General Aspects of Metal Ions as Signaling Agents in Health and Disease
Abstract
:1. Introduction
2. Magnesium
3. Calcium
4. Zinc
5. Copper
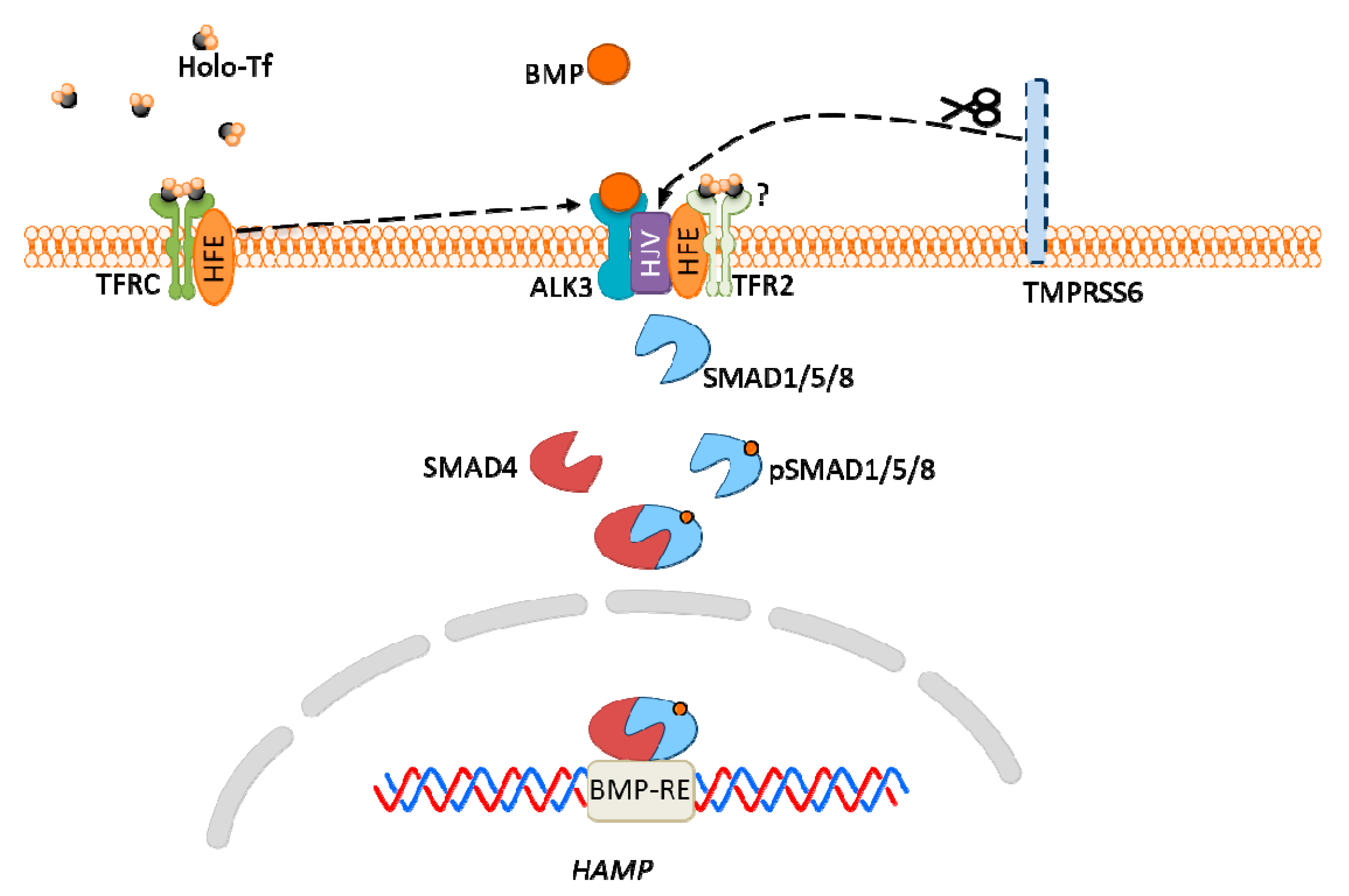
6. Iron
7. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ross, B.; Mehtal, S.; Zhang, J. Molecular tools for acute spatiotemporal manipulation of signal transduction. Curr. Opin. Chem. Biol. 2016, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Lqbal, J.; Zaidi, M.; Avadhani, N.G. Cell signaling. Mol. Integr. Physiol. Musculoskelet. Syst. 2010, 1211, 3–8. [Google Scholar] [CrossRef]
- Penner, R.; Neher, E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J. Exp. Biol. 1988, 139, 329–345. [Google Scholar] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamano, H.; Koike, Y.; Nakada, H.; Shakushi, Y.; Takeda, A. Significance of synaptic Zn2+ signaling in zincergic and non-zincergic synapses in the hippocampus in cognition. J. Trace Elem. Med. Biol. 2016, 38, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Heja, L.; Simon, A.; Jablonkai, I.; Kovacs, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, C.; Weth, A.; Walcher, S.; Lax, C.; Baumgartner, W. Modeling of Zinc Dynamics in the Synaptic Cleft: Implications for Cadherin Mediated Adhesion and Synaptic Plasticity. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamano, H.; Morioka, H.; Nishio, R.; Takeuchi, A.; Takeda, A. Blockade of Rapid Influx of Extracellular Zn2+ into Nigral Dopaminergic Neurons Overcomes Paraquat-Induced Parkinson’s Disease in Rats. Mol. Neurobiol. 2019, 56, 4539–4548. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef]
- Ashraf, A.; Michaelides, C.; Walker, T.A.; Ekonomou, A.; Suessmilch, M.; Sriskanthanathan, A.; Abraha, S.; Parkes, A.; Parkes, H.G.; Geraki, K.; et al. Regional Distributions of Iron, Copper and Zinc and Their Relationships With Glia in a Normal Aging Mouse Model. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Prasad, R. Regional Distribution of Copper, Zinc and Iron in Brain of Wistar Rat Model for Non-Wilsonian Brain Copper Toxicosis. Indian J. Clin. Biochem. 2016, 31, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.; Lemberg, K.; Lamprecht, M.; Skouta, R.; Zaitsev, E.; Gleason, C.; Patel, D.; Bauer, A.; Cantley, A.; Yang, W.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Gimenez-Mascarell, P.; Gonzalez-Recio, I.; Fernandez-Rodriguez, C.; Oyenarte, I.; Mueller, D.; Luz Martinez-Chantar, M.; Alfonso Martinez-Cruz, L. Current Structural Knowledge on the CNNM Family of Magnesium Transport Mediators. Int. J. Mol. Sci. 2019, 20, 1135. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; You, J.; Zhao, N.; Xu, H. Magnesium Regulates Endothelial Barrier Functions through TRPM7, MagT1, and S1P1. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Yatsimirsky, K.B. ELECTRONIC-STRUCTURE, HYDRATION ENERGY AND STABILITY OF METAL AQUAIONS. Teor. I Eksperimentalnaya Khimiya 1994, 30, 1–11. [Google Scholar] [CrossRef]
- Binding, Transport and Storage of Metal Ions in Biological Cells; Royal Society of Chemistry: London, UK, 2014; Volume 2, pp. 1–911. [CrossRef] [Green Version]
- Kolisek, M.; Montezano, A.C.; Sponder, G.; Anagnostopoulou, A.; Vormann, J.; Touyz, R.M.; Aschenbach, J.R. PARK7/DJ-1 dysregulation by oxidative stress leads to magnesium deficiency: Implications in degenerative and chronic diseases. Clin. Sci. 2015, 129, 1143–1150. [Google Scholar] [CrossRef]
- Shahi, A.; Aslani, S.; Ataollahi, M.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Romani, A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 2007, 458, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [Green Version]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. Embo J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef]
- Schindl, R.; Weghuber, J.; Romanin, C.; Schweyen, R.J. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys. J. 2007, 93, 3872–3883. [Google Scholar] [CrossRef] [Green Version]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. Am. J. Physiol. Cell Physiol. 2008, 294, C495–C502. [Google Scholar] [CrossRef] [Green Version]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, F.-Y.; Lenardo, M.J.; Chaigne-Delalande, B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnes. Res. 2011, 24, S109–S114. [Google Scholar] [CrossRef]
- Holmes, D.; Carroll, K.; Brodeur, S.; Pashine, A. Characterizing the intracellular magnesium transporter MagT1 in murine lymphocyte function. J. Immunol. 2016, 196. [Google Scholar]
