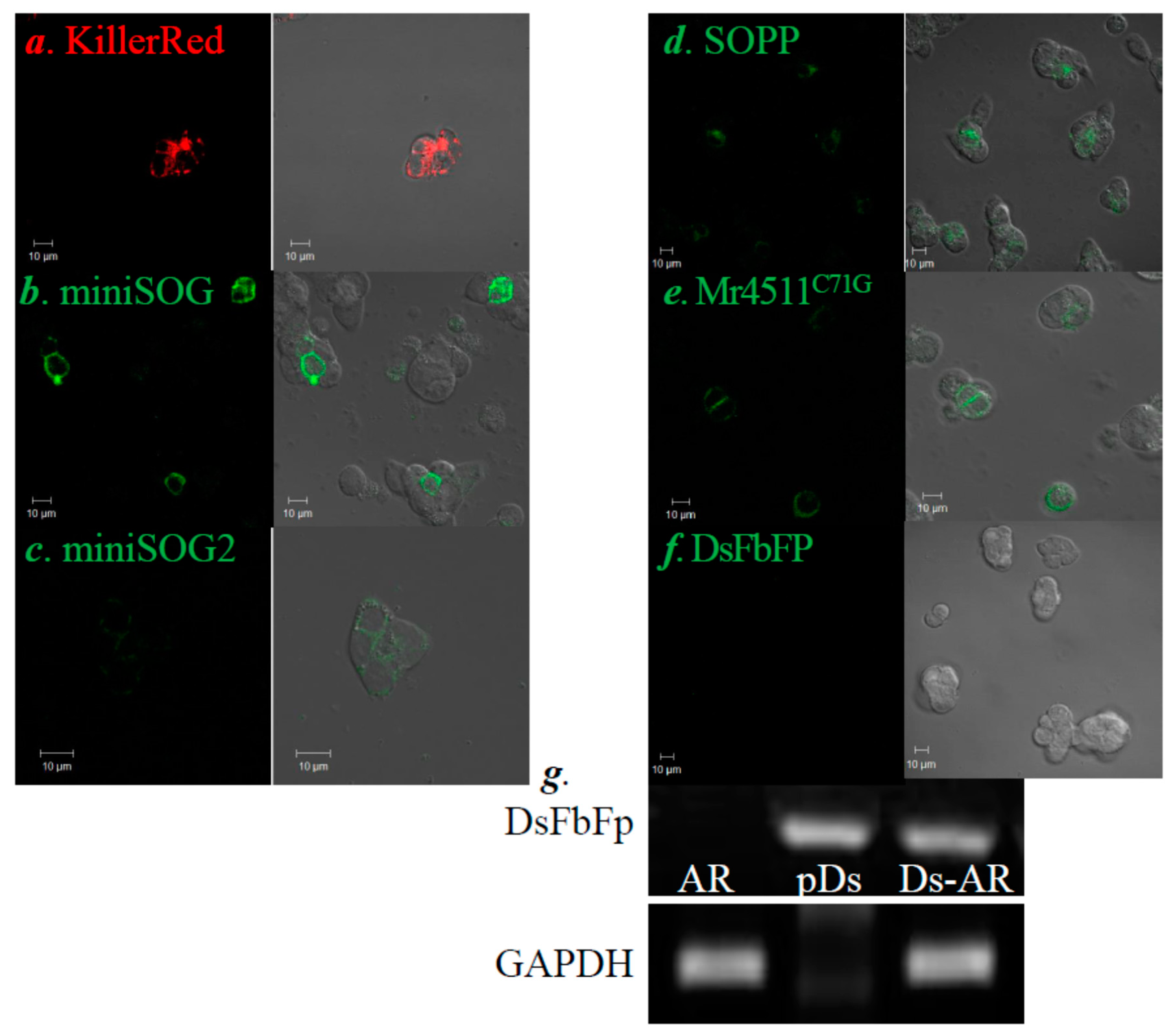
3.1. PM-Delimited Photodynamic CCK1R Activation in AR4-2J Cells with GEPP KillerRed, miniSOG, miniSOG2, SOPP, Mr4511, and DsFbFP
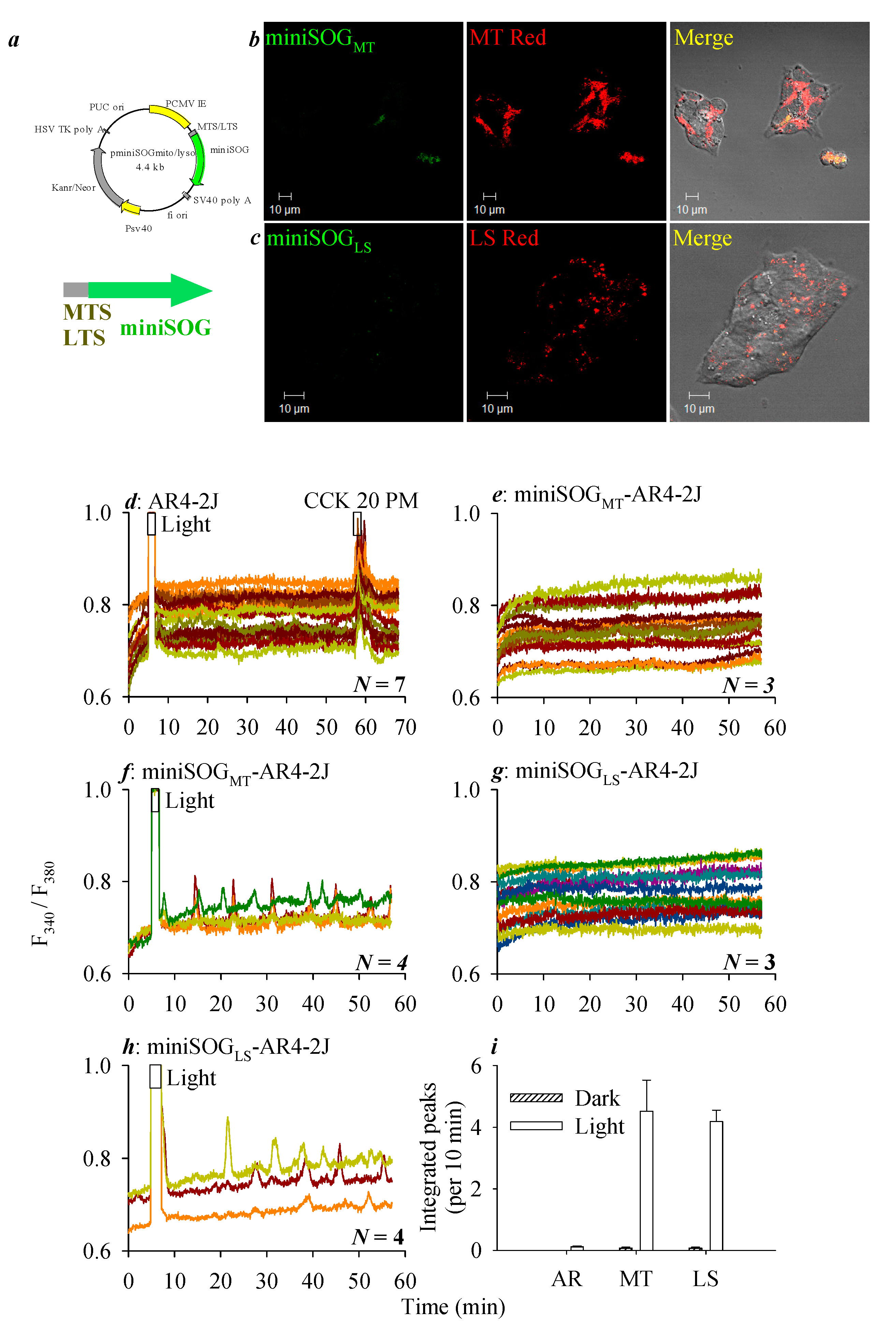
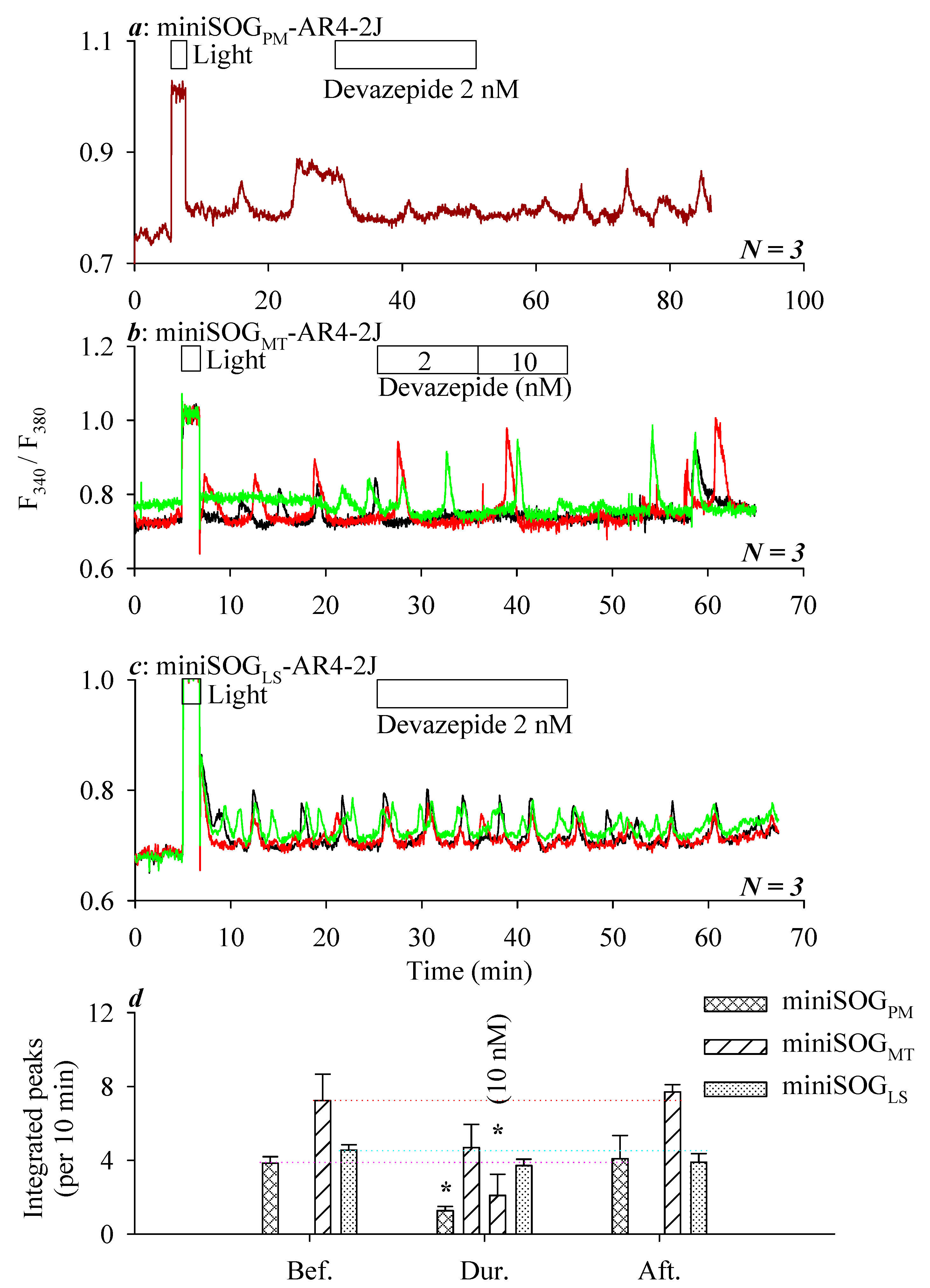
3.2. miniSOG Photodynamic Action in AR4-2J Cells at PM, MT, or LS with Graded Sensivitivity towards Extracellular CCK1R Antagonist
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Photosensitizer | No. AA | Chromophore | λex (nm) | λem (nm) | ϕ1O2 | ϕfluo |
|---|---|---|---|---|---|---|
| KillerRed | 239 | QYG | 585 | 610 | 0.008 | 0.25 |
| SuperNova GreenSuperNova | 239 239 | QYG QWG | 579 440 | 610 510 | 0.022 ND | 0.30 0.23 |
| KillerOrange | 239 | QWG | 512 | 550 | ND | 0.42 |
| TagRFP | 237 | MYG | 555 | 584 | 0.004 | 0.48 |
| miniSOG | 106 | FMN | 448 | 500 | 0.03 | 0.45 |
| AsLOV2 | 175 | FMN | 448 | 492 | ND | ND |
| miniSOG2 | 106 | FMN | 430 | 503 | ND | ND |
| Mr4511C71S/G Pp2FbFPL30M DsFbFP EcFbFP CreiLOV | 164 148 138 135 119 | FMN FMN FMN FMN FMN | 450 449 449 448 449 | 495 495 498 496 497 | 0.17/0.19 0.09 0.33 0.07 0.04 | ND 0.25 0.35 0.44 0.32 |
| miniSOGQ103L/V | 106 | FMN | 440 | 487 | 0.25/0.39 | 0.43 |
| SOPP2/3 phiSOG phiSOGQ103V | 106 218 218 | FMN FMN FMN | 440 449 444 | 487 498 497 | 0.51/0.61 0.02 0.15 | 0.41/0.39 0.36 0.35 |
| Plasmid Name | Backbone | Insert/Replacement |
|---|---|---|
| pKillerRedPM | pKillerRedPM | KillerRed |
| MLCCMRRTKQVEKNDEDQKIMGSEGGPALFQSDMTFKIFIDGEVNGQKFTIVADGSSKFPHGDFNVHAVCETGKLPMSWKPICHLIQYGEPFFARYPDGISHFAQECFPEGLSIDRTVRFENDGTMTSHHTYELDDTCVVSRITVNCDGFQPDGPIMRDQLVDILPNETHMFPHGPNAVRQLAFIGFTTADGGLMMGHFDSKMTFNGSRAIEIPGPHFVTIITKQMRDTSDKRDHVCQREVAYAHSVPRITSAIGSDED | ||
| pminiSOGPM | pKillerRedPM | miniSOG |
| MLCCMRRTKQVEKNDEDQKIMEKSFVITDPRLPDNPIIFASDGFLELTEYSREEILGRNGRFLQGPETDQATVQKIRDAIRDQREITVQLINYTKSGKKFWNLLHLQPMRDQKGELQYFIGVQLDG | ||
| pminiSOGMT | pminiSOGMT | MT localization sequence |
| MSVLTPLLLRGLTGSARRLPVPRAKIHSLGDPPVATMEKSFVITDPRLPDNPIIFASDGFLELTEYSREEILGRNGRFLQGPETDQATVQKIRDAIRDQREITVQLINYTKSGKKFWNLLHLQPMRDQKGELQYFIGVQLDG | ||
| pminiSOGLS | pminiSOGLS | LS localization sequence |
| KGQGSMDEGTADERAPLIRTMEKSFVITDPRLPDNPIIFASDGFLELTEYSREEILGRNGRFLQGPETDQATVQKIRDAIRDQREITVQLINYTKSGKKFWNLLHLQPMRDQKGELQYFIGVQLDG | ||
| pminiSOG2PM | pminiSOGPM | miniSOG2 |
| MLCCMRRTKQVEKNDEDQKIMEKSFVITDPRLPDNPIIFASDSFLELTEYSREEILGRNPRFLRGPETDQATVQKIHDAIRDQREITVQLINYTKSGKKFWNLFRLQPIRDQKGELQYFIGVQLDG | ||
| pSOPPPM | pminiSOGPM | SOPP |
| MLCCMRRTKQVEKNDEDQKIMEKSFVITDPRLPDNPIIFASDGFLELTEYSREEILGRNGRFLQGPETDQATVQKIRDAIRDQREITVQLINYTKSGKKFWNLLHLQPMRDQKGELQYFIGVLLDG | ||
| pMr4511C71GPM | pminiSOGPM | Mr4511C71G |
| MLCCMRRTKQVEKNDEDQKIMETGGTATSHVPDELKAESHRGDPFAAAVRATRMPMIITDPAQHDNPIVFVNDAFLKLTGYTRMEVVGRNGRFLQGPDTEAAAVDRLRAAIRREEDIRVDLLNYRKDGSTFQNALYVGPVRDEAGRVVYFFASQLDVSEHYALTAEIERLKAALAEAEAKLAAR | ||
| pDsFbFPPM | pminiSOGPM | DsFbFP |
| MLCCMRRTKQVEKNDEDQKIMRRHYRDLIRNTPMPDTPQDIADLRALLDEDEAEMSVVFSDPSQPDNPMIYVSDAFLVQTGYTLEEVLGRNARFLQGPDTNPHAVEAIRQGLKAETRFTIDILNYRKDGSAFVNRLRIRPIYDPEGNLMFFAGAQNPVLE | ||
References
