IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis
Abstract
1. Introduction
2. IL-17A: A Key Proinflammatory Cytokine and Therapeutic Target in ESRD Patients on PD
2.1. IL-17A Producing Cells: Th17 Immune Cells and Other Cells Involved in PD-Induced Damage
2.1.1. Th17 Cells
2.1.2. γδ T Cells
2.1.3. Neutrophils
2.1.4. Mast Cells
2.1.5. MAIT Cells
2.2. Role of IL-17A-Expressing Cells in CKD Patients
2.3. Role of IL-17A-Expressing Cells in the Peritoneum Exposed to PDF
2.3.1. PDF-Induced Peritoneal Activation of Th17/IL-17A Axis in Preclinical Models
2.3.2. Local Production of IL-17A in Long-Term PD Patients
3. Peritoneal Impact of IL-17A
3.1. IL-17A as a Mediator of Peritoneal Fibrosis through Activation of Inflammatory Pathways
3.2. IL-17A in Mesothelial Cells
3.3. IL-17A in Peritoneal Fibrosis
3.4. IL-17A in Peritonitis
3.5. IL-17A and Macrophage Functions
3.6. IL-17A in Angiogenesis
4. Pharmacological Interference with the Th17 Immune Responses in PD
4.1. Blockade of the Renin-Angiotensin System
4.2. HMG-CoA Reductase Inhibitors (Statins)
4.3. mTOR Inhibition
4.4. Cyclooxygenase-2 Inhibition
4.5. Peroxisome Proliferator-Activated Receptor-γ Agonists
4.6. Alanyl-Glutamine PD Fluids Supplementation
4.7. Vitamin D and Related Drugs
5. MicroRNAs in Th17 and PD
6. Beyond the Peritoneum
7. Clinical Targeting of IL-17A
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; Remón, C.; Ossorio, M.; Fernández-Perpén, A.; Sánchez-Tomero, J.A.; Cirugeda, A.; de Sousa, E.; Sandoval, P.; et al. Biocompatible dialysis solutions preserve peritoneal mesothelial cell and vessel wall integrity. A case-control study on human biopsies. Perit. Dial. Int. 2016, 36, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bajo, M.A.; del Peso, G.; Yu, X.; Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Mortier, S.; De Vriese, A.S.; Van de Voorde, J.; Schaub, T.P.; Passlick-Deetjen, J.; Lameire, N.H. Hemodynamic Effects of Peritoneal Dialysis Solutions on the Rat Peritoneal Membrane: Role of Acidity, Buffer Choice, Glucose Concentration, and Glucose Degradation Products. J. Am. Soc. Nephrol. 2002, 13, 480–489. [Google Scholar]
- Jörres, A. Novel peritoneal dialysis solutions-What are the clinical implications? Blood Purif. 2012, 33, 153–159. [Google Scholar] [CrossRef]
- Ortiz, A.; Wieslander, A.; Linden, T.; Santamaria, B.; Sanz, A.; Justo, P.; Sanchez-Nino, M.-D.; Benito, A.; Kjellstrand, P. 3,4-DGE is Important for Side Effects in Peritoneal Dialysis What About its Role in Diabetes. Curr. Med. Chem. 2006, 13, 2695–2702. [Google Scholar] [CrossRef]
- Catalan, M.P.; Santamaría, B.; Reyero, A.; Ortiz, A.; Egido, J.; Ortiz, A. 3,4-Di-deoxyglucosone-3-ene promotes leukocyte apoptosis. Kidney Int. 2005, 68, 1303–1311. [Google Scholar] [CrossRef][Green Version]
- Santamaría, B.; Ucero, A.C.; Benito-Martin, A.; Vicent, M.J.; Orzáez, M.; Celdrán, A.; Selgas, R.; Ruíz-Ortega, M.; Ortiz, A. Biocompatibility reduces inflammation-induced apoptosis in mesothelial cells exposed to peritoneal dialysis Fluid. Blood Purif. 2015, 39, 200–209. [Google Scholar] [CrossRef]
- Baroni, G.; Schuinski, A.; de Moraes, T.P.; Meyer, F.; Pecoits-Filho, R. Inflammation and the Peritoneal Membrane: Causes and Impact on Structure and Function during Peritoneal Dialysis. Mediat. Inflamm. 2012, 2012, 912595. [Google Scholar] [CrossRef]
- Velloso, M.S.S.; Otoni, A.; de Paula Sabino, A.; de Castro, W.V.; Pinto, S.W.L.; Marinho, M.A.S.; Rios, D.R.A. Peritoneal dialysis and inflammation. Clin. Chim. Acta 2014, 430, 109–114. [Google Scholar] [CrossRef]
- Devuyst, O.; Margetts, P.J.; Topley, N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Ng, H.Y.; Hsu, C.Y.; Tsai, Y.C.; Yang, Y.K.; Chen, T.C.; Chiou, T.T.Y.; Kuo, C.C.; Lee, W.C.; Hsu, K.T. Proinflammatory cytokines, hepatocyte growth factor and adipokines in peritoneal dialysis patients. Artif. Organs 2010, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sawai, A.; Ito, Y.; Mizuno, M.; Suzuki, Y.; Toda, S.; Ito, I.; Hattori, R.; Matsukawa, Y.; Gotoh, M.; Takei, Y.; et al. Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2010, 26, 2322–2332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parikova, A.; Zweers, M.M.; Struijk, D.G.; Krediet, R.T. Peritoneal effluent markers of inflammation in patients treated with icodextrin-based and glucose-based dialysis solutions. Adv. Perit. Dial. 2003, 19, 186–190. [Google Scholar] [PubMed]
- Sanz, A.B.; Aroeira, L.S.; Bellon, T.; Del Peso, G.; Jimenez-Heffernan, J.; Santamaria, B.; Sanchez-Niño, M.D.; Blanco-Colio, L.M.; Lopez-Cabrera, M.; Ruiz-Ortega, M.; et al. TWEAK promotes peritoneal inflammation. PLoS ONE 2014, 9, e90399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low–glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.L.; Yung, S.; Yim, A.; Wong, K.M.; Tong, K.L.; Wong, K.S.; Li, C.S.; Au, T.C.; Lo, W.K.; Ho, Y.W.; et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: A randomized clinical trial. Am. J. Kidney Dis. 2012, 60, 966–975. [Google Scholar] [CrossRef]
- Schmidt, T.; Luebbe, J.; Paust, H.J.; Panzer, U. Mechanisms and functions of IL-17 signaling in renal autoimmune diseases. Mol. Immunol. 2018, 104, 90–99. [Google Scholar] [CrossRef]
- Wang, E.A.; Suzuki, E.; Maverakis, E.; Adamopoulos, I.E. Targeting IL-17 in psoriatic arthritis. Eur. J. Rheumatol. 2017, 4, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, e60. [Google Scholar] [CrossRef]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Nordlohne, J.; von Vietinghoff, S. Interleukin 17A in atherosclerosis—Regulation and pathophysiologic effector function. Cytokine 2019, 122, 154089. [Google Scholar] [CrossRef]
- Gong, F.; Liu, Z.; Liu, J.; Zhou, P.; Liu, Y.; Lu, X. The paradoxical role of IL-17 in atherosclerosis. Cell. Immunol. 2015, 297, 33–39. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gurney, A.L. IL-17: Prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002, 71, 1–8. [Google Scholar] [CrossRef]