- Wu, N.; Veillette, A. IMMUNOLOGY Magnesium in a signalling role. Nature 2011, 475, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Goytain, A.; Hines, R.M.; Quamme, G.A. Functional characterization of NIPA2, a selective Mg2+ transporter. Am. J. Physiol. Cell Physiol. 2008, 295, C944–C953. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, Y.; Zhang, P.; Wang, J.; Wu, Y.; Wu, X.; Netoff, T.; Jiang, Y. Functional Study of NIPA2 Mutations Identified from the Patients with Childhood Absence Epilepsy. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Liu, N.-N.; Xie, H.; Xiang-wei, W.-S.; Gao, K.; Wang, T.-S.; Jiang, Y.-W. The absence of NIPA2 enhances neural excitability through BK (big potassium) channels. CNS Neurosci. Ther. 2019, 25, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, W.-L.; Yang, B.; Sun, J.; Yang, M.-W. NIPA2 regulates osteoblast function via its effect on apoptosis pathways in type 2 diabetes osteoporosis. Biochem. Biophys. Res. Commun. 2019, 513, 883–890. [Google Scholar] [CrossRef]
- Funato, Y.; Yamazaki, D.; Mizukami, S.; Du, L.; Kikuchi, K.; Miki, H. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014, 124, 5398–5410. [Google Scholar] [CrossRef] [Green Version]
- Funato, Y.; Furutani, K.; Kurachi, Y.; Miki, H. CrossTalk proposal: CNNM proteins are Na+/Mg2+ exchangers playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. Lond. 2018, 596, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Kolisek, M.; Sponder, G.; Pilchova, I.; Cibulka, M.; Tatarkova, Z.; Werner, T.; Racay, P. Magnesium Extravaganza: A Critical Compendium of Current Research into Cellular Mg2+ Transporters Other than TRPM6/7. Rev. Physiol. Biochem. Pharmacol. 2019, 176, 65–105. [Google Scholar] [CrossRef]
- Sponder, G.; Mastrototaro, L.; Kurth, K.; Merolle, L.; Zhang, Z.; Abdulhanan, N.; Smorodchenko, A.; Wolf, K.; Fleig, A.; Penner, R.; et al. Human CNNM2 is not a Mg2+ transporter per se. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1223–1240. [Google Scholar] [CrossRef]
- Hardy, S.; Uetani, N.; Wong, N.; Kostantin, E.; Labbe, D.P.; Begin, L.R.; Mes-Masson, A.; Miranda-Saavedra, D.; Tremblay, M.L. The protein tyrosine phosphatase PRL-2 interacts with the magnesium transporter CNNM3 to promote oncogenesis. Oncogene 2015, 34, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.S.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg center dot ATP-regulated divalent cation channel required for cell viability (vol 411, pg 590, 2001). Nature 2001, 412, 660. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.R.; Xiao, C.Y.; Jia, Y.Y.; Guo, J.L.; Jiang, J.M.; Liu, P.Q. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Stritt, S.; Nurden, P.; Favier, R.; Favier, M.; Ferioli, S.; Gotru, S.K.; van Eeuwijk, J.M.M.; Schulze, H.; Nurden, A.T.; Lambert, M.P.; et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg2+ homeostasis and cytoskeletal architecture. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular Smooth Muscle Cell Differentiation to an Osteogenic Phenotype Involves TRPM7 Modulation by Magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 Disrupts Embryonic Development and Thymopoiesis Without Altering Mg(2+) Homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, A.; Moscheni, C.; Trapani, V.; Wolf, F.I.; Farruggia, G.; Sargenti, A.; Iotti, S.; Maier, J.A.M.; Castiglioni, S. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef] [Green Version]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelbeek, J.; Clark, K.; Venselaar, H.; Huynen, M.A.; van Leeuwen, F.N. The alpha-kinase family: An exceptional branch on the protein kinase tree. Cell. Mol. Life Sci. 2010, 67, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, N.; Lou, L.; Al-Saadi, N.; Tetteh, S.; Runnels, L.W. The kinase activity of the channel-kinase protein TRPM7 regulates stability and localization of the TRPM7 channel in polarized epithelial cells. J. Biol. Chem. 2018, 293, 11491–11504. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; El-Husseini, A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr. Opin. Neurobiol. 2005, 15, 527–535. [Google Scholar] [CrossRef]
- Mies, F.; Spriet, C.; Heliot, L.; Sariban-Sohraby, S. Epithelial Na+ channel stimulation by n-3 fatty acids requires proximity to a membrane-bound A-kinase-anchoring protein complexed with protein kinase A and phosphodiesterase. J. Biol. Chem. 2007, 282, 18339–18347. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.; Platano, D.; Olcese, R.; Costantin, J.L.; Stefani, E.; Birnbaumer, L. Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc. Natl. Acad. Sci. USA 1998, 95, 4690–4695. [Google Scholar] [CrossRef] [Green Version]
- Singaraja, R.R.; Huang, K.; Sanders, S.S.; Milnerwood, A.J.; Hines, R.; Lerch, J.P.; Franciosi, S.; Drisdel, R.C.; Vaid, K.; Young, F.B.; et al. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet. 2011, 20, 3899–3909. [Google Scholar] [CrossRef] [Green Version]
- Goytain, A.; Quamme, G.A. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol. Genom. 2005, 21, 337–342. [Google Scholar] [CrossRef]