- Nishimura, S.; Bilgüvar, K.; Ishigame, K.; Sestan, N.; Günel, M.; Louvi, A. Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development. PLoS ONE 2015, 10, e0124295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, S.; Ozaki, T.; Takeuchi, K.; Unno, K.; Yamoto, K.; Morioka, E.; Takiguchi, S.; Ikeda, M. Functional compensation between cholecystokinin-1 and -2 receptors in murine paraventricular nucleus neurons. J. Biol. Chem. 2012, 287, 39391–39401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, T.; Mohammad, S.; Morioka, E.; Takiguchi, S.; Ikeda, M. Infant satiety depends on transient expression of cholecystokinin-1 receptors on ependymal cells lining the third ventricle in mice. J. Physiol. 2013, 591, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Wada, E.; Battey, J.F.; Wank, S.A. Differential gene-expression of CCKA and CCKB receptors in the rat brain. Mol. Cell Neurosci. 1993, 4, 143–154. [Google Scholar] [CrossRef]
- Hill, D.R.; Shaw, T.M.; Graham, W.; Woodruff, G.N. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J. Neurosci. 1990, 10, 1070–1081. [Google Scholar] [CrossRef] [Green Version]
- Morton, M.F.; Pyati, J.; Dai, H.; Li, L.; Moreno, V.; Shankley, N.P. Molecular cloning, expression and pharmacological characterization of the canine cholecystokinin 1 receptor. Br. J. Pharmacol. 2005, 145, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Mercer, L.D.; Beart, P.M. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokininA receptor. Neurosci. Lett. 1997, 225, 97–100. [Google Scholar] [CrossRef]
- Mercer, L.D.; Beart, P.M. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci. Lett. 2004, 359, 109–113. [Google Scholar] [CrossRef]
- Yoshimichi, G.; Lo, C.C.; Tamashiro, K.L.; Ma, L.; Lee, D.M.; Begg, D.P.; Liu, M.; Sakai, R.R.; Woods, S.C.; Yoshimatsu, H.; et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Broberger, C.; Holmberg, K.; Shi, T.J.; Dockray, G.; Hökfelt, T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001, 903, 128–140. [Google Scholar] [CrossRef]
- Bucinskaite, V.; Kurosawa, M.; Lundeberg, T. Exogenous cholecystokinin-8 reduces vagal efferent nerve activity in rats through CCKA receptors. Br. J. Pharm. 2000, 129, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- Glatzle, J.; Wang, Y.; Adelson, D.W.; Kalogeris, T.J.; Zittel, T.T.; Tso, P.; Wei, J.Y.; Raybould, H.E. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J. Physiol. 2003, 550, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Zhou, S.; Owyang, C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: Implications for leptin as a regulator of short-term satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G217–G227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussa, B.M.; Sartor, D.M.; Verberne, A.J. Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. Eur J. Pharm. 2008, 601, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.M.; Zheng, H.; Ward, S.M.; Berthoud, H.R. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001, 305, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.M.; Zheng, H.; Berthoud, H.R. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat. Rec. 2002, 266, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Akiyoshi, J.; Kiyota, A.; Katsuragi, S.; Tsutsumi, T.; Isogawa, K.; Nagayama, H. Increased anxiety behavior in OLETF rats without cholecystokinin-A receptor. Brain Res. Bull. 2000, 53, 789–792. [Google Scholar] [CrossRef]
