General Aspects of Metal Ions as Signaling Agents in Health and Disease
Abstract
1. Introduction
2. Magnesium
3. Calcium
4. Zinc
5. Copper
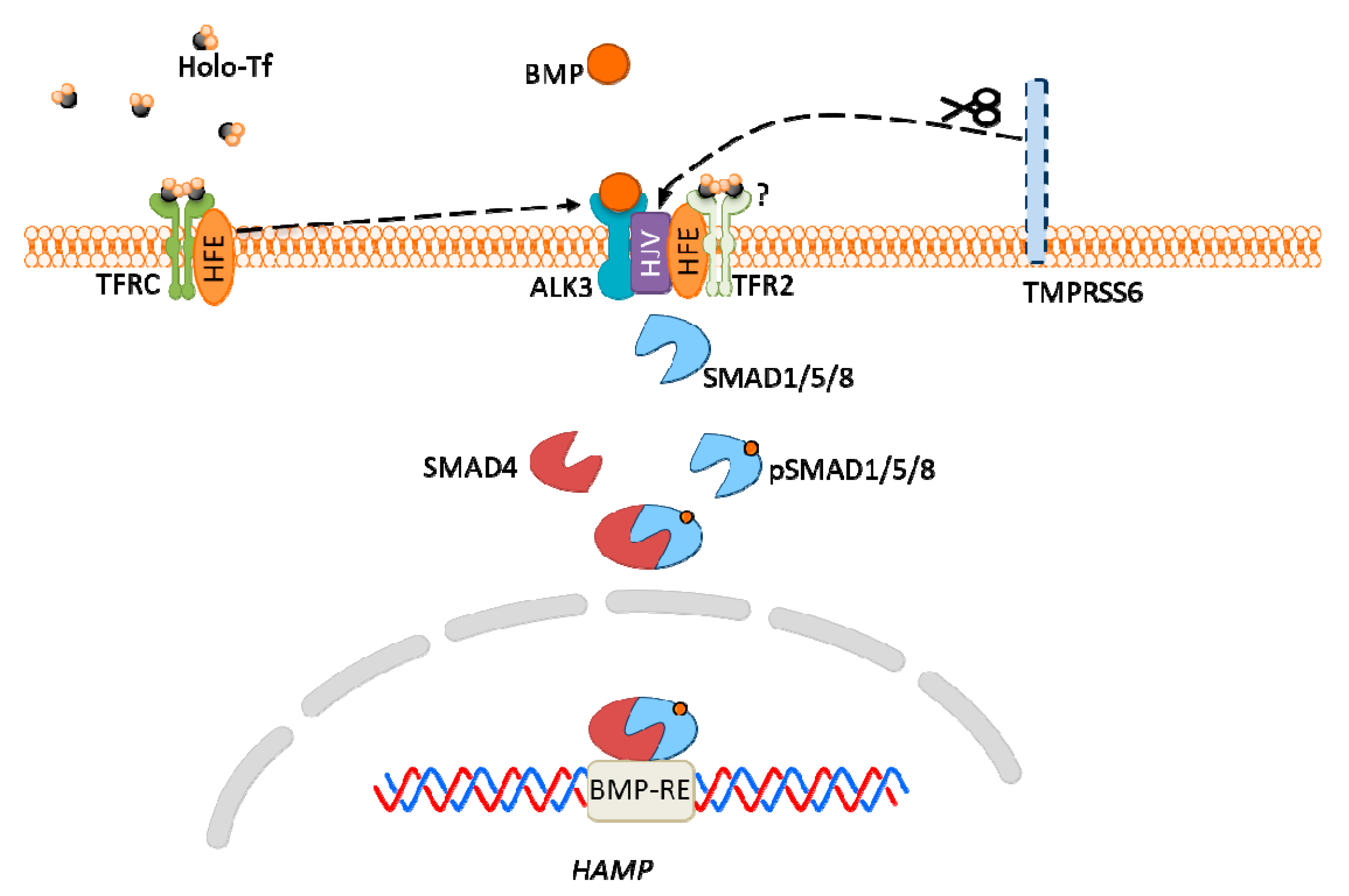
6. Iron
7. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ross, B.; Mehtal, S.; Zhang, J. Molecular tools for acute spatiotemporal manipulation of signal transduction. Curr. Opin. Chem. Biol. 2016, 34, 135–142. [Google Scholar] [CrossRef]
- Lqbal, J.; Zaidi, M.; Avadhani, N.G. Cell signaling. Mol. Integr. Physiol. Musculoskelet. Syst. 2010, 1211, 3–8. [Google Scholar] [CrossRef]
- Penner, R.; Neher, E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J. Exp. Biol. 1988, 139, 329–345. [Google Scholar] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Tamano, H.; Koike, Y.; Nakada, H.; Shakushi, Y.; Takeda, A. Significance of synaptic Zn2+ signaling in zincergic and non-zincergic synapses in the hippocampus in cognition. J. Trace Elem. Med. Biol. 2016, 38, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Heja, L.; Simon, A.; Jablonkai, I.; Kovacs, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Weth, A.; Walcher, S.; Lax, C.; Baumgartner, W. Modeling of Zinc Dynamics in the Synaptic Cleft: Implications for Cadherin Mediated Adhesion and Synaptic Plasticity. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamano, H.; Morioka, H.; Nishio, R.; Takeuchi, A.; Takeda, A. Blockade of Rapid Influx of Extracellular Zn2+ into Nigral Dopaminergic Neurons Overcomes Paraquat-Induced Parkinson’s Disease in Rats. Mol. Neurobiol. 2019, 56, 4539–4548. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef]
- Ashraf, A.; Michaelides, C.; Walker, T.A.; Ekonomou, A.; Suessmilch, M.; Sriskanthanathan, A.; Abraha, S.; Parkes, A.; Parkes, H.G.; Geraki, K.; et al. Regional Distributions of Iron, Copper and Zinc and Their Relationships With Glia in a Normal Aging Mouse Model. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Pal, A.; Prasad, R. Regional Distribution of Copper, Zinc and Iron in Brain of Wistar Rat Model for Non-Wilsonian Brain Copper Toxicosis. Indian J. Clin. Biochem. 2016, 31, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.; Lemberg, K.; Lamprecht, M.; Skouta, R.; Zaitsev, E.; Gleason, C.; Patel, D.; Bauer, A.; Cantley, A.; Yang, W.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Gimenez-Mascarell, P.; Gonzalez-Recio, I.; Fernandez-Rodriguez, C.; Oyenarte, I.; Mueller, D.; Luz Martinez-Chantar, M.; Alfonso Martinez-Cruz, L. Current Structural Knowledge on the CNNM Family of Magnesium Transport Mediators. Int. J. Mol. Sci. 2019, 20, 1135. [Google Scholar] [CrossRef]
- Zhu, D.; You, J.; Zhao, N.; Xu, H. Magnesium Regulates Endothelial Barrier Functions through TRPM7, MagT1, and S1P1. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Yatsimirsky, K.B. ELECTRONIC-STRUCTURE, HYDRATION ENERGY AND STABILITY OF METAL AQUAIONS. Teor. I Eksperimentalnaya Khimiya 1994, 30, 1–11. [Google Scholar] [CrossRef]
- Binding, Transport and Storage of Metal Ions in Biological Cells; Royal Society of Chemistry: London, UK, 2014; Volume 2, pp. 1–911. [CrossRef]
- Kolisek, M.; Montezano, A.C.; Sponder, G.; Anagnostopoulou, A.; Vormann, J.; Touyz, R.M.; Aschenbach, J.R. PARK7/DJ-1 dysregulation by oxidative stress leads to magnesium deficiency: Implications in degenerative and chronic diseases. Clin. Sci. 2015, 129, 1143–1150. [Google Scholar] [CrossRef]
- Shahi, A.; Aslani, S.; Ataollahi, M.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Romani, A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 2007, 458, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. Embo J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef]
- Schindl, R.; Weghuber, J.; Romanin, C.; Schweyen, R.J. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys. J. 2007, 93, 3872–3883. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. Am. J. Physiol. Cell Physiol. 2008, 294, C495–C502. [Google Scholar] [CrossRef]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6. [Google Scholar] [CrossRef]
- Li, F.-Y.; Lenardo, M.J.; Chaigne-Delalande, B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnes. Res. 2011, 24, S109–S114. [Google Scholar] [CrossRef]
- Holmes, D.; Carroll, K.; Brodeur, S.; Pashine, A. Characterizing the intracellular magnesium transporter MagT1 in murine lymphocyte function. J. Immunol. 2016, 196. [Google Scholar]