- Kolisek, M.; Nestler, A.; Vormann, J.; Schweigel-Roentgen, M. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am. J. Physiol. Cell Physiol. 2012, 302, C318–C326. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ramirez, M.; Rodriguez-Moran, M.; Reyes-Romero, M.A.; Guerrero-Romero, F. Effect of oral magnesium supplementation on the transcription of TRPM6, TRPM7, and SLC41A1 in individuals newly diagnosed of pre-hypertension. A randomized, double-blind, placebo-controlled trial. Magnes. Res. 2017, 30, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Hotta, K.; Suzuki, K.; Oka, K. GABA-Induced Intracellular Mg2+ Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr. Biol. 2018, 28, 3984–3991.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncrief, M.B.C.; Maguire, M.E. Magnesium transport in prokaryotes. J. Biol. Inorg. Chem. 1999, 4, 523–527. [Google Scholar] [CrossRef]
- Moore, E.W. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J. Clin. Investig. 1970, 49, 318–334. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium microdomains: Organization and function. Cell Calcium 2006, 40, 405–412. [Google Scholar] [CrossRef]
- Rimessi, A.; Pedriali, G.; Vezzani, B.; Tarocco, A.; Marchi, S.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Interorganellar calcium signaling in the regulation of cell metabolism: A cancer perspective. Semin. Cell Dev. Biol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Munaron, L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int. J. Mol. Med. 2002, 10, 671–676. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Faouzi, M.; Kilch, T.; Horgen, F.D.; Fleig, A.; Penner, R. The TRPM7 channel kinase regulates store-operated calcium entry. J. Physiol. Lond. 2017, 595, 3165–3180. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lu, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Chu, P.; Gu, J.; Zhang, H.; Feng, R.; Wen, X.; Wang, D.; Xiong, W.; Wang, T.; Yin, S. The influence of Ca2+ concentration on voltage-dependent L-type calcium channels’ expression in the marbled eel (Anguilla marmorata). Gene 2020, 722. [Google Scholar] [CrossRef]
- Brown, B.M.; Shim, H.; Christophersen, P.; Wulff, H. Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 219–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Guerini, D. The Ca2+ pumps and the Na+/Ca2+ exchangers. Biometals 1998, 11, 319–330. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodriguez-Moreno, A. Calcium Dynamics and Synaptic Plasticity. Adv. Exp. Med. Biol. 2020, 1131, 965–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Ma, J.; Parrington, J.; Calcraft, P.J.; Galione, A.; Evans, A.M. Calcium signaling via two-pore channels: Local or global, that is the question. Am. J. Physiol. Cell Physiol. 2010, 298, C430–C441. [Google Scholar] [CrossRef] [Green Version]
- Fracchia, K.M.; Pai, C.Y.; Walsh, C.M. Modulation of T cell metabolism and function through calcium signaling. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Li, H.Y.; Zhang, Y.; Li, C.; Li, K.; Ai, K.T.; Yang, J.L. Ca2+-Calcineurin Axis-Controlled NFAT Nuclear Translocation Is Crucial for Optimal T Cell Immunity in an Early Vertebrate. J. Immunol. 2020, 204, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001, 19, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Leger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.-P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santulli, G.; Marks, A.R. Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging. Curr. Mol. Pharmacol. 2015, 8, 206–222. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, M.H.; Havelka, A.M.; Brnjic, S.; Shoshan, M.C.; Linder, S. Charting calcium-regulated apoptosis pathways using chemical biology: Role of calmodulin kinase II. Bmc Chem. Biol. 2008, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Danese, A.; Patergnani, S.; Bonora, M.; Wieckowski, M.R.; Previati, M.; Giorgi, C.; Pinton, P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim. Biophys. Acta Bioenergy 2017, 1858, 615–627. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, C.; Lian, Y.J.; Zhang, H.F.; Meng, X.H.; Yu, M.Y.; Xie, N.C. Role of the mitochondrial calcium uniporter in Mg2+-free-induced epileptic hippocampal neuronal apoptosis. Int. J. Neurosci. 2020, 9. [Google Scholar] [CrossRef]
- Lukas, T.J.; Haiech, J.; Lau, W.; Craig, T.A.; Zimmer, W.E.; Shattuck, R.L.; Shoemaker, M.O.; Watterson, D.M. CALMODULIN AND CALMODULIN-REGULATED PROTEIN-KINASES AS TRANSDUCERS OF INTRACELLULAR CALCIUM SIGNALS. Cold Spring Harb. Symp. Quant. Biol. 1988, 53, 185–193. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Jaiswal, S.; Lu, H.P. Compressive-force induced activation of apo-calmodulin in protein signalling. Phys. Chem. Chem. Phys. PCCP 2020, 22, 1092–1096. [Google Scholar] [CrossRef]