- von Vietinghoff, S.; Ley, K. Interleukin 17 in vascular inflammation. Cytokine Growth Factor Rev. 2010, 21, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Krstic, J.; Obradovic, H.; Kukolj, T.; Mojsilovic, S.; Okic-Dordevic, I.; Bugarski, D.; Santibanez, J. An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling. Protein Pept. Lett. 2015, 22, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or foe in organ fibrosis. Cytokine 2019, 120, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Awaji, M.; Saxena, S.; Varney, M.L.; Sharma, B.; Singh, R.K. IL-17–CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am. J. Pathol. 2020, 190, 222–233. [Google Scholar] [CrossRef]
- Witowski, J.; Kamhieh-Milz, J.; Kawka, E.; Catar, R.; Jörres, A. IL-17 in Peritoneal Dialysis-Associated Inflammation and Angiogenesis: Conclusions and Perspectives. Front. Physiol. 2018, 9, 1694. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Liu, X.; Awar, L.; Mcmickle, A.; Bai, F.; Nagarajan, S.; Yu, S. IL-17 induces expression of vascular cell adhesion molecule through signalling pathway of NF-κB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand. J. Immunol. 2013, 77, 230–237. [Google Scholar] [CrossRef]
- Pietrowski, E.; Bender, B.; Huppert, J.; White, R.; Luhmann, H.J.; Kuhlmann, C.R.W. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J. Vasc. Res. 2010, 48, 52–58. [Google Scholar] [CrossRef]
- Gu, C.; Wu, L.; Li, X. IL-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Díez, R.; Aroeira, L.S.; Orejudo, M.; Bajo, M.A.; Heffernan, J.J.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Ortiz, A.; Gonzalez-Mateo, G.; López-Cabrera, M.; et al. IL-17A is a novel player in dialysis-induced peritoneal damage. Kidney Int. 2014, 86, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Gagliani, N.; Huber, S. Basic aspects of T helper cell differentiation. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2017; Volume 1514, pp. 19–30. [Google Scholar]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Cosmi, L.; Maggi, L.; Santarlasci, V.; Liotta, F.; Annunziato, F. T helper cells plasticity in inflammation. Cytom. Part A 2014, 85, 36–42. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Mesquita, D.; Kirsztajn, G.M.; Franco, M.F.; Reis, L.A.; Perazzio, S.F.; Mesquita, F.V.; da Silva Ferreira, V.; Andrade, L.E.C.; de Souza, A.W.S. CD4+ T helper cells and regulatory T cells in active lupus nephritis: An imbalance towards a predominant Th1 response? Clin. Exp. Immunol. 2018, 191, 50–59. [Google Scholar] [CrossRef]
- Waite, J.C.; Skokos, D. Th17 Response and Inflammatory Autoimmune Diseases. Int. J. Inflamm. 2012, 2012, 819467. [Google Scholar] [CrossRef]
- Romagnani, S. Type 1 T helper and type 2 T helper cells: Functions, regulation and role in protection and disease. Int. J. Clin. Lab. Res. 1992, 21, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Dupage, M.; Bluestone, J.A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.S.; Ward, W.R.; Dobson, H. Lack of LH response to oestradiol treatment in cows with cystic ovarian disease and effect of progesterone treatment or manual rupture. Res. Vet. Sci. 1991, 51, 180–184. [Google Scholar] [CrossRef]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupé, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Volpe, E. Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 348. [Google Scholar] [CrossRef]
- Harris, T.J.; Grosso, J.F.; Yen, H.-R.; Xin, H.; Kortylewski, M.; Albesiano, E.; Hipkiss, E.L.; Getnet, D.; Goldberg, M.V.; Maris, C.H.; et al. Cutting Edge: An In Vivo Requirement for STAT3 Signaling in T H 17 Development and T H 17-Dependent Autoimmunity. J. Immunol. 2007, 179, 4313–4317. [Google Scholar] [CrossRef]
- Robert, M.; Miossec, P. Effects of Interleukin 17 on the cardiovascular system. Autoimmun. Rev. 2017, 16, 984–991. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the immune system in hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef]
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Liappas, G.; Gónzalez-Mateo, G.T.; Majano, P.; Sánchez-Tomero, J.A.; Ruiz-Ortega, M.; Rodrigues Díez, R.; Martín, P.; Sanchez-Díaz, R.; Selgas, R.; López-Cabrera, M.; et al. T Helper 17/Regulatory T Cell Balance and Experimental Models of Peritoneal Dialysis-Induced Damage. Biomed Res. Int. 2015, 2015, 416480. [Google Scholar] [CrossRef] [PubMed]
- Boldizsar, F.; Tarjanyi, O.; Nemeth, P.; Mikecz, K.; Glant, T.T. Th1/Th17 polarization and acquisition of an arthritogenic phenotype in arthritis-susceptible BALB/c, but not in MHC-matched, arthritis-resistant DBA/2 mice. Int. Immunol. 2009, 21, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Liu, J.J.; Pai, M.H.; Tsou, S.S.; Yeh, S.L. Alanyl-glutamine administration suppresses Th17 and reduces inflammatory reaction in dextran sulfate sodium-induced acute colitis. Int. Immunopharmacol. 2013, 17, 1–8. [Google Scholar] [CrossRef]
- Shiromizu, C.M.; Jancic, C.C. γδ T lymphocytes: An effector cell in autoimmunity and infection. Front. Immunol. 2018, 9, 2389. [Google Scholar] [CrossRef]
- Fenoglio, D.; Poggi, A.; Catellani, S.; Battaglia, F.; Ferrera, A.; Setti, M.; Murdaca, G.; Zocchi, M.R. Vδ1 T lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood 2009, 113, 6611–6618. [Google Scholar] [CrossRef]
- Hirata, T.; Osuga, Y.; Takamura, M.; Saito, A.; Hasegawa, A.; Koga, K.; Yoshino, O.; Hirota, Y.; Harada, M.; Taketani, Y. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertil. Steril. 2011, 96, 113–117. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Zhang, C.; Li, P.; Cui, W.; Hao, J.; Ma, X.; Yin, Z.; Du, J. γδT cell-derived interleukin-17A via an interleukin-1β- dependent mechanism mediates cardiac Injury and Fibrosis in hypertension. Hypertension 2014, 64, 305–314. [Google Scholar] [CrossRef]