- Wu, N.; Veillette, A. IMMUNOLOGY Magnesium in a signalling role. Nature 2011, 475, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Goytain, A.; Hines, R.M.; Quamme, G.A. Functional characterization of NIPA2, a selective Mg2+ transporter. Am. J. Physiol. Cell Physiol. 2008, 295, C944–C953. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, Y.; Zhang, P.; Wang, J.; Wu, Y.; Wu, X.; Netoff, T.; Jiang, Y. Functional Study of NIPA2 Mutations Identified from the Patients with Childhood Absence Epilepsy. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Liu, N.-N.; Xie, H.; Xiang-wei, W.-S.; Gao, K.; Wang, T.-S.; Jiang, Y.-W. The absence of NIPA2 enhances neural excitability through BK (big potassium) channels. CNS Neurosci. Ther. 2019, 25, 865–875. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.-L.; Yang, B.; Sun, J.; Yang, M.-W. NIPA2 regulates osteoblast function via its effect on apoptosis pathways in type 2 diabetes osteoporosis. Biochem. Biophys. Res. Commun. 2019, 513, 883–890. [Google Scholar] [CrossRef]
- Funato, Y.; Yamazaki, D.; Mizukami, S.; Du, L.; Kikuchi, K.; Miki, H. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014, 124, 5398–5410. [Google Scholar] [CrossRef]
- Funato, Y.; Furutani, K.; Kurachi, Y.; Miki, H. CrossTalk proposal: CNNM proteins are Na+/Mg2+ exchangers playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. Lond. 2018, 596, 743–746. [Google Scholar] [CrossRef]
- Kolisek, M.; Sponder, G.; Pilchova, I.; Cibulka, M.; Tatarkova, Z.; Werner, T.; Racay, P. Magnesium Extravaganza: A Critical Compendium of Current Research into Cellular Mg2+ Transporters Other than TRPM6/7. Rev. Physiol. Biochem. Pharmacol. 2019, 176, 65–105. [Google Scholar] [CrossRef]
- Sponder, G.; Mastrototaro, L.; Kurth, K.; Merolle, L.; Zhang, Z.; Abdulhanan, N.; Smorodchenko, A.; Wolf, K.; Fleig, A.; Penner, R.; et al. Human CNNM2 is not a Mg2+ transporter per se. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1223–1240. [Google Scholar] [CrossRef]
- Hardy, S.; Uetani, N.; Wong, N.; Kostantin, E.; Labbe, D.P.; Begin, L.R.; Mes-Masson, A.; Miranda-Saavedra, D.; Tremblay, M.L. The protein tyrosine phosphatase PRL-2 interacts with the magnesium transporter CNNM3 to promote oncogenesis. Oncogene 2015, 34, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.S.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg center dot ATP-regulated divalent cation channel required for cell viability (vol 411, pg 590, 2001). Nature 2001, 412, 660. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.R.; Xiao, C.Y.; Jia, Y.Y.; Guo, J.L.; Jiang, J.M.; Liu, P.Q. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Stritt, S.; Nurden, P.; Favier, R.; Favier, M.; Ferioli, S.; Gotru, S.K.; van Eeuwijk, J.M.M.; Schulze, H.; Nurden, A.T.; Lambert, M.P.; et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg2+ homeostasis and cytoskeletal architecture. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular Smooth Muscle Cell Differentiation to an Osteogenic Phenotype Involves TRPM7 Modulation by Magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 Disrupts Embryonic Development and Thymopoiesis Without Altering Mg(2+) Homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Moscheni, C.; Trapani, V.; Wolf, F.I.; Farruggia, G.; Sargenti, A.; Iotti, S.; Maier, J.A.M.; Castiglioni, S. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Middelbeek, J.; Clark, K.; Venselaar, H.; Huynen, M.A.; van Leeuwen, F.N. The alpha-kinase family: An exceptional branch on the protein kinase tree. Cell. Mol. Life Sci. 2010, 67, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Lou, L.; Al-Saadi, N.; Tetteh, S.; Runnels, L.W. The kinase activity of the channel-kinase protein TRPM7 regulates stability and localization of the TRPM7 channel in polarized epithelial cells. J. Biol. Chem. 2018, 293, 11491–11504. [Google Scholar] [CrossRef]
- Huang, K.; El-Husseini, A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr. Opin. Neurobiol. 2005, 15, 527–535. [Google Scholar] [CrossRef]
- Mies, F.; Spriet, C.; Heliot, L.; Sariban-Sohraby, S. Epithelial Na+ channel stimulation by n-3 fatty acids requires proximity to a membrane-bound A-kinase-anchoring protein complexed with protein kinase A and phosphodiesterase. J. Biol. Chem. 2007, 282, 18339–18347. [Google Scholar] [CrossRef]
- Qin, N.; Platano, D.; Olcese, R.; Costantin, J.L.; Stefani, E.; Birnbaumer, L. Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc. Natl. Acad. Sci. USA 1998, 95, 4690–4695. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Huang, K.; Sanders, S.S.; Milnerwood, A.J.; Hines, R.; Lerch, J.P.; Franciosi, S.; Drisdel, R.C.; Vaid, K.; Young, F.B.; et al. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet. 2011, 20, 3899–3909. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol. Genom. 2005, 21, 337–342. [Google Scholar] [CrossRef]
- Kolisek, M.; Nestler, A.; Vormann, J.; Schweigel-Roentgen, M. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am. J. Physiol. Cell Physiol. 2012, 302, C318–C326. [Google Scholar] [CrossRef]