- Song, Z.X.; Chen, Q.; Ding, Q.; Zheng, F.; Li, C.W.; Xu, L.P.; Wang, H.B. Function of Ca2+-/calmodulin-dependent protein kinase IV in Ca2+-stimulated neuronal signaling and behavior. Sci. China Life Sci. 2015, 58, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Murao, K.; Sayo, Y.; Imachi, H.; Cao, W.M.; Ohtsuka, S.; Niimi, M.; Tokumitsu, H.; Inuzuka, H.; Wong, N.C.W.; et al. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes 2004, 53, 1475–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalobo, A.; Berchtold, M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020, 21, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoorn, E.J.; Zietse, R. Disorders of calcium and magnesium balance: A physiology-based approach. Pediatr. Nephrol. 2013, 28, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Bertoni-Freddari, C.; Marcellini, F.; Malavolta, M. Brain, aging and neurodegeneration: Role of zinc ion availability. Prog. Neurobiol. 2005, 75, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Klitenick, M.A.; Manton, W.I.; Kirkpatrick, J.B. CYTOARCHITECTONIC DISTRIBUTION OF ZINC IN THE HIPPOCAMPUS OF MAN AND THE RAT. Brain Res. 1983, 273, 335–339. [Google Scholar] [CrossRef]
- Lee, H.C.; Edmonds, M.E.; Duncan, F.E.; O’Halloran, T.V.; Woodruff, T.K. Zinc exocytosis is sensitive to myosin light chain kinase inhibition in mouse and human eggs. Mol. Hum. Reprod. 2020, 26, 228–239. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuhiro, K.; Dean, J. Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy. Dev. Cell 2018, 46, 627–640.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomianka, L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience 1992, 48, 325–352. [Google Scholar] [CrossRef]
- Haug, F.M.S. Electron microscopical localization of zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie 1967, 8, 355–368. [Google Scholar] [CrossRef]
- Evstratova, A.; Toth, K. Information processing and synaptic plasticity at hippocampal mossy fiber terminals. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.A.; Zhang, X.-L.; Sullivan, A.P.; Vose, L.R.; Moghadam, A.A.; Fried, V.A.; Stanton, P.K. Zinc enhances hippocampal long-term potentiation at CA1 synapses through NR2B containing NMDA receptors. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Bastos, F.M.C.; Lopes, S.A.; Corceiro, V.N.; Matias, C.M.; Dionisio, J.C.; Sampaio dos Aidos, F.D.S.; Mendes, P.J.; Santos, R.M.; Quinta-Ferreira, R.M.; Emilia Quinta-Ferreira, M. Postsynaptic zinc potentiation elicited by KCl depolarization at hippocampal mossy fiber synapses. Gen. Physiol. Biophys. 2017, 36, 289–296. [Google Scholar] [CrossRef]
- Takeda, A.; Fuke, S.; Ando, M.; Oku, N. Positive modulation of long-term potentiation at hippocampal ca1 synapses by low micromolar concentrations of zinc. Neuroscience 2009, 158, 585–591. [Google Scholar] [CrossRef]
- Takeda, A.; Ando, M.; Kanno, S.; Oku, N. Unique response of zinc in the hippocampus to behavioral stress and attenuation of subsequent mossy fiber long-term potentiation. Neurotoxicology 2009, 30, 712–717. [Google Scholar] [CrossRef]
- Vogt, K.; Mellor, J.; Tong, G.; Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 2000, 26, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Kay, A.R. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J. Neurosci. 2003, 23, 6847–6855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, A.R.; Toth, K. Is Zinc a Neuromodulator? Sci. Signal. 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hough, C.J.; Frederickson, C.J.; Sarvey, J.M. Induction of mossy fiber -> CA3 long-term potentiation requires translocation of synaptically released Zn2+. J. Neurosci. 2001, 21, 8015–8025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, J.C.; Hollopeter, G.; Thomas, S.A.; Froelick, G.J.; Palmiter, R.D. Disruption of the metallothionein-III gene in mice: Analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J. Neurosci. 1997, 17, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Palumaa, P.; Eriste, E.; Njunkova, O.; Pokras, L.; Jornvall, H.; Sillard, R. Brain-specific metallothionein-3 has higher metal-binding capacity than ubiquitous metallothioneins and binds metals noncooperatively. Biochemistry 2002, 41, 6158–6163. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [Green Version]
- Ben Mimouna, S.; Le Charpentier, T.; Lebon, S.; Van Steenwinckel, J.; Messaoudi, I.; Gressens, P. Involvement of the synapse-specific zinc transporter ZnT3 in cadmium-induced hippocampal neurotoxicity. J. Cell. Physiol. 2019, 234, 15872–15884. [Google Scholar] [CrossRef]
- Quinta-Ferreira, M.E.; Sampaio dos Aidos, F.D.S.; Matias, C.M.; Mendes, P.J.; Dionisio, J.C.; Santos, R.M.; Rosario, L.M.; Quinta-Ferreira, R.M. Modelling zinc changes at the hippocampal mossy fiber synaptic cleft. J. Comput. Neurosci. 2016, 41, 323–337. [Google Scholar] [CrossRef]