- Saleh, M.A.; Norlander, A.E.; Madhur, M.S. Inhibition of Interleukin-17A, But Not Interleukin-17F, Signaling Lowers Blood Pressure, and Reduces End-Organ Inflammation in Angiotensin II–Induced Hypertension. JACC Basic to Transl. Sci. 2016, 1, 606–616. [Google Scholar] [CrossRef]
- Orejudo, M.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Garcia-Redondo, A.; Santos-Sánchez, L.; Rández-Garbayo, J.; Cannata-Ortiz, P.; Ramos, A.M.; Ortiz, A.; Selgas, R.; et al. Interleukin 17A participates in renal inflammation associated to experimental and human hypertension. Front. Pharmacol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Rei, M.; Goncąlves-Sousa, N.; Lancą, T.; Thompson, R.G.; Mensurado, S.; Balkwill, F.R.; Kulbe, H.; Pennington, D.J.; Silva-Santos, B. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl. Acad. Sci. USA 2014, 111, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, X.; Shibata, K.; Yamada, H.; Muta, H.; Podack, E.R.; Yoshikai, Y. CD30 is required for activation of a unique subset of interleukin- 17A-Producing γδT Cells in innate immunity against mycobacterium bovis bacillus calmette-guérin infection. Infect. Immun. 2013, 81, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yamada, H.; Hara, H.; Kishihara, K.; Yoshikai, Y. Resident Vδ1 + γδ T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J. Immunol. 2007, 178, 4466–4472. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Hua, L.; Meng, X.; Xiao, Y.; Hao, X.; Guo, S.; Zhao, P.; Wang, L.; Dong, B.; Yu, Y.; et al. Correlation of Surface Toll-Like Receptor 9 Expression with IL-17 Production in Neutrophils during Septic Peritonitis in Mice Induced by E. coli. Mediat. Inflamm. 2016, 2016, 3296307. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef]
- Li, R.H.L.; Tablin, F. A comparative review of neutrophil extracellular traps in sepsis. Front. Vet. Sci. 2018, 5, 291. [Google Scholar] [CrossRef]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Hueber, A.J.; Asquith, D.L.; Miller, A.M.; Reilly, J.; Kerr, S.; Leipe, J.; Melendez, A.J.; McInnes, I.B. Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 2010, 184, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, H.; Zhang, G.; Lin, X.; Chen, C.; Sun, J.; Zhang, Y.; Zhang, Q.; Yu, J. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS ONE 2014, 9, e106834. [Google Scholar] [CrossRef][Green Version]
- Kenna, T.J.; Brown, M.A. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 2013, 9, 375–379. [Google Scholar] [CrossRef]
- Noordenbos, T.; Blijdorp, I.; Chen, S.; Stap, J.; Mul, E.; Cañete, J.D.; Lubberts, E.; Yeremenko, N.; Baeten, D. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J. Leukoc. Biol. 2016, 100, 453–462. [Google Scholar] [CrossRef]
- Jiménez-Heffernan, J.A.; Bajo, M.A.; Perna, C.; Del Peso, G.; Larrubia, J.R.; Gamallo, C.; Sánchez-Tomero, J.A.; López-Cabrera, M.; Selgas, R. Mast cell quantification in normal peritoneum and during peritoneal dialysis treatment. Arch. Pathol. Lab. Med. 2006, 130, 1188–1192. [Google Scholar] [CrossRef]
- Zareie, M.; Fabbrini, P.; Hekking, L.H.P.; Keuning, E.D.; Ter Wee, P.M.; Beelen, R.H.J.; Van Den Born, J. Novel role for mast cells in omental tissue remodeling and cell recruitment in experimental peritoneal dialysis. J. Am. Soc. Nephrol. 2006, 17, 3447–3457. [Google Scholar] [CrossRef]
- Rönnberg, E.; Johnzon, C.F.; Calounova, G.; Garcia Faroldi, G.; Grujic, M.; Hartmann, K.; Roers, A.; Guss, B.; Lundequist, A.; Pejler, G. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Dahdah, A.; Gautier, G.; Attout, T.; Fiore, F.; Lebourdais, E.; Msallam, R.; Daëron, M.; Monteiro, R.C.; Benhamou, M.; Charles, N.; et al. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J. Clin. Investig. 2014, 124, 4577–4589. [Google Scholar] [CrossRef]
- Bradding, P.; Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef]
- Zareie, M.; Hekking, L.H.; Driesprong, B.A.; ter Wee, P.M.; Beelen, R.H.; van den Born, J. Accumulation of omental mast cells during peritoneal dialysis. Perit. Dial. Int. 2001, 21 (Suppl. 3), 373–376. [Google Scholar] [CrossRef]
- Alscher, D.M.; Braun, N.; Biegger, D.; Fritz, P. Peritoneal Mast Cells in Peritoneal Dialysis Patients, Particularly in Encapsulating Peritoneal Sclerosis Patients. Am. J. Kidney Dis. 2007, 49, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Kazama, I.; Baba, A.; Endo, Y.; Toyama, H.; Ejima, Y.; Matsubara, M.; Tachi, M. Mast cell involvement in the progression of peritoneal fibrosis in rats with chronic renal failure. Nephrology 2015, 20, 609–616. [Google Scholar] [CrossRef]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161 hi IL-17-secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Reantragoon, R.; Corbett, A.J.; Sakala, I.G.; Gherardin, N.A.; Furness, J.B.; Chen, Z.; Eckle, S.B.G.; Uldrich, A.P.; Birkinshaw, R.W.; Patel, O.; et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013, 210, 2305–2320. [Google Scholar] [CrossRef]
- Ibidapo-obe, O.; Stengel, S.; Köse-Vogel, N.; Quickert, S.; Reuken, P.A.; Busch, M.; Bauer, M.; Stallmach, A.; Bruns, T. Mucosal-Associated Invariant T Cells Redistribute to the Peritoneal Cavity During Spontaneous Bacterial Peritonitis and Contribute to Peritoneal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 661–677. [Google Scholar] [CrossRef]
- Summers, S.A.; Steinmetz, O.M.; Li, M.; Kausman, J.Y.; Semple, T.; Edgtton, K.L.; Borza, D.B.; Braley, H.; Holdsworth, S.R.; Kitching, A.R. Th1 and Th17 cells induce proliferative glomerulonephritis. J. Am. Soc. Nephrol. 2009, 20, 2518–2524. [Google Scholar] [CrossRef]
- Ghali, J.; Holdsworth, S.; Kitching, A. Targeting IL-17 and IL-23 in Immune Mediated Renal Disease. Curr. Med. Chem. 2015, 22, 4341–4365. [Google Scholar] [CrossRef]