- Rodriguez-Ramirez, M.; Rodriguez-Moran, M.; Reyes-Romero, M.A.; Guerrero-Romero, F. Effect of oral magnesium supplementation on the transcription of TRPM6, TRPM7, and SLC41A1 in individuals newly diagnosed of pre-hypertension. A randomized, double-blind, placebo-controlled trial. Magnes. Res. 2017, 30, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Hotta, K.; Suzuki, K.; Oka, K. GABA-Induced Intracellular Mg2+ Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr. Biol. 2018, 28, 3984–3991.e5. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed]
- Moncrief, M.B.C.; Maguire, M.E. Magnesium transport in prokaryotes. J. Biol. Inorg. Chem. 1999, 4, 523–527. [Google Scholar] [CrossRef]
- Moore, E.W. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J. Clin. Investig. 1970, 49, 318–334. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium microdomains: Organization and function. Cell Calcium 2006, 40, 405–412. [Google Scholar] [CrossRef]
- Rimessi, A.; Pedriali, G.; Vezzani, B.; Tarocco, A.; Marchi, S.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Interorganellar calcium signaling in the regulation of cell metabolism: A cancer perspective. Semin. Cell Dev. Biol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Munaron, L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int. J. Mol. Med. 2002, 10, 671–676. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Faouzi, M.; Kilch, T.; Horgen, F.D.; Fleig, A.; Penner, R. The TRPM7 channel kinase regulates store-operated calcium entry. J. Physiol. Lond. 2017, 595, 3165–3180. [Google Scholar] [CrossRef]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lu, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Chu, P.; Gu, J.; Zhang, H.; Feng, R.; Wen, X.; Wang, D.; Xiong, W.; Wang, T.; Yin, S. The influence of Ca2+ concentration on voltage-dependent L-type calcium channels’ expression in the marbled eel (Anguilla marmorata). Gene 2020, 722. [Google Scholar] [CrossRef]
- Brown, B.M.; Shim, H.; Christophersen, P.; Wulff, H. Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Guerini, D. The Ca2+ pumps and the Na+/Ca2+ exchangers. Biometals 1998, 11, 319–330. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodriguez-Moreno, A. Calcium Dynamics and Synaptic Plasticity. Adv. Exp. Med. Biol. 2020, 1131, 965–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Ma, J.; Parrington, J.; Calcraft, P.J.; Galione, A.; Evans, A.M. Calcium signaling via two-pore channels: Local or global, that is the question. Am. J. Physiol. Cell Physiol. 2010, 298, C430–C441. [Google Scholar] [CrossRef]
- Fracchia, K.M.; Pai, C.Y.; Walsh, C.M. Modulation of T cell metabolism and function through calcium signaling. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Li, H.Y.; Zhang, Y.; Li, C.; Li, K.; Ai, K.T.; Yang, J.L. Ca2+-Calcineurin Axis-Controlled NFAT Nuclear Translocation Is Crucial for Optimal T Cell Immunity in an Early Vertebrate. J. Immunol. 2020, 204, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001, 19, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Leger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.-P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Marks, A.R. Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging. Curr. Mol. Pharmacol. 2015, 8, 206–222. [Google Scholar] [CrossRef]
- Olofsson, M.H.; Havelka, A.M.; Brnjic, S.; Shoshan, M.C.; Linder, S. Charting calcium-regulated apoptosis pathways using chemical biology: Role of calmodulin kinase II. Bmc Chem. Biol. 2008, 8, 2. [Google Scholar] [CrossRef]
- Danese, A.; Patergnani, S.; Bonora, M.; Wieckowski, M.R.; Previati, M.; Giorgi, C.; Pinton, P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim. Biophys. Acta Bioenergy 2017, 1858, 615–627. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, C.; Lian, Y.J.; Zhang, H.F.; Meng, X.H.; Yu, M.Y.; Xie, N.C. Role of the mitochondrial calcium uniporter in Mg2+-free-induced epileptic hippocampal neuronal apoptosis. Int. J. Neurosci. 2020, 9. [Google Scholar] [CrossRef]
- Lukas, T.J.; Haiech, J.; Lau, W.; Craig, T.A.; Zimmer, W.E.; Shattuck, R.L.; Shoemaker, M.O.; Watterson, D.M. CALMODULIN AND CALMODULIN-REGULATED PROTEIN-KINASES AS TRANSDUCERS OF INTRACELLULAR CALCIUM SIGNALS. Cold Spring Harb. Symp. Quant. Biol. 1988, 53, 185–193. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Jaiswal, S.; Lu, H.P. Compressive-force induced activation of apo-calmodulin in protein signalling. Phys. Chem. Chem. Phys. PCCP 2020, 22, 1092–1096. [Google Scholar] [CrossRef]