- Blakemore, L.J.; Trombley, P.Q. Mechanisms of zinc modulation of olfactory bulb AMPA receptors. Neuroscience 2019, 410, 160–175. [Google Scholar] [CrossRef]
- Ha, H.T.T.; Leal-Ortiz, S.; Lalwani, K.; Kiyonaka, S.; Hamachi, I.; Mysore, S.P.; Montgomery, J.M.; Garner, C.C.; Huguenard, J.R.; Kim, S.A. Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.; Chung, J.-M. Modulation of Dopaminergic Neuronal Excitability by Zinc through the Regulation of Calcium-related Channels. Exp. Neurobiol. 2019, 28, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Ekstein, D.; Benninger, F.; Daninos, M.; Pitsch, J.; van Loo, K.M.J.; Becker, A.J.; Yaari, Y. Zinc induces long-term upregulation of T-type calcium current in hippocampal neurons in vivo. J. Physiol. Lond. 2012, 590, 5895–5905. [Google Scholar] [CrossRef] [PubMed]
- Sindreu, C.; Bayes, A.; Altafaj, X.; Perez-Clausell, J. Zinc transporter-1 concentrates at the postsynaptic density of hippocampal synapses. Mol. Brain 2014, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellone, M.; Pelucchi, S.; Alberti, L.; Geneazzani, A.A.; Di Luca, M.; Gardoni, F. Zinc transporter-1: A novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 2015, 132, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Schulien, A.J.; Justice, J.A.; Di Maio, R.; Wills, Z.P.; Shah, N.H.; Aizenman, E. Zn2+-induced Ca2+ release via ryanodine receptors triggers calcineurin-dependent redistribution of cortical neuronal Kv2.1 K+ channels. J. Physiol. Lond. 2016, 594, 2647–2659. [Google Scholar] [CrossRef] [Green Version]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef] [Green Version]
- Bredesen, D.E. Metabolic profiling distinguishes three subtypes of Alzheimer’s disease. Aging-Us 2015, 7, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, R.; Ide-Ektessabi, A.; Ikeda, K.; Mizuno, Y.; Fujisawa, S.; Takeuchi, T.; Ohta, T. Investigation of cellular metallic elements in single neurons of human brain tissues. Neuroreport 2002, 13, 1817–1820. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Gardner, B.; Dieriks, B.V.; Cameron, S.; Mendis, L.H.S.; Turner, C.; Faull, R.L.M.; Curtis, M.A. Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Samudralwar, D.L.; Diprete, C.C.; Ni, B.F.; Ehmann, W.D.; Markesbery, W.R. Elemental imbalances in the olfactory pathway in Alzheimers-diseasE. J. Neurol. Sci. 1995, 130, 139–145. [Google Scholar] [CrossRef]
- Lim, J.H.; Davis, G.E.; Wang, Z.; Li, V.; Wu, Y.; Rue, T.C.; Storm, D.R. Zicam-Induced Damage to Mouse and Human Nasal Tissue. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [Green Version]
- Eom, J.-W.; Lee, J.-M.; Koh, J.-Y.; Kim, Y.-H. AMP-activated protein kinase contributes to zinc-induced neuronal death via activation by LKB1 and induction of Bim in mouse cortical cultures. Mol. Brain 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Shi, W.; Zhao, Y.; Ji, X.; Liu, K.J. Zinc accumulation in mitochondria promotes ischemia-induced BBB disruption through Drp1-dependent mitochondria fission. Toxicol. Appl. Pharmacol. 2019, 377. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, N.B.; Stanika, R.I.; Kazanina, G.; Villanueva, I.; Andrews, S.B. The interactive role of zinc and calcium in mitochondrial dysfunction and neurodegeneration. J. Neurochem. 2014, 128, 592–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharaf, M.S.; Van den Heuvel, M.R.; Stevens, D.; Kamunde, C. Zinc and calcium modulate mitochondrial redox state and morphofunctional integrity. Free Radic. Biol. Med. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yuan, L.; He, W.; Yang, X. Zinc ions regulate opening of tight junction favouring efflux of macromolecules via the GSK3 beta/snail-mediated pathway. Metallomics 2018, 10, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Vasudevaraju, P.; Bharathi, T.J.; Shamasundar, N.M.; Subba Rao, K.; Balaraj, B.M.; Ksj, R.; Ts, S.R. New evidence on iron, copper accumulation and zinc depletion and its correlation with DNA integrity in aging human brain regions. Indian J. Psychiatry 2010, 52, 140–144. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.-x.; Li, L.-x.; Liu, Y.-q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018, 179, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H. Significance of the degree of synaptic Zn2+ signaling in cognition. Biometals 2016, 29, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kucma, M.; Szewczyk, B.; Sadlik, K.; Piekoszewski, W.; Trela, F.; Opoka, W.; Poleszak, E.; Pilc, A.; Nowak, G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013, 151, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Kotarska, K.; Siwek, A.; Olech, L.; Kuter, K. Antidepressant activity of zinc: Further evidence for the involvement of the serotonergic system. Pharmacol. Rep. 2017, 69, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General Aspects of Metal Toxicity. Curr. Med. Chem. 2014, 21, 3721–3740. [Google Scholar] [CrossRef] [PubMed]