- Dolff, S.; Witzke, O.; Wilde, B. Th17 cells in renal inflammation and autoimmunity. Autoimmun. Rev. 2019, 18, 129–136. [Google Scholar] [CrossRef]
- Nogueira, E.; Hamour, S.; Sawant, D.; Henderson, S.; Mansfield, N.; Chavele, K.-M.; Pusey, C.D.; Salama, A.D. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2010, 25, 2209–2217. [Google Scholar] [CrossRef]
- Krohn, S.; Nies, J.F.; Kapffer, S.; Schmidt, T.; Riedel, J.H.; Kaffke, A.; Peters, A.; Borchers, A.; Steinmetz, O.M.; Krebs, C.F.; et al. IL-17C/IL-17 receptor E signaling in CD4+ T cells promotes T H 17 cell-driven glomerular inflammation. J. Am. Soc. Nephrol. 2018, 29, 1210–1222. [Google Scholar] [CrossRef]
- Kwan, B.C.-H.; Tam, L.-S.; Lai, K.-B.; Lai, F.M.-M.; Li, E.K.-M.; Wang, G.; Chow, K.-M.; Li, P.K.-T.; Szeto, C.-C. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009, 48, 1491–1497. [Google Scholar] [CrossRef]
- de Oliveira Peliçari, K.; Postal, M.; Sinicato, N.A.; Peres, F.A.; Fernandes, P.T.; Marini, R.; Costallat, L.T.L.; Appenzeller, S. Serum interleukin-17 levels are associated with nephritis in childhood-onset systemic lupus erythematosus. Clinics (Sao Paulo) 2015, 70, 313–317. [Google Scholar] [CrossRef]
- Kalavrizioti, D.; Gerolymos, M.; Rodi, M.; Kalliakmani, P.; Provatopoulou, S.; Eleftheriadis, T.; Mouzaki, A.; Goumenos, D.S. T helper (Th)-cytokines in the urine of patients with primary glomerulonephritis treated with immunosuppressive drugs: Can they predict outcome? Cytokine 2015, 76, 260–269. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Emerging roles of the Th17/IL-17-axis in glomerulonephritis. Cytokine 2016, 77, 238–244. [Google Scholar] [CrossRef]
- Krebs, C.F.; Lange, S.; Niemann, G.; Rosendahl, A.; Lehners, A.; Meyer-Schwesinger, C.; Stahl, R.A.K.; Benndorf, R.A.; Velden, J.; Paust, H.J.; et al. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension 2014, 63, 565–571. [Google Scholar] [CrossRef]
- Lavoz, C.; Matus, Y.S.; Orejudo, M.; Carpio, J.D.; Droguett, A.; Egido, J.; Mezzano, S.; Ruiz-Ortega, M. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney Int. 2019, 95, 1418–1432. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.J.; Chen, X.; Kwan, T.; Chadban, S.J.; Wu, H. Interleukin 17A promotes diabetic kidney injury. Sci. Rep. 2019, 9, 2264. [Google Scholar] [CrossRef]
- Lavoz, C.; Rayego-Mateos, S.; Orejudo, M.; Opazo-Ríos, L.; Marchant, V.; Marquez-Exposito, L.; Tejera-Muñoz, A.; Navarro-González, J.F.; Droguett, A.; Ortiz, A.; et al. Could IL-17A Be a Novel Therapeutic Target in Diabetic Nephropathy? J. Clin. Med. 2020, 9, 272. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Li, P.K.T.; Ng, J.K.C.; Mcintyre, C.W. Inflammation and Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 54–65. [Google Scholar] [CrossRef]
- Zamauskaite, A.; Yaqoob, M.M.; Madrigal, J.A.; Cohen, S.B. The frequency of Th2 type cells increases with time on peritoneal dialysis in patients with diabetic nephropathy. Eur. Cytokine Netw. 1999, 10, 219–226. [Google Scholar]
- Zhu, X.; Li, S.; Zhang, Q.; Zhu, D.; Xu, Y.; Zhang, P.; Han, J.; Duan, Z.; Gao, J.; Ou, Y. Correlation of increased Th17/Treg cell ratio with endoplasmic reticulum stress in chronic kidney disease. Medicine (United States) 2018, 97, e10748. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Hu, K.; Lv, G.; Fu, Y.; Ayana, D.A.; Zhao, P.; Jiang, Y. The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Romanova, Y.; Laikov, A.; Markelova, M.; Khadiullina, R.; Makseev, A.; Hasanova, M.; Rizvanov, A.; Khaiboullina, S.; Salafutdinov, I. Proteomic analysis of human serum from patients with chronic kidney disease. Biomolecules 2020, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, H.; Zhang, L.; Yang, X.; Zhang, M.; Zhu, X.; Ji, X.; Wang, H. Role of interleukin 17 in TGF-β signaling-mediated renal interstitial fibrosis. Cytokine 2018, 106, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Díez, R.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Suarez-Alvarez, B.; Lavoz, C.; Stark Aroeira, L.; Sánchez-López, E.; Orejudo, M.; Alique, M.; Lopez-Larrea, C.; et al. The C-terminal module IV of connective tissue growth factor is a novel immune modulator of the Th17 response. Lab. Investig. 2013, 93, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.; Kabiri, R.; Bode, M.; Cai, A.; Klinge, S.; Ehmke, H.; Mittrücker, H.W.; Wenzel, U.O. Adaptive immunity and IL-17A are not involved in the progression of chronic kidney disease after 5/6 nephrectomy in mice. Br. J. Pharmacol. 2019, 176, 2002–2014. [Google Scholar] [CrossRef]
- Liappas, G.; González-Mateo, G.T.; Sánchez-Díaz, R.; Lazcano, J.J.; Lasarte, S.; Matesanz-Marín, A.; Zur, R.; Ferrantelli, E.; Ramírez, L.G.; Aguilera, A.; et al. Immune-regulatory molecule CD69 controls peritoneal fibrosis. J. Am. Soc. Nephrol. 2016, 27, 3561–3576. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; Keuning, E.D.; Talhout, W.; Paauw, N.J.; van Ittersum, F.J.; ter Wee, P.M.; Beelen, R.H.J.; Vervloet, M.G.; Ferrantelli, E. Differences in peritoneal response after exposure to low-GDP bicarbonate/lactate-buffered dialysis solution compared to conventional dialysis solution in a uremic mouse model. Int. Urol. Nephrol. 2018, 50, 1151–1161. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, T.Y.; Lin, C.Y. Kinetics and involvement of interleukin-17 in the outcome of peritonitis in nondiabetic patients undergoing peritoneal dialysis. J. Chin. Med. Assoc. 2011, 74, 11–15. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Mateo, G.T.; Loureiro, J.; Jimenez-Hefferman, J.A.; Bajo, M.-A.; Selgas, R.; Lopez-Cabrera, M.; Aroeira, L.S. Chronic exposure of mouse peritoneum to peritoneal dialysis fluid: Structural and functional alterations of the peritoneal membrane. Perit. Dial. Int. 2009, 29, 227–230. [Google Scholar] [CrossRef]