- Song, Z.X.; Chen, Q.; Ding, Q.; Zheng, F.; Li, C.W.; Xu, L.P.; Wang, H.B. Function of Ca2+-/calmodulin-dependent protein kinase IV in Ca2+-stimulated neuronal signaling and behavior. Sci. China Life Sci. 2015, 58, 6–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, X.; Murao, K.; Sayo, Y.; Imachi, H.; Cao, W.M.; Ohtsuka, S.; Niimi, M.; Tokumitsu, H.; Inuzuka, H.; Wong, N.C.W.; et al. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes 2004, 53, 1475–1481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villalobo, A.; Berchtold, M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Zietse, R. Disorders of calcium and magnesium balance: A physiology-based approach. Pediatr. Nephrol. 2013, 28, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Bertoni-Freddari, C.; Marcellini, F.; Malavolta, M. Brain, aging and neurodegeneration: Role of zinc ion availability. Prog. Neurobiol. 2005, 75, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Klitenick, M.A.; Manton, W.I.; Kirkpatrick, J.B. CYTOARCHITECTONIC DISTRIBUTION OF ZINC IN THE HIPPOCAMPUS OF MAN AND THE RAT. Brain Res. 1983, 273, 335–339. [Google Scholar] [CrossRef]
- Lee, H.C.; Edmonds, M.E.; Duncan, F.E.; O’Halloran, T.V.; Woodruff, T.K. Zinc exocytosis is sensitive to myosin light chain kinase inhibition in mouse and human eggs. Mol. Hum. Reprod. 2020, 26, 228–239. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, K.; Dean, J. Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy. Dev. Cell 2018, 46, 627–640.e5. [Google Scholar] [CrossRef] [PubMed]
- Slomianka, L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience 1992, 48, 325–352. [Google Scholar] [CrossRef]
- Haug, F.M.S. Electron microscopical localization of zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie 1967, 8, 355–368. [Google Scholar] [CrossRef]
- Evstratova, A.; Toth, K. Information processing and synaptic plasticity at hippocampal mossy fiber terminals. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef][Green Version]
- Sullivan, J.A.; Zhang, X.-L.; Sullivan, A.P.; Vose, L.R.; Moghadam, A.A.; Fried, V.A.; Stanton, P.K. Zinc enhances hippocampal long-term potentiation at CA1 synapses through NR2B containing NMDA receptors. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Bastos, F.M.C.; Lopes, S.A.; Corceiro, V.N.; Matias, C.M.; Dionisio, J.C.; Sampaio dos Aidos, F.D.S.; Mendes, P.J.; Santos, R.M.; Quinta-Ferreira, R.M.; Emilia Quinta-Ferreira, M. Postsynaptic zinc potentiation elicited by KCl depolarization at hippocampal mossy fiber synapses. Gen. Physiol. Biophys. 2017, 36, 289–296. [Google Scholar] [CrossRef]
- Takeda, A.; Fuke, S.; Ando, M.; Oku, N. Positive modulation of long-term potentiation at hippocampal ca1 synapses by low micromolar concentrations of zinc. Neuroscience 2009, 158, 585–591. [Google Scholar] [CrossRef]
- Takeda, A.; Ando, M.; Kanno, S.; Oku, N. Unique response of zinc in the hippocampus to behavioral stress and attenuation of subsequent mossy fiber long-term potentiation. Neurotoxicology 2009, 30, 712–717. [Google Scholar] [CrossRef]
- Vogt, K.; Mellor, J.; Tong, G.; Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 2000, 26, 187–196. [Google Scholar] [CrossRef]
- Kay, A.R. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J. Neurosci. 2003, 23, 6847–6855. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.R.; Toth, K. Is Zinc a Neuromodulator? Sci. Signal. 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hough, C.J.; Frederickson, C.J.; Sarvey, J.M. Induction of mossy fiber -> CA3 long-term potentiation requires translocation of synaptically released Zn2+. J. Neurosci. 2001, 21, 8015–8025. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.C.; Hollopeter, G.; Thomas, S.A.; Froelick, G.J.; Palmiter, R.D. Disruption of the metallothionein-III gene in mice: Analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J. Neurosci. 1997, 17, 1271–1281. [Google Scholar] [CrossRef]
- Palumaa, P.; Eriste, E.; Njunkova, O.; Pokras, L.; Jornvall, H.; Sillard, R. Brain-specific metallothionein-3 has higher metal-binding capacity than ubiquitous metallothioneins and binds metals noncooperatively. Biochemistry 2002, 41, 6158–6163. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef]
- Ben Mimouna, S.; Le Charpentier, T.; Lebon, S.; Van Steenwinckel, J.; Messaoudi, I.; Gressens, P. Involvement of the synapse-specific zinc transporter ZnT3 in cadmium-induced hippocampal neurotoxicity. J. Cell. Physiol. 2019, 234, 15872–15884. [Google Scholar] [CrossRef]
- Quinta-Ferreira, M.E.; Sampaio dos Aidos, F.D.S.; Matias, C.M.; Mendes, P.J.; Dionisio, J.C.; Santos, R.M.; Rosario, L.M.; Quinta-Ferreira, R.M. Modelling zinc changes at the hippocampal mossy fiber synaptic cleft. J. Comput. Neurosci. 2016, 41, 323–337. [Google Scholar] [CrossRef]
- Blakemore, L.J.; Trombley, P.Q. Mechanisms of zinc modulation of olfactory bulb AMPA receptors. Neuroscience 2019, 410, 160–175. [Google Scholar] [CrossRef]