- Wen, D.; Sun, D.; Zang, G.; Hao, L.; Liu, X.; Yu, F.; Ma, C.; Cong, B. Cholecystokinin octapeptide induces endogenous opioid-dependent anxiolytic effects in morphine-withdrawal rats. Neuroscience 2014, 277, 14–25. [Google Scholar] [CrossRef]
- Zhu, G.; Yan, J.; Smith, W.W.; Moran, T.H.; Bi, S. Roles of dorsomedial hypothalamic cholecystokinin signaling in the controls of meal patterns and glucose homeostasis. Physiol. Behav. 2012, 105, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Vermeulen, R.; Hokfelt, T.; Horne, M.K.; Stanic, D. Female mice lacking cholecystokinin 1 receptors have compromised neurogenesis, and fewer dopaminergic cells in the olfactory bulb. Front. Cell Neurosci. 2013, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Akiyoshi, J.; Kai, K.; Ishii, N.; Kodama, K.; Tsutsumi, T.; Isogawa, K.; Nagayama, H. Spatial memory impairment in OLETF rats without cholecystokinin-A receptor. Neuropeptides 2003, 37, 271–276. [Google Scholar] [CrossRef]
- Lo, C.C.; Davidson, W.S.; Hibbard, S.K.; Georgievsky, M.; Lee, A.; Tso, P.; Woods, S.C. Intraperitoneal CCK and fourth-intraventricular Apo AIV require both peripheral and NTS CCK1R to reduce food intake in male rats. Endocrinology 2014, 155, 1700–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, I.C.; Meddle, S.L.; Wilson, P.W.; Wardle, C.A.; Law, A.S.; Bishop, V.R.; Hindar, C.; Robertson, G.W.; Burt, D.W.; Ellison, S.J.; et al. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E909–E921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Takiguchi, S.; Sato, N.; Kanai, S.; Kawanami, T.; Yoshida, Y.; Miyasaka, K.; Takata, Y.; Funakoshi, A.; Noda, T. Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: A study in CCK-A receptor knockout mice. JPN J. Physiol. 2001, 51, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takiguchi, S.; Suzuki, S.; Sato, Y.; Kanai, S.; Miyasaka, K.; Jimi, A.; Shinozaki, H.; Takata, Y.; Funakoshi, A.; Kono, A.; et al. Role of CCK-A receptor for pancreatic function in mice: A study in CCK-A receptor knockout mice. Pancreas 2002, 24, 276–283. [Google Scholar] [CrossRef]
- Schmidt, W.E.; Schenk, S.; Nustede, R.; Holst, J.J.; Fölsch, U.R.; Creutzfeldt, W. Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology 1994, 107, 1610–1620. [Google Scholar] [CrossRef]
- Whited, K.L.; Thao, D.; Lloyd, K.C.; Kopin, A.S.; Raybould, H.E. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G156–G162. [Google Scholar] [CrossRef] [Green Version]
- Varga, G.; Bálint, A.; Burghardt, B.; D'Amato, M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br. J. Pharm. 2004, 141, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.J.; Kanno, T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J. Physiol. 1997, 504, 47–55. [Google Scholar] [CrossRef] [Green Version]