- Martin, P.; Gomez, M.; Lamana, A.; Cruz-Adalia, A.; Ramirez-Huesca, M.; Ursa, M.A.; Yanez-Mo, M.; Sanchez-Madrid, F. CD69 Association with Jak3/Stat5 Proteins Regulates Th17 Cell Differentiation. Mol. Cell. Biol. 2010, 30, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Maksic, D.; Vasilijic, S.; Colic, M.; Stankovic-Popovic, V.; Bokonjic, D. Systemic and intraperitoneal proinflammatory cytokine profiles in patients on continuous ambulatory peritoneal dialysis. Adv. Perit. Dial. 2009, 25, 50–55. [Google Scholar] [PubMed]
- Xiao, J.; Gong, Y.; Chen, Y.; Yu, D.; Wang, X.; Zhang, X.; Dou, Y.; Liu, D.; Cheng, G.; Lu, S.; et al. IL-6 promotes epithelial-to-mesenchymal transition of human peritoneal mesothelial cells possibly through the JAK2/STAT3 signaling pathway. Am. J. Physiol. Renal Physiol. 2017, 313, F310–F318. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Carvalho, M.J.; Stenvinkel, P.; Lindholm, B.; Heimbürger, O. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit. Dial. Int. 2006, 26, 53–63. [Google Scholar] [CrossRef]
- Schmitt, C.P.; Aufricht, C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2017, 32, 1835–1843. [Google Scholar] [CrossRef]
- Witowski, J.; Ksiązek, K.; Warnecke, C.; Kuźlan, M.; Korybalska, K.; Tayama, H.; Wiśniewska-Elnur, J.; Pawlaczyk, K.; Trómińska, J.; Brȩborowicz, A.; et al. Role of mesothelial cell-derived granulocyte colony-stimulating factor in interleukin-17-induced neutrophil accumulation in the peritoneum. Kidney Int. 2007, 71, 514–525. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Xing, X.; Wan, L.; Li, M. Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res. Ther. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Xing, X.; Cui, X.; Li, M. Interleukin-17A promotes functional activation of systemic sclerosis patient-derived dermal vascular smooth muscle cells by extracellular-regulated protein kinases signalling pathway. Arthritis Res. Ther. 2014, 16, 4223. [Google Scholar] [CrossRef][Green Version]
- Witowski, J.; Pawlaczyk, K.; Breborowicz, A.; Scheuren, A.; Kuzlan-Pawlaczyk, M.; Wisniewska, J.; Polubinska, A.; Friess, H.; Gahl, G.M.; Frei, U.; et al. IL-17 Stimulates Intraperitoneal Neutrophil Infiltration through the Release of GROα Chemokine from Mesothelial Cells. J. Immunol. 2000, 165, 5814–5821. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ryu, H.M.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. The role of Toll-like receptor 4 in high-glucose-induced inflammatory and fibrosis markers in human peritoneal mesothelial cells. Int. Urol. Nephrol. 2017, 49, 171–181. [Google Scholar] [CrossRef]
- Ruiz-Carpio, V.; Sandoval, P.; Aguilera, A.; Albar-Vizcaíno, P.; Perez-Lozano, M.L.; González-Mateo, G.T.; Acuña-Ruiz, A.; García-Cantalejo, J.; Botías, P.; Bajo, M.A.; et al. Genomic reprograming analysis of the Mesothelial to mesenchymal transition identifies biomarkers in peritoneal dialysis patients. Sci. Rep. 2017, 7, 44942. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Lara-Pezzi, E.; Selgas, R.; Ramirez-Huesca, M.; Dominguez-Jimenez, C.; Jimenez-Heffernan, J.A.; Aguilera, A.; Sanchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cabrera, M. Mesenchymal Conversion of Mesothelial Cells Is a Key Event in the Pathophysiology of the Peritoneum during Peritoneal Dialysis. Adv. Med. 2014, 2014, 473134. [Google Scholar] [CrossRef]
- Del Peso, G.; Jiménez-Heffernan, J.A.; Bajo, M.A.; Aroeira, L.S.; Aguilera, A.; Fernández-Perpén, A.; Cirugeda, A.; Castro, M.J.; De Gracia, R.; Sánchez-Villanueva, R.; et al. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int. 2008, 73, s26–s33. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, L.S.; Loureiro, J.; Gonzalez-Mateo, G.T.; Fernandez-Millara, V.; del Peso, G.; Sanchez-Tomero, J.A.; Ruiz-Ortega, M.; Bajo, M.A.; Lopez-Cabrera, M.; Selgas, R. Characterization of epithelial-to-mesenchymal transition of mesothelial cells in a mouse model of chronic peritoneal exposure to high glucose dialysate. Perit. Dial. Int. 2008, 28 (Suppl. 5), 29–33. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Lozano, M.L.P.; Cerezo, A.; López-Cabrera, M.; Del Pozo, M.A. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-κB/Snail1 pathway. DMM Dis. Model. Mech. 2008, 1, 264–274. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Xu, Y.; Shen, Z.; Cheng, H.; Cheng, F.; Liu, X.; Wang, R. RhoA/Rho-kinase triggers epithelial-mesenchymal transition in mesothelial cells and contributes to the pathogenesis of dialysis-related peritoneal fibrosis. Oncotarget 2018, 9, 14397–14412. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Z.; Liu, H.; Zhu, J.; Liu, F.; Chen, G. Transition of mesothelial cell to fibroblast in peritoneal dialysis: EMT, stem cell or bystander? Perit. Dial. Int. 2015, 35, 14–25. [Google Scholar] [CrossRef]
- Aguilera, A.; Yáñez-Mo, M.; Selgas, R.; Sánchez-Madrid, F.; López-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 2005, 6, 262–268. [Google Scholar]
- Selgas, R.; Bajo, A.; Jimenez-Heffernan, J.A.; Sanchez-Tomero, J.A.; Del Peso, G.; Aguilera, A.; Lopez-Cabrera, M. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transplant. 2006, 21 (Suppl. 2), ii2–ii7. [Google Scholar] [CrossRef]
- Busnadiego, O.; Loureiro-Álvarez, J.; Sandoval, P.; Lagares, D.; Dotor, J.; Pérez-Lozano, M.L.; López-Armada, M.J.; Lamas, S.; López-Cabrera, M.; Rodríguez-Pascual, F. A pathogenetic role for endothelin-1 in peritoneal dialysis-associated fibrosis. J. Am. Soc. Nephrol. 2015, 26, 173–182. [Google Scholar] [CrossRef]