- Ha, H.T.T.; Leal-Ortiz, S.; Lalwani, K.; Kiyonaka, S.; Hamachi, I.; Mysore, S.P.; Montgomery, J.M.; Garner, C.C.; Huguenard, J.R.; Kim, S.A. Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Noh, J.; Chung, J.-M. Modulation of Dopaminergic Neuronal Excitability by Zinc through the Regulation of Calcium-related Channels. Exp. Neurobiol. 2019, 28, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Ekstein, D.; Benninger, F.; Daninos, M.; Pitsch, J.; van Loo, K.M.J.; Becker, A.J.; Yaari, Y. Zinc induces long-term upregulation of T-type calcium current in hippocampal neurons in vivo. J. Physiol. Lond. 2012, 590, 5895–5905. [Google Scholar] [CrossRef] [PubMed]
- Sindreu, C.; Bayes, A.; Altafaj, X.; Perez-Clausell, J. Zinc transporter-1 concentrates at the postsynaptic density of hippocampal synapses. Mol. Brain 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Mellone, M.; Pelucchi, S.; Alberti, L.; Geneazzani, A.A.; Di Luca, M.; Gardoni, F. Zinc transporter-1: A novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 2015, 132, 159–168. [Google Scholar] [CrossRef]
- Schulien, A.J.; Justice, J.A.; Di Maio, R.; Wills, Z.P.; Shah, N.H.; Aizenman, E. Zn2+-induced Ca2+ release via ryanodine receptors triggers calcineurin-dependent redistribution of cortical neuronal Kv2.1 K+ channels. J. Physiol. Lond. 2016, 594, 2647–2659. [Google Scholar] [CrossRef]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef]
- Bredesen, D.E. Metabolic profiling distinguishes three subtypes of Alzheimer’s disease. Aging-Us 2015, 7, 595–600. [Google Scholar] [CrossRef]
- Ishihara, R.; Ide-Ektessabi, A.; Ikeda, K.; Mizuno, Y.; Fujisawa, S.; Takeuchi, T.; Ohta, T. Investigation of cellular metallic elements in single neurons of human brain tissues. Neuroreport 2002, 13, 1817–1820. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Gardner, B.; Dieriks, B.V.; Cameron, S.; Mendis, L.H.S.; Turner, C.; Faull, R.L.M.; Curtis, M.A. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Samudralwar, D.L.; Diprete, C.C.; Ni, B.F.; Ehmann, W.D.; Markesbery, W.R. Elemental imbalances in the olfactory pathway in Alzheimers-diseasE. J. Neurol. Sci. 1995, 130, 139–145. [Google Scholar] [CrossRef]
- Lim, J.H.; Davis, G.E.; Wang, Z.; Li, V.; Wu, Y.; Rue, T.C.; Storm, D.R. Zicam-Induced Damage to Mouse and Human Nasal Tissue. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Eom, J.-W.; Lee, J.-M.; Koh, J.-Y.; Kim, Y.-H. AMP-activated protein kinase contributes to zinc-induced neuronal death via activation by LKB1 and induction of Bim in mouse cortical cultures. Mol. Brain 2016, 9. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, W.; Zhao, Y.; Ji, X.; Liu, K.J. Zinc accumulation in mitochondria promotes ischemia-induced BBB disruption through Drp1-dependent mitochondria fission. Toxicol. Appl. Pharmacol. 2019, 377. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, N.B.; Stanika, R.I.; Kazanina, G.; Villanueva, I.; Andrews, S.B. The interactive role of zinc and calcium in mitochondrial dysfunction and neurodegeneration. J. Neurochem. 2014, 128, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.S.; Van den Heuvel, M.R.; Stevens, D.; Kamunde, C. Zinc and calcium modulate mitochondrial redox state and morphofunctional integrity. Free Radic. Biol. Med. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yuan, L.; He, W.; Yang, X. Zinc ions regulate opening of tight junction favouring efflux of macromolecules via the GSK3 beta/snail-mediated pathway. Metallomics 2018, 10, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Vasudevaraju, P.; Bharathi, T.J.; Shamasundar, N.M.; Subba Rao, K.; Balaraj, B.M.; Ksj, R.; Ts, S.R. New evidence on iron, copper accumulation and zinc depletion and its correlation with DNA integrity in aging human brain regions. Indian J. Psychiatry 2010, 52, 140–144. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.-x.; Li, L.-x.; Liu, Y.-q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018, 179, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H. Significance of the degree of synaptic Zn2+ signaling in cognition. Biometals 2016, 29, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kucma, M.; Szewczyk, B.; Sadlik, K.; Piekoszewski, W.; Trela, F.; Opoka, W.; Poleszak, E.; Pilc, A.; Nowak, G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013, 151, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Kotarska, K.; Siwek, A.; Olech, L.; Kuter, K. Antidepressant activity of zinc: Further evidence for the involvement of the serotonergic system. Pharmacol. Rep. 2017, 69, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General Aspects of Metal Toxicity. Curr. Med. Chem. 2014, 21, 3721–3740. [Google Scholar] [CrossRef] [PubMed]