- An, Y.P.; Xiao, R.; Cui, H.; Cui, Z.J. Selective activation by photodynamic action of cholecystokinin receptor in the freshly isolated rat pancreatic acini. Br. J. Pharm. 2003, 139, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Cholecystokinin 1 receptor-a unique G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Front. Physiol. 2018, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cui, Z.J. NanoLuc bioluminescence-driven photodynamic activation of cholecystokinin 1 receptor with genetically encoded protein photosensitizer miniSOG. Int. J. Mol. Sci. 2020, 21, 3763. [Google Scholar] [CrossRef] [PubMed]
- Bulina, M.E.; Lukyanov, K.A.; Britanova, O.V.; Onichtchouk, D.; Lukyanov, S.; Chudakov, D.M. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat. Protoc. 2006, 1, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, N.V.; Lukyanov, K.A.; Chudakov, D.M.; Martynov, V.I.; Popov, V.O.; Kovalchuk, M.V.; Wlodawer, A.; Dauter, Z.; Pletnev, V. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J. Biol. Chem. 2009, 284, 32028–32039. [Google Scholar] [CrossRef] [Green Version]
- Vegh, R.B.; Solntsev, K.M.; Kuimova, M.K.; Cho, S.; Liang, Y.; Loo BLTolbert, L.M.; Bommarius, A.S. Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chem. Commun. 2011, 47, 4887–4889. [Google Scholar] [CrossRef]
- Petrova, N.V.; Luzhin, A.V.; Serebrovskaya, E.O.; Ryumina, A.P.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Inducing cellular senescence in vitro by using genetically encoded photosensitizers. Aging Us 2016, 8, 2449. [Google Scholar] [CrossRef] [Green Version]
- Onukwufor, J.O.; Trewin, A.J.; Baran, T.M.; Almast, A.; Foster, T.H.; Wojtovich, A.P. Quantification of reactive oxygen species production by the red fluorescent proteins KillerRed, SuperNova and mCherry. Free Radic. Biol. Med. 2019, 147, 1–7. [Google Scholar] [CrossRef]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011, 9, e1001041. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pulido, A.; Cortajarena, A.L.; Torra, J.; Ruiz-González, R.; Nonell, S.; Flors, C. Assessing the potential of photosensitizing flavoproteins as tags for correlative microscopy. Chem. Commun. (CAMB) 2016, 52, 8405–8408. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Sann, S.B.; Zhou, K.; Nabavi, S.; Proulx, C.D.; Malinow, R.; Jin, Y.; Tsien, R.Y. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI). Neuron 2013, 79, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Stawicki, T.M.; Goncharov, A.; Jin, Y. Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. Elife 2013, 2, e01180. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Su, Z.; Liu, Q.; Zhou, L. State-dependent and site-directed photodynamic transformation of HCN2 channel by singlet oxygen. J. Gen. Physiol. 2014, 143, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.N.; Li, Y.; Cui, Z.J. Photodynamic physiology—Photonanomanipulations in cellular physiology with protein photosensitisers. Front. Physiol. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, K.; Matsuda, T.; Sakai, N.; Fu, D.; Noda, M.; Uchiyama, S.; Kotera, I.; Arai, Y.; Horiuchi, M.; Fukui, K. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013, 3, 2629. [Google Scholar] [CrossRef] [Green Version]
- Riani, Y.D.; Matsuda, T.; Takemoto, K.; Nagai, T. Green monomeric photosensitizing fluorescent protein for photo-inducible protein inactivation and cell ablation. BMC Biol. 2018, 16, 50. [Google Scholar] [CrossRef] [Green Version]