- Loureiro, J.; Schilte, M.; Aguilera, A.; Albar-Vizcaino, P.; Ramirez-Huesca, M.; Perez-Lozano, M.L.; Gonzalez-Mateo, G.; Aroeira, L.S.; Selgas, R.; Mendoza, L.; et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol. Dial. Transplant. 2010, 25, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jiang, Z.; Zhang, H.; Zhu, H.; Zhou, S.F.; Yu, X. NADPH oxidase-dependent formation of reactive oxygen species contributes to angiotensin II-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. Int. J. Mol. Med. 2011, 28, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hi, B.L.; Ha, H. Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis. J. Korean Med. Sci. 2007, 22, 943–945. [Google Scholar]
- Xing, X.; Yang, J.; Yang, X.; Wei, Y.; Zhu, L.; Gao, D.; Li, M. IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway. PLoS ONE 2013, 8, e85032. [Google Scholar] [CrossRef]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schüler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Nikolaev, A.; Reißig, S.; et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef]
- Wu, J.; Saleh, M.A.; Kirabo, A.; Itani, H.A.; Montaniel, K.R.C.; Xiao, L.; Chen, W.; Mernaugh, R.L.; Cai, H.; Bernstein, K.E.; et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Investig. 2016, 126, 50–67. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Perez Lozano, M.L.; Pellinen, T.; Sandoval, P.; Lopez-Cabrera, M.; del Pozo, M.A. Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells. PLoS ONE 2012, 7, e31492. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Foronda, M.; Perez-Lozano, M.L.; Sánchez-Perales, S.; López-Cabrera, M.; Del Pozo, M.Á. p38 maintains E-cadherin expression by modulating TAK1-NF-κB during epithelial-to-mesenchymal transition. J. Cell Sci. 2010, 123, 4321–4331. [Google Scholar] [CrossRef]
- Strippoli, R.; Loureiro, J.; Moreno, V.; Benedicto, I.; Pérez Lozano, M.L.; Barreiro, O.; Pellinen, T.; Minguet, S.; Foronda, M.; Osteso, M.T.; et al. Caveolin-1 deficiency induces a MEK - ERK 1/2-Snail-1-dependent epithelial–mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol. Med. 2015, 7, 102–123. [Google Scholar] [CrossRef]
- Dudas, P.L.; Sague, S.L.; Elloso, M.M.; Farrell, F.X. Proinflammatory/profibrotic effects of interleukin-17A on human proximal tubule epithelium. Nephron Exp. Nephrol. 2011, 117, e114–e123. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Li, M.-M.; Shen, J.; Liu, F.; Cao, J.-Y.; Jin, S.; Yu, Y. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-κB/ZEB1 signal pathway. Am. J. Cancer Res. 2015, 5, 1169–1179. [Google Scholar] [PubMed]
- Orejudo, M.; García-Redondo, A.B.; Rodrigues-Diez, R.R.; Rodrigues-Díez, R.; Santos-Sanchez, L.; Tejera-Muñoz, A.; Egido, J.; Selgas, R.; Salaices, M.; Briones, A.M.; et al. Interleukin-17A induces vascular remodeling of small arteries and blood pressure elevation. Clin. Sci. (Lond.) 2020, 134, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Madala, S.K.; Ramalingam, T.R.; Gochuico, B.R.; Rosas, I.O.; Cheever, A.W.; Wynn, T.A. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010, 207, 535–552. [Google Scholar] [CrossRef]
- Mi, S.; Li, Z.; Yang, H.-Z.; Liu, H.; Wang, J.-P.; Ma, Y.-G.; Wang, X.-X.; Liu, H.-Z.; Sun, W.; Hu, Z.-W. Blocking IL-17A Promotes the Resolution of Pulmonary Inflammation and Fibrosis Via TGF-β1–Dependent and –Independent Mechanisms. J. Immunol. 2011, 187, 3003–3014. [Google Scholar] [CrossRef]
- Cipolla, E.; Fisher, A.J.; Gu, H.; Mickler, E.A.; Agarwal, M.; Wilke, C.A.; Kim, K.K.; Moore, B.B.; Vittal, R. IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J. 2017, 31, 5543–5556. [Google Scholar] [CrossRef]
- Speeckaert, R.; Lambert, J.; Grine, L.; Van Gele, M.; De Schepper, S.; van Geel, N. The many faces of interleukin-17 in inflammatory skin diseases. Br. J. Dermatol. 2016, 175, 892–901. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhao, Q.; Chen, M. Role of Interleukin-17 in Pathogenesis of Intestinal Fibrosis in Mice. Dig. Dis. Sci. 2019, 65, 1971–1979. [Google Scholar] [CrossRef]
- Mehrotra, P.; Collett, J.A.; McKinney, S.D.; Stevens, J.; Ivancic, C.M.; Basile, D.P. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: Compensatory role of natural killer cells in athymic rats. Am. J. Physiol. Ren. Physiol. 2017, 312, F385–F397. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Tan, R.J.; Zhou, D.; Coleman, B.M.; Jawale, C.V.; Liu, Y.; Biswas, P.S. IL-17 Receptor Signaling Negatively Regulates the Development of Tubulointerstitial Fibrosis in the Kidney. Mediat. Inflamm. 2018, 2018, 5103672. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Chen, L.; Bea, F.; Wangler, S.; Celik, S.; Lasitschka, F.; Wang, Y.; Böckler, D.; Katus, H.A.; Dengler, T.J. Inhibition of IL-17A Attenuates Atherosclerotic Lesion Development in ApoE-Deficient Mice. J. Immunol. 2009, 183, 8167–8175. [Google Scholar] [CrossRef] [PubMed]
- Truchetet, M.E.; Brembilla, N.C.; Montanari, E.; Lonati, P.; Raschi, E.; Zeni, S.; Fontao, L.; Meroni, P.L.; Chizzolini, C. Interleukin-17A+ Cell Counts Are Increased in Systemic Sclerosis Skin and Their Number Is Inversely Correlated with the Extent of Skin Involvement. Arthritis Rheum. 2013, 65, 1347–1356. [Google Scholar] [CrossRef]
- Nakashima, T.; Jinnin, M.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; et al. Impaired IL-17 Signaling Pathway Contributes to the Increased Collagen Expression in Scleroderma Fibroblasts. J. Immunol. 2012, 188, 3573–3583. [Google Scholar] [CrossRef]
- Braun, R.K.; Ferrick, C.; Neubauer, P.; Sjoding, M.; Sterner-Kock, A.; Kock, M.; Putney, L.; Ferrick, D.A.; Hyde, D.M.; Love, R.B. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation 2008, 31, 167–179. [Google Scholar] [CrossRef]
- Ramani, K.; Garg, A.V.; Jawale, C.V.; Conti, H.R.; Whibley, N.; Jackson, E.K.; Shiva, S.S.; Horne, W.; Kolls, J.K.; Gaffen, S.L.; et al. The Kallikrein-Kinin System: A Novel Mediator of IL-17-Driven Anti-Candida Immunity in the Kidney. PLoS Pathog. 2016, 12, e1005952. [Google Scholar] [CrossRef]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Pérez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodríguez-Pascual, F.; et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef]