- Doreulee, N.; Yanovsky, Y.; Haas, H.L. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus 1997, 7, 666–669. [Google Scholar] [CrossRef]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef] [Green Version]
- Gaier, E.D.; Miller, M.B.; Ralle, M.; Aryal, D.; Wetsel, W.C.; Mains, R.E.; Eipper, B.A. Peptidylglycine alpha-amidating monooxygenase heterozygosity alters brain copper handling with region specificity. J. Neurochem. 2013, 127, 605–619. [Google Scholar] [CrossRef]
- Schmidt, K.; Ralle, M.; Schaffer, T.; Jayakanthan, S.; Bari, B.; Muchenditsi, A.; Lutsenko, S. ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine--hydroxylase. J. Biol. Chem. 2018, 293, 20085–20098. [Google Scholar] [CrossRef] [Green Version]
- Kapkaeva, M.R.; Popova, O.V.; Kondratenko, R.V.; Rogozin, P.D.; Genrikhs, E.E.; Stelmashook, E.V.; Skrebitsky, V.G.; Khaspekov, L.G.; Isaev, N.K. Effects of copper on viability and functional properties of hippocampal neurons in vitro. Exp. Toxicol. Pathol. 2017, 69, 259–264. [Google Scholar] [CrossRef]
- Kardos, J.; Kovacs, I.; Hajos, F.; Kalman, M.; Simonyi, M. Nerve-endings from rat-brain tissue release copper upon depolarization—A possible role in regulating neuronal excitability. Neurosci. Lett. 1989, 103, 139–144. [Google Scholar] [CrossRef]
- Gaier, E.D.; Rodriguiz, R.M.; Zhou, J.; Ralle, M.; Wetsel, W.C.; Eipper, B.A.; Mains, R.E. In vivo and in vitro analyses of amygdalar function reveal a role for copper. J. Neurophysiol. 2014, 111, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Weber, N.L.; Smith, J.P. Copper Inhibits NMDA Receptor-Independent LTP and Modulates the Paired-Pulse Ratio after LTP in Mouse Hippocampal Slices. Int. J. Alzheimer’s Dis. 2011, 2011, 864753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maryon, E.B.; Molloy, S.A.; Yu, K.I.H.; Kaplan, J.H. Rate and Regulation of Copper Transport by Human Copper Transporter 1 (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Copper accumulation by cultured astrocytes. Neurochem. Int. 2010, 56, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, D.; Kang, Y.J. Copper chaperone for superoxide dismutase-1 transfers copper to mitochondria but does not affect cytochrome c oxidase activity. Exp. Biol. Med. 2013, 238, 1017–1023. [Google Scholar] [CrossRef]
- Vest, K.E.; Paskavitz, A.L.; Lee, J.B.; Padilla-Benavides, T. Dynamic changes in copper homeostasis and post-transcriptional regulation of Atp7a during myogenic differentiation. Metallomics 2018, 10, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Horning, M.S.; Trombley, P.Q. Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J. Neurophysiol. 2001, 86, 1652–1660. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, C.; Baranowska-Bosiacka, I.; Gavazzo, P. Multiple effects of copper on NMDA receptor currents. Brain Res. 2014, 1542, 20–31. [Google Scholar] [CrossRef]
- Meramat, A.; Rajab, N.F.; Shahar, S.; Sharif, R.A. DNA damage, copper and lead associates with cognitive function among older adults. J. Nutr. Health Aging 2017, 21, 539–545. [Google Scholar] [CrossRef]
- Deibel, M.A.; Ehmann, W.D.; Markesbery, W.R. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106–1119. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N. Copper deficiency myelopathy (human swayback). Mayo Clin. Proc. 2006, 81, 1371–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlief, M.L.; West, T.; Craig, A.M.; Holtzman, D.M.; Gitlin, J.D. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. USA 2006, 103, 14919–14924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, C.; Magaki, S.; Schrag, M.; Ghosh, M.C.; Kirsch, W.M. Iron Regulatory Protein 2 is Involved in Brain Copper Homeostasis. J. Alzheimers Dis. 2009, 18, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C.; Munoz, B.; Sepulveda, F.J.; Urrutia, J.; Quiroz, M.; Luza, S.; De Ferrari, G.V.; Aguayo, L.G.; Opazo, C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011, 119, 78–88. [Google Scholar] [CrossRef]
- Yu, H.; Wang, D.; Zou, L.; Zhang, Z.; Xu, H.; Zhu, F.; Ren, X.; Xu, B.; Yuan, J.; Liu, J.; et al. Proteomic alterations of brain subcellular organelles caused by low-dose copper exposure: Implication for Alzheimer’s disease. Arch. Toxicol. 2018, 92, 1363–1382. [Google Scholar] [CrossRef]
- Xu, J.; Begley, P.; Church, S.J.; Patassini, S.; McHarg, S.; Kureishy, N.; Hollywood, K.A.; Waldvogel, H.J.; Liu, H.; Zhang, S.; et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: Metabolic basis for dementia. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.H.; Hogl, S.; Narayanan, U.; Zhang, L.; McHarg, S.; Xu, J.; Gong, D.; et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc. Diabetol. 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Xie, F.; Muzik, O. Alteration of Copper Fluxes in Brain Aging: A Longitudinal Study in Rodent Using (CuCl2)-Cu-64-PET/CT. Aging Dis. 2018, 9, 109–118. [Google Scholar] [CrossRef]