- Doreulee, N.; Yanovsky, Y.; Haas, H.L. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus 1997, 7, 666–669. [Google Scholar] [CrossRef]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef]
- Gaier, E.D.; Miller, M.B.; Ralle, M.; Aryal, D.; Wetsel, W.C.; Mains, R.E.; Eipper, B.A. Peptidylglycine alpha-amidating monooxygenase heterozygosity alters brain copper handling with region specificity. J. Neurochem. 2013, 127, 605–619. [Google Scholar] [CrossRef]
- Schmidt, K.; Ralle, M.; Schaffer, T.; Jayakanthan, S.; Bari, B.; Muchenditsi, A.; Lutsenko, S. ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine--hydroxylase. J. Biol. Chem. 2018, 293, 20085–20098. [Google Scholar] [CrossRef]
- Kapkaeva, M.R.; Popova, O.V.; Kondratenko, R.V.; Rogozin, P.D.; Genrikhs, E.E.; Stelmashook, E.V.; Skrebitsky, V.G.; Khaspekov, L.G.; Isaev, N.K. Effects of copper on viability and functional properties of hippocampal neurons in vitro. Exp. Toxicol. Pathol. 2017, 69, 259–264. [Google Scholar] [CrossRef]
- Kardos, J.; Kovacs, I.; Hajos, F.; Kalman, M.; Simonyi, M. Nerve-endings from rat-brain tissue release copper upon depolarization—A possible role in regulating neuronal excitability. Neurosci. Lett. 1989, 103, 139–144. [Google Scholar] [CrossRef]
- Gaier, E.D.; Rodriguiz, R.M.; Zhou, J.; Ralle, M.; Wetsel, W.C.; Eipper, B.A.; Mains, R.E. In vivo and in vitro analyses of amygdalar function reveal a role for copper. J. Neurophysiol. 2014, 111, 1927–1939. [Google Scholar] [CrossRef]
- Salazar-Weber, N.L.; Smith, J.P. Copper Inhibits NMDA Receptor-Independent LTP and Modulates the Paired-Pulse Ratio after LTP in Mouse Hippocampal Slices. Int. J. Alzheimer’s Dis. 2011, 2011, 864753. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Yu, K.I.H.; Kaplan, J.H. Rate and Regulation of Copper Transport by Human Copper Transporter 1 (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Copper accumulation by cultured astrocytes. Neurochem. Int. 2010, 56, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, D.; Kang, Y.J. Copper chaperone for superoxide dismutase-1 transfers copper to mitochondria but does not affect cytochrome c oxidase activity. Exp. Biol. Med. 2013, 238, 1017–1023. [Google Scholar] [CrossRef]
- Vest, K.E.; Paskavitz, A.L.; Lee, J.B.; Padilla-Benavides, T. Dynamic changes in copper homeostasis and post-transcriptional regulation of Atp7a during myogenic differentiation. Metallomics 2018, 10, 309–322. [Google Scholar] [CrossRef]
- Horning, M.S.; Trombley, P.Q. Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J. Neurophysiol. 2001, 86, 1652–1660. [Google Scholar] [CrossRef]
- Marchetti, C.; Baranowska-Bosiacka, I.; Gavazzo, P. Multiple effects of copper on NMDA receptor currents. Brain Res. 2014, 1542, 20–31. [Google Scholar] [CrossRef]
- Meramat, A.; Rajab, N.F.; Shahar, S.; Sharif, R.A. DNA damage, copper and lead associates with cognitive function among older adults. J. Nutr. Health Aging 2017, 21, 539–545. [Google Scholar] [CrossRef]
- Deibel, M.A.; Ehmann, W.D.; Markesbery, W.R. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106–1119. [Google Scholar] [CrossRef]
- Kumar, N. Copper deficiency myelopathy (human swayback). Mayo Clin. Proc. 2006, 81, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Schlief, M.L.; West, T.; Craig, A.M.; Holtzman, D.M.; Gitlin, J.D. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. USA 2006, 103, 14919–14924. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Magaki, S.; Schrag, M.; Ghosh, M.C.; Kirsch, W.M. Iron Regulatory Protein 2 is Involved in Brain Copper Homeostasis. J. Alzheimers Dis. 2009, 18, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Munoz, B.; Sepulveda, F.J.; Urrutia, J.; Quiroz, M.; Luza, S.; De Ferrari, G.V.; Aguayo, L.G.; Opazo, C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011, 119, 78–88. [Google Scholar] [CrossRef]
- Yu, H.; Wang, D.; Zou, L.; Zhang, Z.; Xu, H.; Zhu, F.; Ren, X.; Xu, B.; Yuan, J.; Liu, J.; et al. Proteomic alterations of brain subcellular organelles caused by low-dose copper exposure: Implication for Alzheimer’s disease. Arch. Toxicol. 2018, 92, 1363–1382. [Google Scholar] [CrossRef]
- Xu, J.; Begley, P.; Church, S.J.; Patassini, S.; McHarg, S.; Kureishy, N.; Hollywood, K.A.; Waldvogel, H.J.; Liu, H.; Zhang, S.; et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: Metabolic basis for dementia. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.H.; Hogl, S.; Narayanan, U.; Zhang, L.; McHarg, S.; Xu, J.; Gong, D.; et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc. Diabetol. 2014, 13. [Google Scholar] [CrossRef]
- Peng, F.; Xie, F.; Muzik, O. Alteration of Copper Fluxes in Brain Aging: A Longitudinal Study in Rodent Using (CuCl2)-Cu-64-PET/CT. Aging Dis. 2018, 9, 109–118. [Google Scholar] [CrossRef]