- Salzer, W.L. Peritoneal dialysis-related peritonitis: Challenges and solutions. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 173–186. [Google Scholar] [CrossRef]
- Szeto, C.C.; Li, P.K.T. Peritoneal dialysis–associated peritonitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1100–1105. [Google Scholar] [CrossRef]
- Hurst, S.M.; Wilkinson, T.S.; McLoughlin, R.M.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, K.B.; Lam, C.W.K.; Chan, T.M.; Li, F.K.; Leung, J.C.K. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 2000, 35, 644–652. [Google Scholar] [CrossRef]
- Chung, D.R.; Kasper, D.L.; Panzo, R.J.; Chtinis, T.; Grusby, M.J.; Sayegh, M.H.; Tzianabos, A.O. CD4+ T Cells Mediate Abscess Formation in Intra-abdominal Sepsis by an IL-17-Dependent Mechanism. J. Immunol. 2003, 170, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.G.; O’Keeffe, K.M.; Lalor, S.J.; Maher, B.M.; Mills, K.H.G.; McLoughlin, R.M. Staphylococcus aureus Infection of Mice Expands a Population of Memory γδ T Cells That Are Protective against Subsequent Infection. J. Immunol. 2014, 192, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Lou, J.; Zhu, J.; He, M.; Deng, X.; Cai, Z. Neutralisation of Peritoneal IL-17A Markedly Improves the Prognosis of Severe Septic Mice by Decreasing Neutrophil Infiltration and Proinflammatory Cytokines. PLoS ONE 2012, 7, e46506. [Google Scholar] [CrossRef]
- Wang, H.H.; Lin, C.Y. Interleukin-12 and -18 levels in peritoneal dialysate effluent correlate with the outcome of peritonitis in patients undergoing peritoneal dialysis: Implications for the type I/type II T-cell immune response. Am. J. Kidney Dis. 2005, 46, 328–338. [Google Scholar] [CrossRef]
- Lin, C.Y.; Roberts, G.W.; Kift-Morgan, A.; Donovan, K.L.; Topley, N.; Eberl, M. Pathogen-specific local immune fingerprints diagnose bacterial infection in peritoneal dialysis patients. J. Am. Soc. Nephrol. 2013, 24, 2002–2009. [Google Scholar] [CrossRef]
- Zhang, J.; Friberg, I.M.; Kift-Morgan, A.; Parekh, G.; Morgan, M.P.; Liuzzi, A.R.; Lin, C.Y.; Donovan, K.L.; Colmont, C.S.; Morgan, P.H.; et al. Machine-learning algorithms define pathogen-specific local immune fingerprints in peritoneal dialysis patients with bacterial infections. Kidney Int. 2017, 92, 179–191. [Google Scholar] [CrossRef]
- Habib, S.M.; Abrahams, A.C.; Korte, M.R.; Zietse, R.; De Vogel, L.L.; Boer, W.H.; Dendooven, A.; Van Groningen, M.C.C.; Betjes, M.G.H. CD4-positive T cells and M2 macrophages dominate the peritoneal infiltrate of patients with encapsulating peritoneal sclerosis. PLoS ONE 2015, 10, e0120174. [Google Scholar] [CrossRef][Green Version]
- Hu, W.; Jiang, Z.; Zhang, Y.; Liu, Q.; Fan, J.; Luo, N.; Dong, X.; Yu, X. Characterization of infiltrating macrophages in high glucose-induced peritoneal fibrosis in rats. Mol. Med. Rep. 2012, 6, 93–99. [Google Scholar] [CrossRef]
- Fernandez de Castro, M.; Selgas, R.; Jimenez, C.; Auxiliadora Bajo, M.; Martinez, V.; Romero, J.R.; de Alvaro, F.; Vara, F. Cell populations present in the nocturnal peritoneal effluent of patients on continuous ambulatory peritoneal dialysis and their relationship with peritoneal function and incidence of peritonitis. Perit. Dial. Int. 1994, 14, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Mak, T.S.K.; Lan, H.Y. Macrophages in Renal Fibrosis. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 285–303. [Google Scholar]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Wang, Y.; Harris, D.C.H. Macrophages in renal disease. J. Am. Soc. Nephrol. 2011, 22, 21–27. [Google Scholar] [CrossRef]
- Kinsey, G.R. Macrophage dynamics in AKI to CKD progression. J. Am. Soc. Nephrol. 2014, 25, 209–211. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Liao, C.-T.; Andrews, R.; Wallace, L.E.; Khan, M.W.A.; Kift-Morgan, A.; Topley, N.; Fraser, D.J.; Taylor, P.R. Peritoneal macrophage heterogeneity is associated with different peritoneal dialysis outcomes. Kidney Int. 2017, 91, 1088–1103. [Google Scholar] [CrossRef]
- Fieren, M.W.J.A. Mechanisms Regulating Cytokine Release from Peritoneal Macrophages during Continuous Ambulatory Peritoneal Dialysis. Blood Purif. 1996, 14, 179–187. [Google Scholar] [CrossRef]
- Bellon, T.; Martinez, V.; Lucendo, B.; del Peso, G.; Castro, M.J.; Aroeira, L.S.; Rodriguez-Sanz, A.; Ossorio, M.; Sanchez-Villanueva, R.; Selgas, R.; et al. Alternative activation of macrophages in human peritoneum: Implications for peritoneal fibrosis. Nephrol. Dial. Transplant 2011, 26, 2995–3005. [Google Scholar] [CrossRef]
- Ossorio, M.; Martinez, V.; Bajo, M.-A.; Del Peso, G.; Castro, M.-J.; Romero, S.; Selgas, R.; Bellon, T. Prominent Levels of the Profibrotic Chemokine CCL18 during Peritonitis: In Vitro Downregulation by Vitamin D Receptor Agonists. Biomed Res. Int. 2018, 2018, 6415892. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, E. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J. Leukoc. Biol. 2005, 78, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsu, H.; Lin, C.C.; Pan, S.Y.; Liu, S.Y.; Wu, C.F.; Tsai, P.Z.; Liao, C.T.; Cheng, H.T.; Chiang, W.C.; et al. Inflammatory macrophages switch to CCL17-expressing phenotype and promote peritoneal fibrosis. J. Pathol. 2020, 250, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, M.; Liu, Y.; Sun, W.; Shi, J.; Ni, J.; Wang, Q. A pathogenetic role for M1 macrophages in peritoneal dialysis-associated fibrosis. Mol. Immunol. 2018, 94, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.P.; Su, N.; Fan, J.J.; Ruan, Y.P.; Peng, W.X.; Li, Y.F.; Yu, X.Q. The role of peritoneal alternatively activated macrophages in the process of peritoneal fibrosis related to peritoneal dialysis. Int. J. Mol. Sci. 2013, 14, 10369–10382. [Google Scholar] [CrossRef] [PubMed]