- Famitafreshi, H.; Karimian, M. Modulation of catalase, copper and zinc in the hippocampus and the prefrontal cortex in social isolation-induced depression in male rats. Acta Neurobiol. Exp. 2019, 79, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Han, O. Molecular mechanism of intestinal iron absorption. Metallomics 2011, 3, 103–109. [Google Scholar] [CrossRef]
- Inoue, K.; Nakai, Y.; Ueda, S.; Kamigaso, S.; Ohta, K.; Hatakeyama, M.; Hayashi, Y.; Otagiri, M.; Yuasa, H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G660–G668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laftah, A.; Latunde-Dada, G.; Fakih, S.; Hider, R.; Simpson, R.; Mckie, A. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br. J. Nutr. 2009, 101, 1150–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, S.; Garrick, M.; Arredondo, M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell Physiol. 2012, 302, C1780–C1785. [Google Scholar] [CrossRef] [PubMed]
- Lawen, A.; Lane, D. Mammalian Iron Homeostasis in Health and Disease: Uptake, Storage, Transport, and Molecular Mechanisms of Action. Antioxid. Redox Signal. 2013, 18, 2473–2507. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Pyrkosz-Bulska, M.; Szebesczyk, A.; Ostrowska, M. Iron Chelating Strategies in Systemic Metal Overload, Neurodegeneration and Cancer. Curr. Med. Chem. 2014, 21, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Et Biophys. Acta-Gen. Subj. 2012, 1820, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Yee, J. Hepcidin. Adv. Chronic Kidney Dis. 2019, 26, 298–305. [Google Scholar] [CrossRef]
- Rochette, L.; Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C. The iron-regulatory hormone hepcidin: A possible therapeutic target? Pharmacol. Ther. 2015, 146, 35–52. [Google Scholar] [CrossRef]
- Corradini, E.; Meynard, D.; Wu, Q.; Chen, S.; Ventura, P.; Pietrangelo, A.; Babitt, J. Serum and Liver Iron Differently Regulate the Bone Morphogenetic Protein 6 (BMP6)-SMAD Signaling Pathway in Mice. Hepatology 2011, 54, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Corradini, E.; Rozier, M.; Meynard, D.; Odhiambo, A.; Lin, H.; Feng, Q.; Migas, M.; Britton, R.; Babitt, J.; Fleming, R. Iron Regulation of Hepcidin Despite Attenuated Smad1,5,8 Signaling in Mice Without Transferrin Receptor 2 or Hfe. Gastroenterology 2011, 141, 1907–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, J.; Edwards, C.; Acton, R. HFE gene: Structure, function, mutations, and associated iron abnormalities. Gene 2015, 574, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Wu, Q.; Cheng, W.; Liu, W.; Zhao, Y.; Mayeur, C.; Schmidt, P.; Yu, P.; Wang, F.; et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood 2014, 124, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Fillebeen, C.; Daba, A.; Wagner, J.; Sebastiani, G.; Pantopoulos, K. Iron-Dependent Regulation of Hepcidin in Hjv-/- Mice: Evidence That Hemojuvelin Is Dispensable for Sensing Body Iron Levels. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Rubio, A.; Campanella, A.; Gourbeyre, O.; Artuso, I.; Bordini, J.; Gineste, A.; Latour, C.; Besson-Fournier, C.; Lin, H.; et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood 2016, 127, 2327–2336. [Google Scholar] [CrossRef] [Green Version]
- Rishi, G.; Secondes, E.; Subramaniam, V. Hemochromatosis: Evaluation of the dietary iron model and regulation of hepcidin. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2550–2556. [Google Scholar] [CrossRef]
- Taniguchi, R.; Kato, H.; Font, J.; Deshpande, C.; Wada, M.; Ito, K.; Ishitani, R.; Jormakka, M.; Nureki, O. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- di Patti, M.; Polticelli, F.; Cece, G.; Cutone, A.; Felici, F.; Persichini, T.; Musci, G. A structural model of human ferroportin and of its iron binding site. FEBS J. 2014, 281, 2851–2860. [Google Scholar] [CrossRef]
- Ward, D.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.; Leitz, D.; Kraut, S.; Brandenberger, C.; Agrawal, R.; Weissmann, N.; Muhlfeld, C.; Mall, M.; Altamura, S.; Muckenthaler, M. Disruption of the Hepcidin/Ferroportin Regulatory System Causes Pulmonary Iron Overload and Restrictive Lung Disease. Ebiomedicine 2017, 20, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazer, D.; Wilkins, S.; Darshan, D.; Mirciov, C.; Dunn, L.; Anderson, G. Ferroportin Is Essential for Iron Absorption During Suckling, But Is Hyporesponsive to the Regulatory Hormone Hepcidin. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torti, S.; Torti, F. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebber, C.; Muller, F.; Clemente, L.