- Pletneva, N.V.; Pletnev, V.Z.; Sarkisyan, K.S.; Gorbachev, D.A.; Egorov, E.S.; Mishin, A.S.; Lukyanov, K.A.; Dauter, Z.; Pletnev, S. Crystal structure of phototoxic orange fluorescent proteins with a tryptophan-based chromophore. PLoS ONE 2015, 10, e0145740. [Google Scholar] [CrossRef]
- Sarkisyan, K.S.; Zlobovskaya, O.A.; Gorbachev, D.A.; Bozhanova, N.G.; Sharonov, G.V.; Staroverov, D.B.; Egorov, E.S.; Ryabova, A.V.; Solntsev, K.M.; Mishin, A.S.; et al. KillerOrange, a genetically encoded photosensitizer activated by blue and green light. PLoS ONE 2015, 10, e0145287. [Google Scholar] [CrossRef]
- Makhijani, K.; To, T.L.; Ruiz-González, R.; Lafaye, C.; Royant, A.; Shu, X. Precision optogenetic tool for selective single- and multiple-cell ablation in a live animal model system. Cell Chem. Biol. 2017, 24, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-González, R.; Cortajarena, A.L.; Mejias, S.H.; Agut, M.; Nonell, S.; Flors, C. Singlet oxygen generation by the genetically encoded tag miniSOG. J. Am. Chem. Soc. 2013, 135, 9564–9567. [Google Scholar] [CrossRef]
- Westberg, M.; Holmegaard, L.; Pimenta, F.M.; Etzerodt, M.; Ogilby, P.R. Rational design of an efficient, genetically encodable, protein encased singlet oxygen photosensitizer. J. Am. Chem. Soc. 2015, 137, 1632–1642. [Google Scholar] [CrossRef]
- Westberg, M.; Bregnhøj, M.; Etzerodt, M.; Ogilby, P.R. No photon wasted: An efficient and selective singlet oxygen photosensitizing protein. J. Phys. Chem. B 2017, 121, 9366–9371. [Google Scholar] [CrossRef] [PubMed]
- Westberg, M.; Etzerodt, M.; Ogilby, P.R. Rational design of genetically encoded singlet oxygen photosensitizing proteins. Curr. Opin. Struct. Biol. 2019, 57, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pulido, A.; Torra, J.; Mejias, S.H.; Cortajarena, A.L.; Ruiz-Gonzalez, R.; Nonell, S.; Flors, C. Fluorescent flavoprotein heterodimers: Combining photostability with singlet oxygen generation. ChemPhotoChem 2018, 2, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-González, R.; White, J.H.; Agut, M.; Nonell, S.; Flors, C. A genetically-encoded photosensitiser demonstrates killing of bacteria by purely endogenous singlet oxygen. Photochem Photobiol Science 2012, 11, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Burgos-Caminal, A.; Endres, S.; Wingen, M.; Drepper, T.; Gensch, T.; Ruiz-González, R.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent protein Pp2FbFP L30M, a novel derivative of Pseudomonas putida flavin-binding Pp2FbFP. Photochem. Photobiol. Sci. 2015, 14, 280–287. [Google Scholar] [CrossRef]
- Endres, S.; Wingen, M.; Torra, J.; Ruiz-Gonzalez, R.; Polen, T.; Bosio, G.; Bitzenhofer, N.L.; Hilgers, F.; Gensch, T.; Nonell, S.; et al. An optogenetic toolbox of LOV-based photosensitizers for light-driven killing of bacteria. Sci. Rep. 2018, 8, 15021. [Google Scholar] [CrossRef] [Green Version]
- Consiglieri, E.; Xu, Q.Z.; Bregnhoj, M.; Westberg, M.; Ogilby, P.R.; Losi, A. Single mutation in a novel bacterial LOV protein yields a singlet oxygen generator. Photochem. Photobiol. Sci. 2019, 18, 2657–2660. [Google Scholar] [CrossRef]