- Famitafreshi, H.; Karimian, M. Modulation of catalase, copper and zinc in the hippocampus and the prefrontal cortex in social isolation-induced depression in male rats. Acta Neurobiol. Exp. 2019, 79, 184–192. [Google Scholar] [CrossRef]
- Han, O. Molecular mechanism of intestinal iron absorption. Metallomics 2011, 3, 103–109. [Google Scholar] [CrossRef]
- Inoue, K.; Nakai, Y.; Ueda, S.; Kamigaso, S.; Ohta, K.; Hatakeyama, M.; Hayashi, Y.; Otagiri, M.; Yuasa, H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G660–G668. [Google Scholar] [CrossRef] [PubMed]
- Laftah, A.; Latunde-Dada, G.; Fakih, S.; Hider, R.; Simpson, R.; Mckie, A. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br. J. Nutr. 2009, 101, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, S.; Garrick, M.; Arredondo, M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell Physiol. 2012, 302, C1780–C1785. [Google Scholar] [CrossRef] [PubMed]
- Lawen, A.; Lane, D. Mammalian Iron Homeostasis in Health and Disease: Uptake, Storage, Transport, and Molecular Mechanisms of Action. Antioxid. Redox Signal. 2013, 18, 2473–2507. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Pyrkosz-Bulska, M.; Szebesczyk, A.; Ostrowska, M. Iron Chelating Strategies in Systemic Metal Overload, Neurodegeneration and Cancer. Curr. Med. Chem. 2014, 21, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Et Biophys. Acta-Gen. Subj. 2012, 1820, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Agarwal, A.; Yee, J. Hepcidin. Adv. Chronic Kidney Dis. 2019, 26, 298–305. [Google Scholar] [CrossRef]
- Rochette, L.; Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C. The iron-regulatory hormone hepcidin: A possible therapeutic target? Pharmacol. Ther. 2015, 146, 35–52. [Google Scholar] [CrossRef]
- Corradini, E.; Meynard, D.; Wu, Q.; Chen, S.; Ventura, P.; Pietrangelo, A.; Babitt, J. Serum and Liver Iron Differently Regulate the Bone Morphogenetic Protein 6 (BMP6)-SMAD Signaling Pathway in Mice. Hepatology 2011, 54, 273–284. [Google Scholar] [CrossRef]
- Corradini, E.; Rozier, M.; Meynard, D.; Odhiambo, A.; Lin, H.; Feng, Q.; Migas, M.; Britton, R.; Babitt, J.; Fleming, R. Iron Regulation of Hepcidin Despite Attenuated Smad1,5,8 Signaling in Mice Without Transferrin Receptor 2 or Hfe. Gastroenterology 2011, 141, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.; Edwards, C.; Acton, R. HFE gene: Structure, function, mutations, and associated iron abnormalities. Gene 2015, 574, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Wu, Q.; Cheng, W.; Liu, W.; Zhao, Y.; Mayeur, C.; Schmidt, P.; Yu, P.; Wang, F.; et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood 2014, 124, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Fillebeen, C.; Daba, A.; Wagner, J.; Sebastiani, G.; Pantopoulos, K. Iron-Dependent Regulation of Hepcidin in Hjv-/- Mice: Evidence That Hemojuvelin Is Dispensable for Sensing Body Iron Levels. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Rubio, A.; Campanella, A.; Gourbeyre, O.; Artuso, I.; Bordini, J.; Gineste, A.; Latour, C.; Besson-Fournier, C.; Lin, H.; et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood 2016, 127, 2327–2336. [Google Scholar] [CrossRef]
- Rishi, G.; Secondes, E.; Subramaniam, V. Hemochromatosis: Evaluation of the dietary iron model and regulation of hepcidin. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2550–2556. [Google Scholar] [CrossRef]
- Taniguchi, R.; Kato, H.; Font, J.; Deshpande, C.; Wada, M.; Ito, K.; Ishitani, R.; Jormakka, M.; Nureki, O. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- di Patti, M.; Polticelli, F.; Cece, G.; Cutone, A.; Felici, F.; Persichini, T.; Musci, G. A structural model of human ferroportin and of its iron binding site. FEBS J. 2014, 281, 2851–2860. [Google Scholar] [CrossRef]
- Ward, D.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef]
- Neves, J.; Leitz, D.; Kraut, S.; Brandenberger, C.; Agrawal, R.; Weissmann, N.; Muhlfeld, C.; Mall, M.; Altamura, S.; Muckenthaler, M. Disruption of the Hepcidin/Ferroportin Regulatory System Causes Pulmonary Iron Overload and Restrictive Lung Disease. Ebiomedicine 2017, 20, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.; Wilkins, S.; Darshan, D.; Mirciov, C.; Dunn, L.; Anderson, G. Ferroportin Is Essential for Iron Absorption During Suckling, But Is Hyporesponsive to the Regulatory Hormone Hepcidin. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.; Torti, F. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Bebber, C.; Muller, F.; Clemente, L.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.; Pierre, F.; Corpet, D. Heme Iron from Meat and Risk of Colorectal Cancer: A Meta-analysis and a Review of the Mechanisms Involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sornjai, W.; Van Long, F.; Pion, N.; Pasquer, A.; Saurin, J.; Marcel, V.; Diaz, J.; Mertani, H.; Smith, D. Iron and hepcidin mediate human colorectal cancer cell growth. Chem. Biol. Interact. 2020, 319. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.; Weisz, K.; Triner, D.; Xie, L.; Attili, D.; Pant, A.; Gyorffy, B.; Zhan, M.; Carter-Su, C.; et al. Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metab. 2016, 24, 447–461. [Google Scholar] [CrossRef]