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastide, N.; Pierre, F.; Corpet, D. Heme Iron from Meat and Risk of Colorectal Cancer: A Meta-analysis and a Review of the Mechanisms Involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sornjai, W.; Van Long, F.; Pion, N.; Pasquer, A.; Saurin, J.; Marcel, V.; Diaz, J.; Mertani, H.; Smith, D. Iron and hepcidin mediate human colorectal cancer cell growth. Chem. Biol. Interact. 2020, 319. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.; Weisz, K.; Triner, D.; Xie, L.; Attili, D.; Pant, A.; Gyorffy, B.; Zhan, M.; Carter-Su, C.; et al. Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metab. 2016, 24, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Latunde-Dada, G. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Stockwell, B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.; Steinberg, G.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhao, Y.; Guo, J.; Zhao, S.; Song, L.; Fei, C.; Zhang, Z.; Li, X.; Chang, C. Iron overload promotes erythroid apoptosis through regulating HIF-1a/ROS signaling pathway in patients with myelodysplastic syndrome. Leuk. Res. 2017, 58, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kilari, S.; Pullakhandam, R.; Nair, K. Zinc inhibits oxidative stress-induced iron signaling and apoptosis in Caco-2 cells. Free Radic. Biol. Med. 2010, 48, 961–968. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Duan, J.; Yang, L.; Cai, Z.; Zhu, W.; Nie, Y.; He, J.; Xia, C.; Gong, Q.; et al. Bioactive iron oxide nanoparticles suppress osteoclastogenesis and ovariectomy-induced bone loss through regulating the TRAF6-p62-CYLD signaling complex. Acta Biomater. 2020, 103, 281–292. [Google Scholar] [CrossRef]
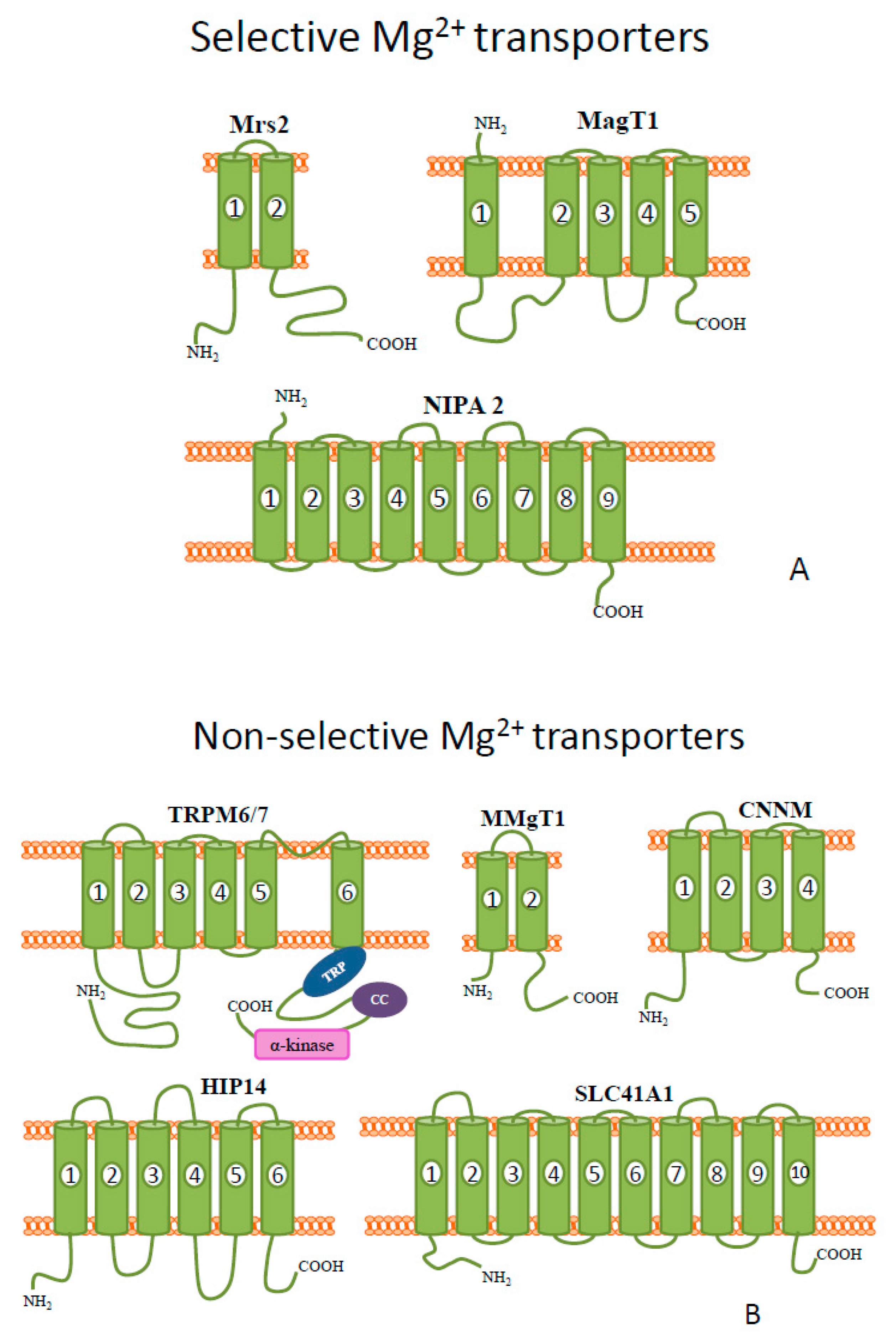
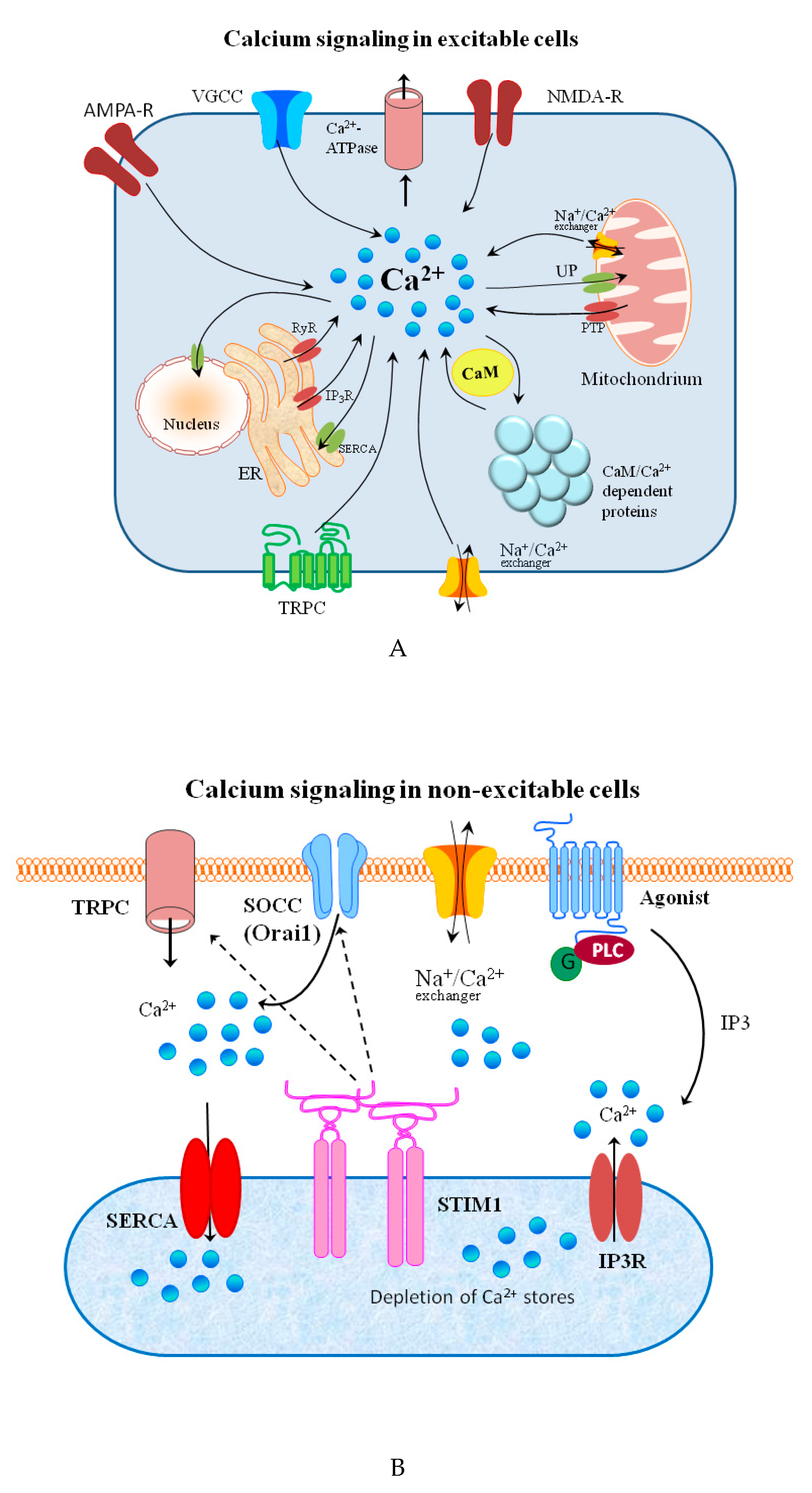
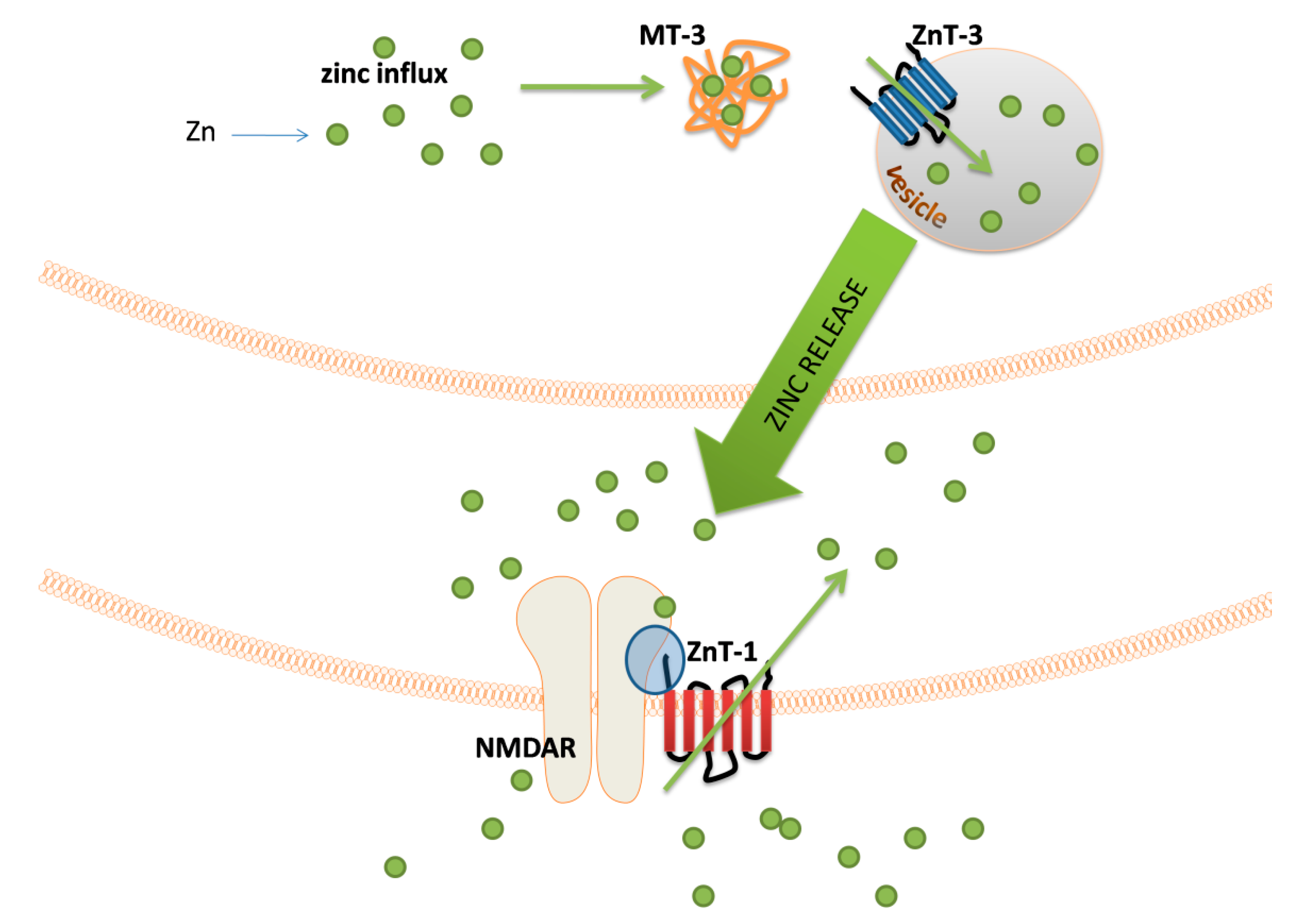
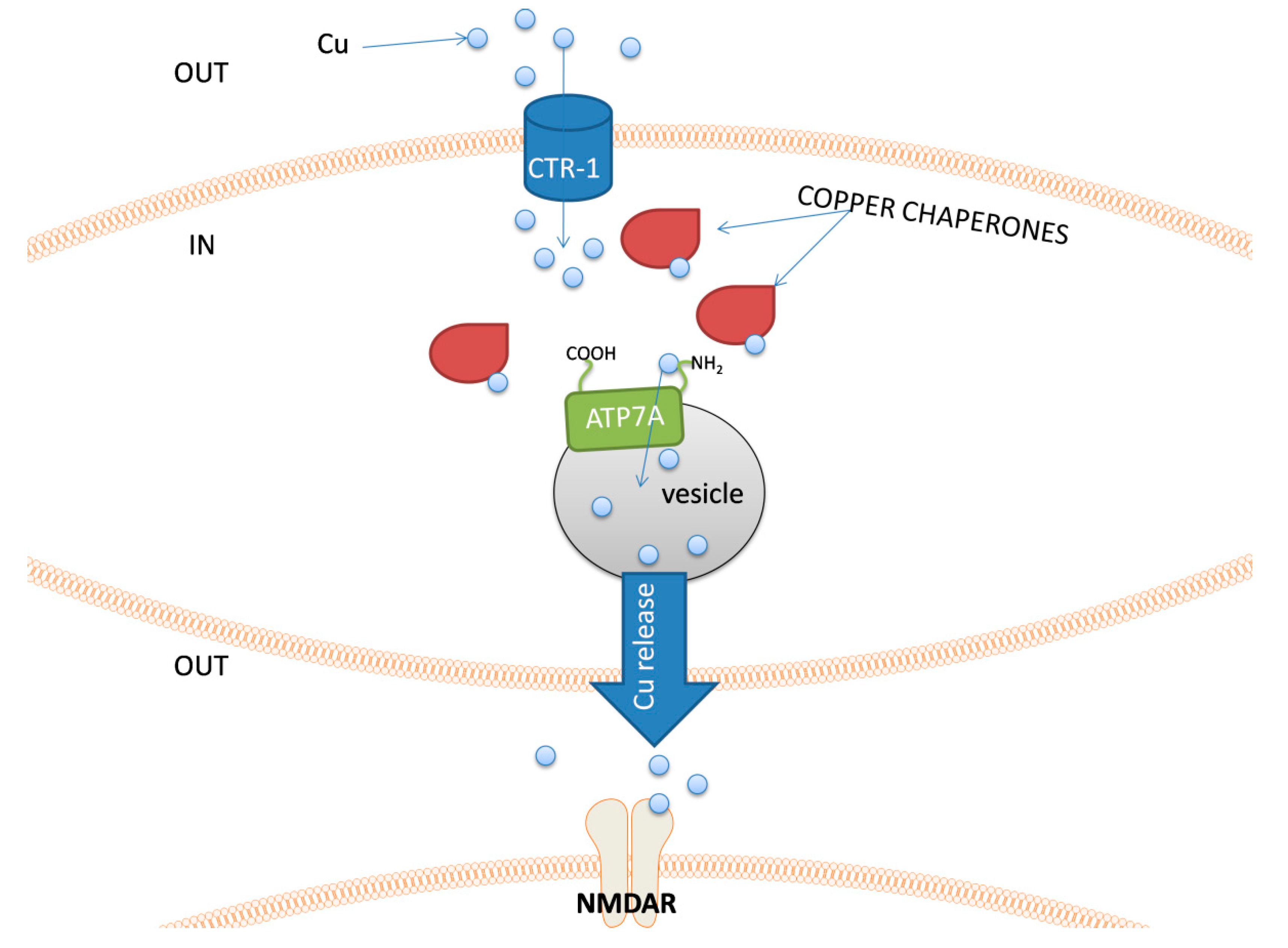
| Metal | Metal Ion Location during Signal Induction | Transporters and Receptors Involved in Sensing | Downstream Results |
|---|---|---|---|
| Mg | Intracellular | HIP14 MRS2 MMgT1/2 | - suppression of ROS toxicity - dynamics of cytoskeleton - ribosomal biosynthesis, regulation of translation via mTOR pathway, - antagonizing the Ca2+ signal - PTP inhibition - repair of DNA damage - inhibition of protein aggregation |
| Extracellular | TRPM6 and TRPM7 MagT1 NIPA SLC41A1(controversial: Na+/Mg2+ transporter or Mg2+ sensor) CNNM (controversial: Na+/Mg2+ transporter or Mg2+ sensor) | - growth factor signaling - membrane stabilization - channel regulation - osteoblast apoptosis | |
| Ca | Intracellular | IP3Rs RYRs NFAT NAADP cADPR Calmodulin STIM1 | - gene transcription - T-cell activation and development - CaMKs activation - insulin synthesis - fertilization - learning and memory |
| Extracellular | TRPMs TRPCs VGCCs Kinases Caspase-3 CaSR Orai1 (Calcium Release-Activated Calcium Modulator 1) | - membrane potential modulation - response to many kinds of stresses - signal transduction - neuronal synaptic transmission - apoptosis - regulation of PTH - cell proliferation, mobility | |
| Zn | Intracellular | MT-3 ZnT-3 ZnT-1 | - modulation of mitochondrial activity - vesicle formation - regulation of postsynaptic signal transduction |
| Extracellular | NMDAR APMAR VGCCs | - synaptic signal transduction and/or neuromodulation | |
| Cu | Intracellular | Chaperones ATP7A | - proper functioning of mitochondria - vesicle formation - regulation of cell redox processes |
| Extracellular | PAM DBH NMDAR AMPAR | - neurotransmitter synthesis modulation - reduced influx of Ca2+ - modulation of GABA and other amino-acids receptors | |
| Fe | Intracellular (LIP) | RE-BP bound to IRE at mRNA CDK1-JAK1-STAT3 pathway PHD2 | - ferritin and FPN1 translation; suppression of translation of TFRC and DMT1 - tumor cell proliferation - apoptosis - ferroptosis |
| Extracellular (iron-Tf) | HFE/TFRC TFR2 | - hepcidin expression |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywoszyńska, K.; Witkowska, D.; Świątek-Kozłowska, J.; Szebesczyk, A.; Kozłowski, H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules 2020, 10, 1417. https://doi.org/10.3390/biom10101417
Krzywoszyńska K, Witkowska D, Świątek-Kozłowska J, Szebesczyk A, Kozłowski H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules. 2020; 10(10):1417. https://doi.org/10.3390/biom10101417
Chicago/Turabian StyleKrzywoszyńska, Karolina, Danuta Witkowska, Jolanta Świątek-Kozłowska, Agnieszka Szebesczyk, and Henryk Kozłowski. 2020. "General Aspects of Metal Ions as Signaling Agents in Health and Disease" Biomolecules 10, no. 10: 1417. https://doi.org/10.3390/biom10101417
APA StyleKrzywoszyńska, K., Witkowska, D., Świątek-Kozłowska, J., Szebesczyk, A., & Kozłowski, H. (2020). General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules, 10(10), 1417. https://doi.org/10.3390/biom10101417