- Lu, H.X.; Mazumder, M.; Jaikaran, A.S.I.; Kumar, A.; Leis, E.K.; Xu, X.L.; Altmann, M.; Cochrane, A.; Woolley, G.A. A yeast system for discovering optogenetic inhibitors of eukaryotic translation initiation. ACS Synth. Biol. 2019, 8, 744–757. [Google Scholar] [CrossRef]
- Petrencakova, M.; Filandr, F.; Hovan, A.; Yassaghi, G.; Man, P.; Kozar, T.; Schwer, M.-S.; Jancura, D.; Pluckthun, A.; Novak, P.; et al. Photoinduced damage of AsLOV2 domain is accompanied by increased singlet oxygen production due to flavin dissociation. Sci. Rep. 2020, 10, 4119. [Google Scholar] [CrossRef] [Green Version]
- Stäubert, C.; Schöneberg, T. GPCR signaling from intracellular membranes—A novel concept. Bioessays 2017, 39, 1700200. [Google Scholar] [CrossRef]
- Hill, S.J.; Watson, S.P. Fluorescence approaches unravel spatial and temporal aspects of GPCR organisation, location, and intracellular signalling. Trends Pharm. Sci. 2018, 39, 91–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.J.; Liang, H.Y.; Jin, W.J.; Cui, Z.J. Substance P conjugated to CdTe quantum dots triggers cytosolic calcium oscillations and induces quantum dots internalization in the pancreatic carcinoma cell line AR4-2J. Anal. Bioanal. Chem. 2011, 400, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Cui, Z.J. Extracellular histones activate plasma membrane Toll-like receptor 9 to trigger calcium oscillations in rat pancreatic acinar tumor cell AR4-2J. Cells 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.S.; Cui, Z.J. Pancreatic stellate cells serve as a brake mechanism on pancreatic acinar cell calcium signaling modulated by methionine sulfoxide reductase expression. Cells 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Wingen, M.; Potzkei, J.; Endres, S.; Casini, G.; Rupprecht, C.; Fahlke, C.; Krauss, U.; Jaeger, K.E.; Drepper, T.; Gensch, T. The photophysics of LOV-based fluorescent proteins--new tools for cell biology. Photochem. Photobiol. Sci. 2014, 13, 875–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.Z.; Cui, Z.J. Permanent photodynamic activation of the cholecystokinin 2 receptor. Biomolecules 2020, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, T.; Tsujimoto, Y. Deleterious effects of mitochondrial ROS generated by KillerRed photodynamic action in human cell lines and C. elegans. J. Photochem. Photobiol. B Biol. 2012, 117, 1–12. [Google Scholar] [CrossRef]
- Yang, J.Y.; Yang, W.Y. Spatiotemporally controlled initiation of Parkin-mediated mitophagy within single cells. Autophagy 2011, 7, 1230–1238. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, N.; Akasaki, K.; Tsuji, H. Two acidic amino acid residues, Asp(470) and Glu(471), contained in the carboxyl cytoplasmic tail of a major lysosomal membrane protein, LGP85/LIMP II, are important for its accumulation in secondary lysosomes. Biochem. Biophys. Res. Commun. 2000, 270, 557–563. [Google Scholar] [CrossRef]
- Drepper, T.; Eggert, T.; Circolone, F.; Heck, A.; Krauss, U.; Guterl, J.K.; Wendorff, M.; Losi, A.; Gärtner, W.; Jaeger, K.E. Reporter proteins for in vivo fluorescence without oxygen. Nat. Biotechnol. 2007, 25, 443–445. [Google Scholar] [CrossRef]