- Latunde-Dada, G. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Yang, W.; Stockwell, B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.; Steinberg, G.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhao, Y.; Guo, J.; Zhao, S.; Song, L.; Fei, C.; Zhang, Z.; Li, X.; Chang, C. Iron overload promotes erythroid apoptosis through regulating HIF-1a/ROS signaling pathway in patients with myelodysplastic syndrome. Leuk. Res. 2017, 58, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kilari, S.; Pullakhandam, R.; Nair, K. Zinc inhibits oxidative stress-induced iron signaling and apoptosis in Caco-2 cells. Free Radic. Biol. Med. 2010, 48, 961–968. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Duan, J.; Yang, L.; Cai, Z.; Zhu, W.; Nie, Y.; He, J.; Xia, C.; Gong, Q.; et al. Bioactive iron oxide nanoparticles suppress osteoclastogenesis and ovariectomy-induced bone loss through regulating the TRAF6-p62-CYLD signaling complex. Acta Biomater. 2020, 103, 281–292. [Google Scholar] [CrossRef]
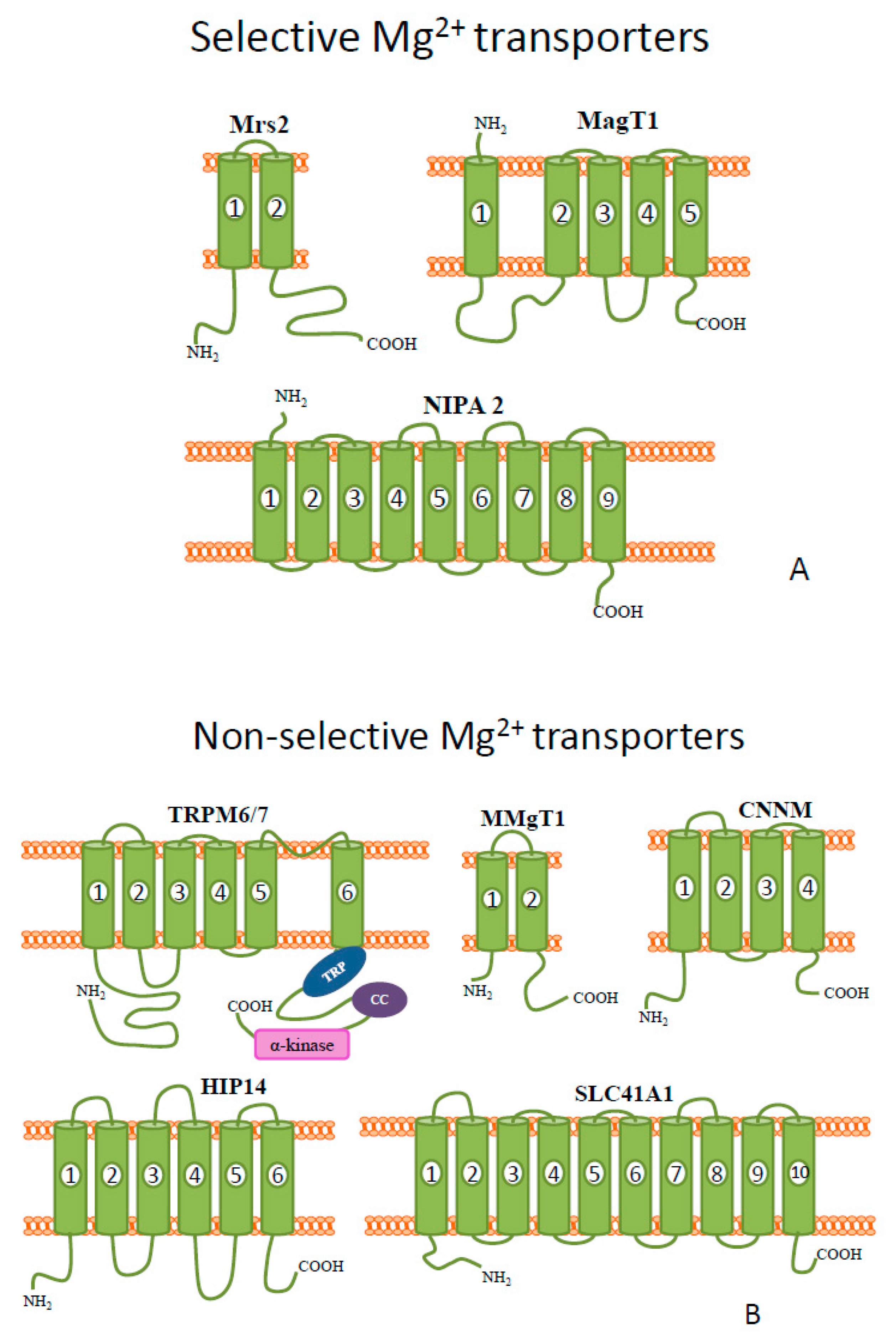
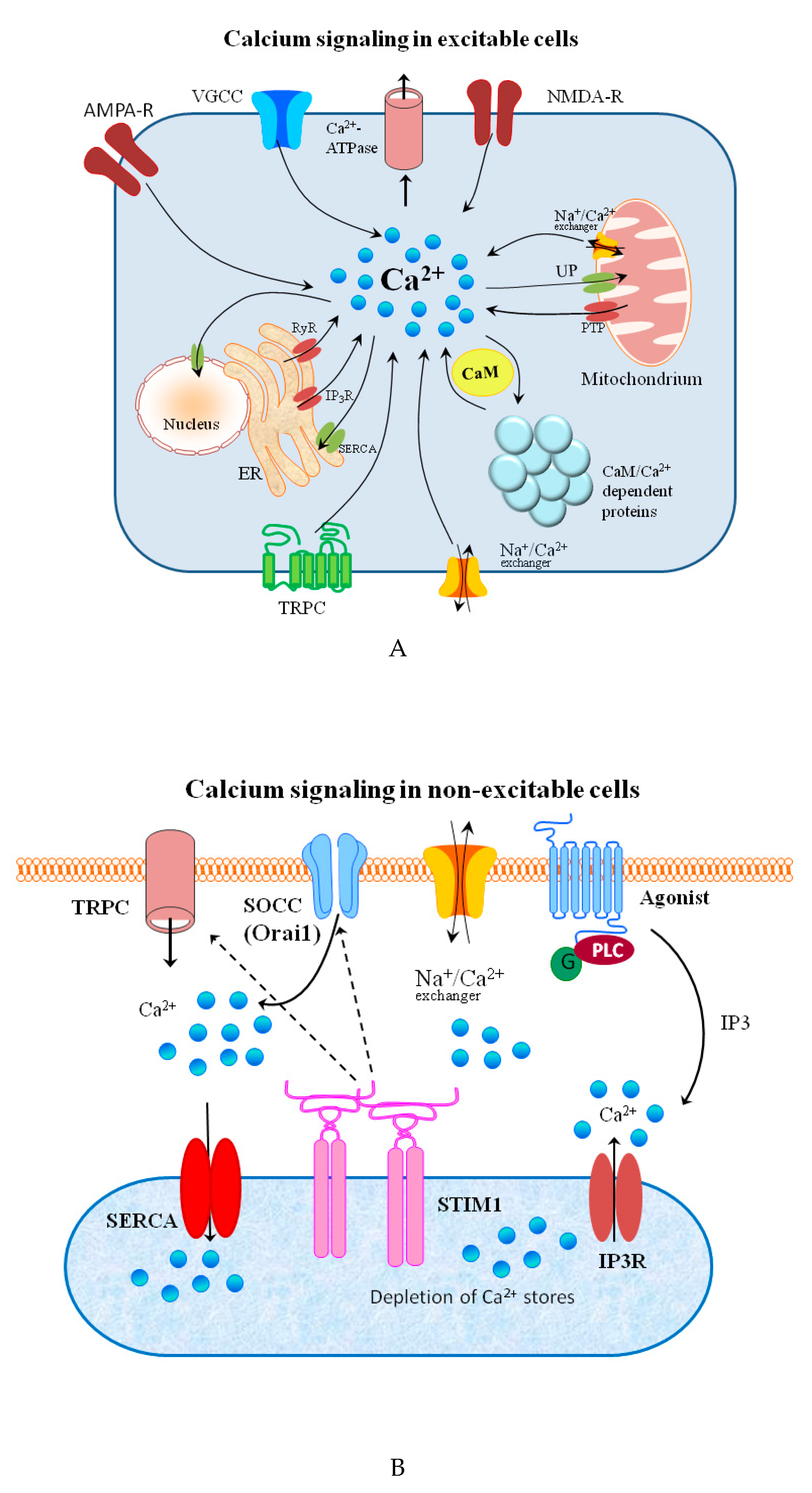
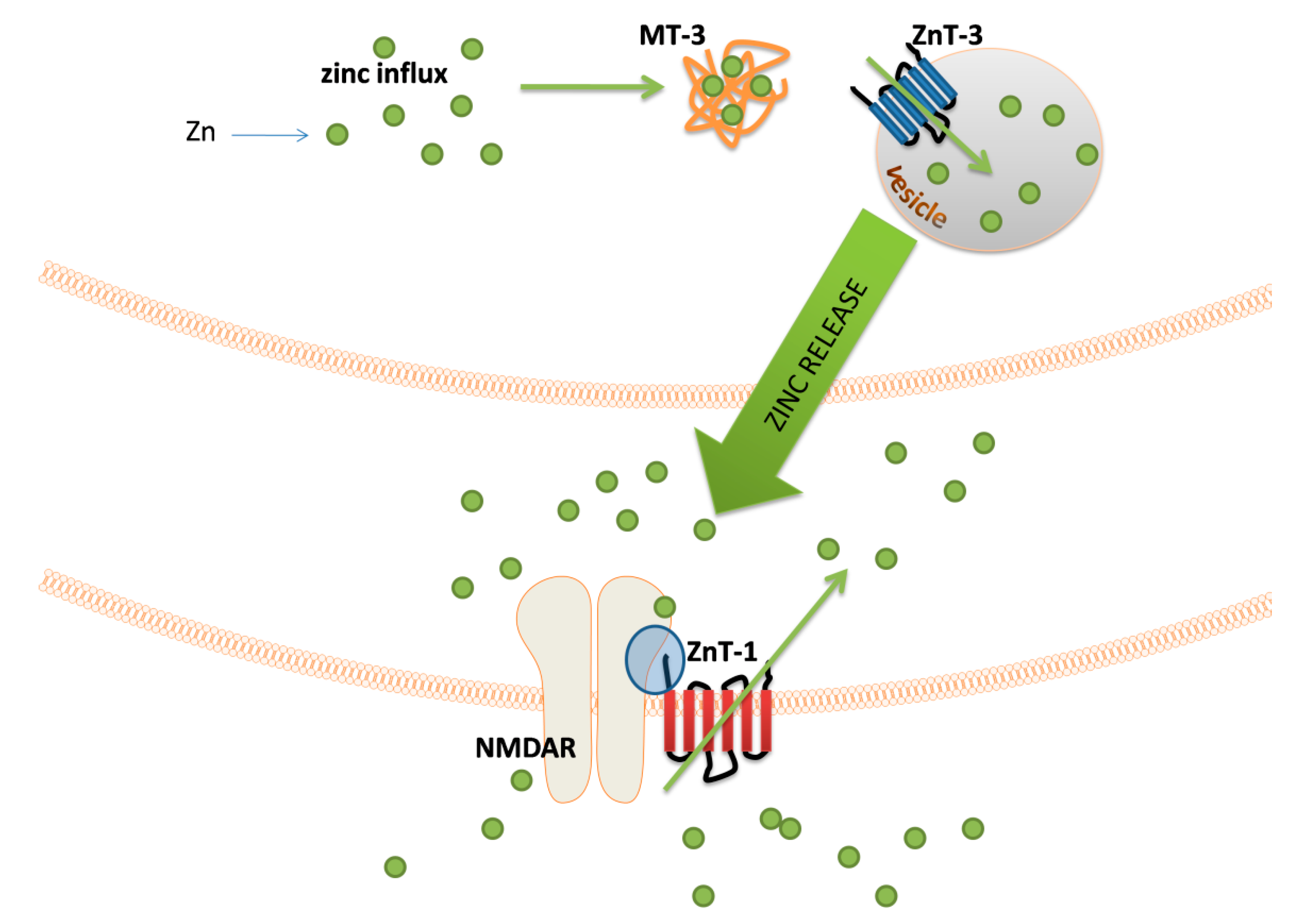
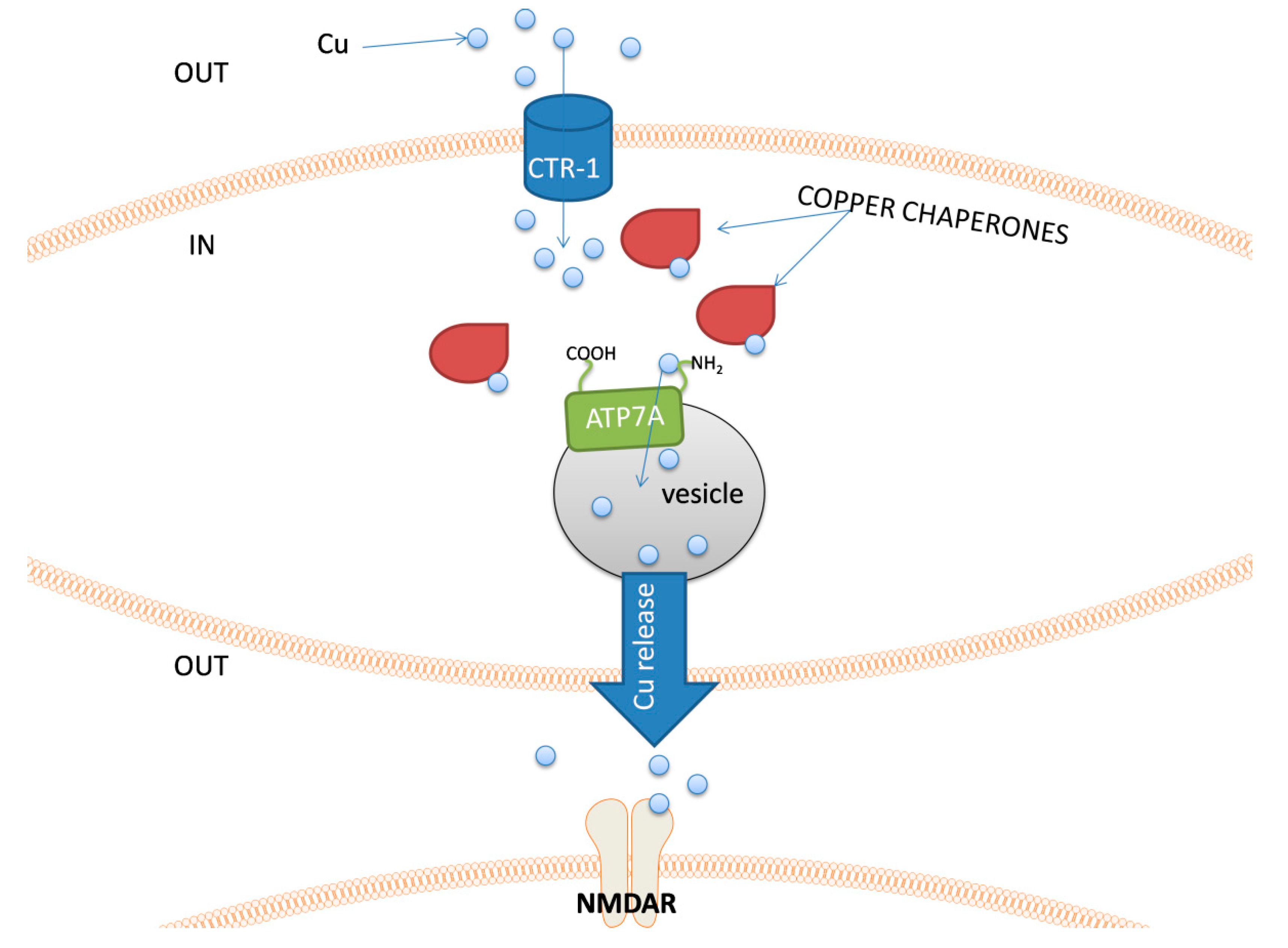
| Metal | Metal Ion Location during Signal Induction | Transporters and Receptors Involved in Sensing | Downstream Results |
|---|---|---|---|
| Mg | Intracellular | HIP14 MRS2 MMgT1/2 | - suppression of ROS toxicity - dynamics of cytoskeleton - ribosomal biosynthesis, regulation of translation via mTOR pathway, - antagonizing the Ca2+ signal - PTP inhibition - repair of DNA damage - inhibition of protein aggregation |
| Extracellular | TRPM6 and TRPM7 MagT1 NIPA SLC41A1(controversial: Na+/Mg2+ transporter or Mg2+ sensor) CNNM (controversial: Na+/Mg2+ transporter or Mg2+ sensor) | - growth factor signaling - membrane stabilization - channel regulation - osteoblast apoptosis | |
| Ca | Intracellular | IP3Rs RYRs NFAT NAADP cADPR Calmodulin STIM1 | - gene transcription - T-cell activation and development - CaMKs activation - insulin synthesis - fertilization - learning and memory |
| Extracellular | TRPMs TRPCs VGCCs Kinases Caspase-3 CaSR Orai1 (Calcium Release-Activated Calcium Modulator 1) | - membrane potential modulation - response to many kinds of stresses - signal transduction - neuronal synaptic transmission - apoptosis - regulation of PTH - cell proliferation, mobility | |
| Zn | Intracellular | MT-3 ZnT-3 ZnT-1 | - modulation of mitochondrial activity - vesicle formation - regulation of postsynaptic signal transduction |
| Extracellular | NMDAR APMAR VGCCs | - synaptic signal transduction and/or neuromodulation | |
| Cu | Intracellular | Chaperones ATP7A | - proper functioning of mitochondria - vesicle formation - regulation of cell redox processes |
| Extracellular | PAM DBH NMDAR AMPAR | - neurotransmitter synthesis modulation - reduced influx of Ca2+ - modulation of GABA and other amino-acids receptors | |
| Fe | Intracellular (LIP) | RE-BP bound to IRE at mRNA CDK1-JAK1-STAT3 pathway PHD2 | - ferritin and FPN1 translation; suppression of translation of TFRC and DMT1 - tumor cell proliferation - apoptosis - ferroptosis |
| Extracellular (iron-Tf) | HFE/TFRC TFR2 | - hepcidin expression |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywoszyńska, K.; Witkowska, D.; Świątek-Kozłowska, J.; Szebesczyk, A.; Kozłowski, H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules 2020, 10, 1417. https://doi.org/10.3390/biom10101417
Krzywoszyńska K, Witkowska D, Świątek-Kozłowska J, Szebesczyk A, Kozłowski H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules. 2020; 10(10):1417. https://doi.org/10.3390/biom10101417
Chicago/Turabian StyleKrzywoszyńska, Karolina, Danuta Witkowska, Jolanta Świątek-Kozłowska, Agnieszka Szebesczyk, and Henryk Kozłowski. 2020. "General Aspects of Metal Ions as Signaling Agents in Health and Disease" Biomolecules 10, no. 10: 1417. https://doi.org/10.3390/biom10101417
APA StyleKrzywoszyńska, K., Witkowska, D., Świątek-Kozłowska, J., Szebesczyk, A., & Kozłowski, H. (2020). General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules, 10(10), 1417. https://doi.org/10.3390/biom10101417