- Pimenta, F.M.; Jensen, R.L.; Breitenbach, T.; Etzerodt, M.; Ogilby, P.R. Oxygen-dependent photochemistry and photophysics of “miniSOG,” a protein-encased flavin. Photochem. Photobiol. 2013, 89, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Lafaye, C.; Signor, L.; Aumonier, S.; Flors, C.; Shu, X.; Nonell, S.; Gotthard, G.; Royant, A. Tailing miniSOG: Structural bases of the complex photophysics of a flavin-binding singlet oxygen photosensitizing protein. Sci. Rep. 2019, 9, 2428. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Guo, X.; Li, J.; Liu, Q.; Kang, F.; Wang, X.; Hu, C.; Liu, H.; Gong, W.; et al. Significant expansion and red-shifting of fluorescent protein chromophore determined through computational design and genetic code expansion. Biophys. Rep. 2018, 4, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Caruso, J.A.; Hubbs, G.; Schnepp, P.; Woods, J.; Fang, J.; Li, C.; Zhang, K.; Stemmer, P.M.; Jena, B.P.; et al. Molecular architecture of mouse and human pancreatic zymogen granules: Protein components and their copy numbers. Biophys. Rep. 2018, 4, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Westermann, B. The mitochondria-plasma membrane contact site. Curr. Opin. Cell Biol. 2015, 35, 1–6. [Google Scholar] [CrossRef]
- Szymański, J.; Janikiewicz, J.; Michalska, B.; Patalas-Krawczyk, P.; Perrone, M.; Ziółkowski, W.; Duszyński, J.; Pinton, P.; Dobrzyń, A.; Więckowski, M.R. Interaction of mitochondria with the endoplasmic reticulum and plasma membrane in calcium homeostasis, lipid trafficking and mitochondrial structure. Int. J. Mol. Sci. 2017, 18, 1576. [Google Scholar] [CrossRef]
- Eisenberg-Bord, M.; Schuldiner, M. Mitochatting—If only we could be a fly on the cell wall. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1469–1480. [Google Scholar] [CrossRef]
- Gatta, A.T.; Levine, T.P. Piecing together the patchwork of contact sites. Trends Cell Biol. 2017, 27, 214–229. [Google Scholar] [CrossRef]
- Tarasova, N.I.; Stauber, R.H.; Choi, J.K.; Hudson, E.A.; Czerwinski, G.; Miller, J.L.; Pavlakis, G.N.; Michejda, C.J.; Wank, S.A. Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A. J. Biol. Chem. 1997, 272, 14817–14824. [Google Scholar] [CrossRef] [Green Version]
- Roettger, B.F.; Rentsch, R.U.; Pinon, D.; Holicky, E.; Hadac, E.; Larkin, J.M.; Miller, L.J. Dual pathways of internalization of the cholecystokinin receptor. J. Cell Biol. 1995, 128, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Cawston, E.E.; Harikumar, K.G.; Miller, L.J. Ligand-induced internalization of the type 1 cholecystokinin receptor independent of recognized signaling activity. Am. J. Physiol. Cell Physiol. 2012, 302, C615–C627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadevosyan, A.; Vaniotis, G.; Allen, B.G.; Hébert, T.E.; Nattel, S. G protein-coupled receptor signalling in the cardiac nuclear membrane: Evidence and possible roles in physiological and pathophysiological function. J. Physiol. 2012, 590, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.J.; Matthews, E.K. Photodynamic modulation of cellular function. Acta Pharm. Sin. 1998, 19, 297–303. [Google Scholar]
- Bovis, M.J.; Woodhams, J.H.; Loizidou, M.; Scheglmann, D.; Bown, S.G.; MacRobert, A.J. Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy. J. Control. Release 2012, 157, 196–205. [Google Scholar] [CrossRef]
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Nowis, D.; Makowski, M.; Stoklosa, T.; Legat, M.; Issat, T.; Golab, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Re, M.; Pampillo, M.; Savard, M.; Dubuc, C.; McArdle, C.A.; Millar, R.P.; Conn, P.M.; Gobeil, F., Jr.; Bhattacharya, M.; Babwah, A.V. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS ONE 2002, 5, e11489. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Djeungoue-Petga, M.-A.; Hebert-Chatelain, E. Linking mitochondria and synaptic transmission: The CB1 receptor. BioEssays 2017, 39, 1700126. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Powelson, E.B.; Whitney, I.E.; Raven, M.A.; Nusinowitz, S.; Jiang, M.; Birnbaumer, L.; Reese, B.E.; Farber, D.B. Involvement of OA1, an intracellular GPCR, and G alpha i3, its binding protein, in melanosomal biogenesis and optic pathway formation. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; von Zastrow, M. GPCR signaling along the endocytic pathway. Curr. Opin. Cell Biol. 2014, 27, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dores, M.R.; Trejo, J. Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond. Traffic 2019, 20, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.D.; Wu, S.C.; Dahl, E.F.; Sazama, A.J.; O'Connell, T.D. Nuclear localization drives α1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal. 2012, 24, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Vaniotis, G.; Allen, B.G.; Hébert, T.E. Nuclear GPCRs in cardiomyocytes: An insider's view of β-adrenergic receptor signaling. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1754–H1764. [Google Scholar] [CrossRef] [Green Version]
- Gwathmey, T.M.; Alzayadneh, E.M.; Pendergrass, K.D.; Chappell, M.C. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R518–R530. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.Y.; Li, Y.; Li, Z.Y.; Cui, Z.J. Permanent photodynamic cholecystokinin 1 receptor activation—Dimer-to-monomer conversion. Cell Mol. Neurobiol. 2018, 38, 1283–1292. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cui, Z.J. Photodynamic Activation of Cholecystokinin 1 Receptor with Different Genetically Encoded Protein Photosensitizers and from Varied Subcellular Sites. Biomolecules 2020, 10, 1423. https://doi.org/10.3390/biom10101423
Li Y, Cui ZJ. Photodynamic Activation of Cholecystokinin 1 Receptor with Different Genetically Encoded Protein Photosensitizers and from Varied Subcellular Sites. Biomolecules. 2020; 10(10):1423. https://doi.org/10.3390/biom10101423
Chicago/Turabian StyleLi, Yuan, and Zong Jie Cui. 2020. "Photodynamic Activation of Cholecystokinin 1 Receptor with Different Genetically Encoded Protein Photosensitizers and from Varied Subcellular Sites" Biomolecules 10, no. 10: 1423. https://doi.org/10.3390/biom10101423
APA StyleLi, Y., & Cui, Z. J. (2020). Photodynamic Activation of Cholecystokinin 1 Receptor with Different Genetically Encoded Protein Photosensitizers and from Varied Subcellular Sites. Biomolecules, 10(10), 1423. https://doi.org/10.3390/biom10101423