- Sikorska- Wiśniewska, M.; Mika, A.; Śledziński, T.; Małgorzewicz, S.; Stepnowski, P.; Rutkowski, B.; Chmielewski, M. Disorders of serum omega-3 fatty acid composition in dialyzed patients, and their associations with fat mass. Ren. Fail. 2017, 39, 406–412. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, X.; Gong, C.; Liu, H.; Liu, F. Protective effects and mechanisms of omega-3 polyunsaturated fatty acid on intestinal injury and macrophage polarization in peritoneal dialysis rats. Nephrology 2019, 24, 1081–1089. [Google Scholar] [CrossRef]
- Sergejeva, S.; Linden, A. Impact of IL-17 on Cells of the Monocyte Lineage in Health and Disease. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 178–186. [Google Scholar] [CrossRef][Green Version]
- Shahrara, S.; Pickens, S.R.; Dorfleutner, A.; Pope, R.M. IL-17 Induces Monocyte Migration in Rheumatoid Arthritis. J. Immunol. 2009, 182, 3884–3891. [Google Scholar] [CrossRef]
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 induces macrophages to M2-like phenotype via NF-κB. Cancer Manag. Res. 2018, 10, 4217–4228. [Google Scholar] [CrossRef]
- Liu, L.; Ge, D.; Ma, L.; Mei, J.; Liu, S.; Zhang, Q.; Ren, F.; Liao, H.; Pu, Q.; Wang, T.; et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J. Thorac. Oncol. 2012, 7, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Q.; Atsuta, I.; Liu, S.; Chen, C.; Shi, S.; Le, A.D. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin. Cancer Res. 2013, 19, 3176–3188. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Nishikawa, K.; Seo, N.; Torii, M.; Ma, N.; Muraoka, D.; Tawara, I.; Masuya, M.; Tanaka, K.; Takei, Y.; Shiku, H.; et al. Interleukin-17 induces an atypical M2-Like macrophage subpopulation that regulates intestinal inflammation. PLoS ONE 2014, 9, e108494. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.X.; Tang, X.; Zhu, J.Y.; Zhang, W.; Tang, W.Q.; Yan, J.; Xu, X.; Liang, H.P. Cytochrome P450 1A1 enhances Arginase-1 expression, which reduces LPS-induced mouse peritonitis by targeting JAK1/STAT6. Cell. Immunol. 2020, 349, 104047. [Google Scholar] [CrossRef]
- Stengel, S.; Quickert, S.; Lutz, P.; Ibidapo-Obe, O.; Steube, A.; Köse-Vogel, N.; Yarbakht, M.; Reuken, P.A.; Busch, M.; Brandt, A.; et al. Peritoneal Level of CD206 Associates With Mortality and an Inflammatory Macrophage Phenotype in Patients With Decompensated Cirrhosis and Spontaneous Bacterial Peritonitis. Gastroenterology 2020, 158, 1745–1761. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Aguilera, A.; Selgas, R.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; Del Peso, G.; Sánchez-Tomero, J.A.; Jiménez-Heffernan, J.A.; et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: Role of vascular endothelial growth factor. Am. J. Kidney Dis. 2005, 46, 938–948. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [PubMed]
- Numata, M.; Nakayama, M.; Nimura, S.; Kawakami, M.; Lindholm, B.; Kawaguchi, Y. Association between an increased surface area of peritoneal microvessels and a high peritoneal solute transport rate. Perit. Dial. Int. 2003, 23, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lozano, M.L.; Sandoval, P.; Rynne-Vidal, Á.; Aguilera, A.; Jiménez-Heffernan, J.A.; Albar-Vizcaíno, P.; Majano, P.L.; Sánchez-Tomero, J.A.; Selgas, R.; López-Cabrera, M. Functional Relevance of the Switch of VEGF Receptors/Co-Receptors during Peritoneal Dialysis-Induced Mesothelial to Mesenchymal Transition. PLoS ONE 2013, 8, e60776. [Google Scholar] [CrossRef]
- Numasaki, M.; Fukushi, J.I.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef]
- Huang, Q.; Duan, L.; Qian, X.; Fan, J.; Lv, Z.; Zhang, X.; Han, J.; Wu, F.; Guo, M.; Hu, G.; et al. IL-17 Promotes Angiogenic Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung Adenocarcinoma. Sci. Rep. 2016, 6, 36551. [Google Scholar] [CrossRef]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, J.; Wu, X.; Chen, Y.; Yuan, W.; Chen, H. Interleukin-17 upregulates vascular endothelial growth factor by activating the JAK/STAT pathway in nucleus pulposus cells. Jt. Bone Spine 2017, 84, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp. Cell Res. 2011, 317, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Addison, C.L.; Daniel, T.O.; Burdick, M.D.; Liu, H.; Ehlert, J.E.; Xue, Y.Y.; Buechi, L.; Walz, A.; Richmond, A.; Strieter, R.M. The CXC Chemokine Receptor 2, CXCR2, Is the Putative Receptor for ELR + CXC Chemokine-Induced Angiogenic Activity. J. Immunol. 2000, 165, 5269–5277. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Wu, S.; Tan, H.; Sun, Y.; Liu, X.; Si, S.; Xu, L.; Huang, J.; Zhou, W.; et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol. Med. Rep. 2019, 20, 1065–1074. [Google Scholar] [CrossRef]
- Wei, Z.W.; Xia, G.K.; Wu, Y.; Chen, W.; Xiang, Z.; Schwarz, R.E.; Brekken, R.A.; Awasthi, N.; He, Y.L.; Zhang, C.H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015, 359, 335–343. [Google Scholar] [CrossRef]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates VEGF expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappa B through the CBM (Carma3/Bcl10/Matl1) complex. J. Biol. Chem. 2008, 284, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Yoshida, H.; Wakabayashi, Y.; Chinen, T.; Saeki, K.; Nakaya, M.; Takaesu, G.; Hori, S.; Yoshimura, A.; Kobayashi, T. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J. Biol. Chem. 2008, 283, 17003–17008. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. (Hradec Kralove) 2010, 53, 73–77. [Google Scholar]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-Β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef]
- Cravedi, P.; Remuzzi, G.; Ruggenenti, P. Targeting the Renin Angiotensin System in Dialysis Patients. Semin. Dial. 2011, 24, 290–297. [Google Scholar] [CrossRef]
- Noh, H.; Ha, H.; Yu, M.R.; Kim, Y.O.; Kim, J.H.; Lee, H.B. Angiotensin II mediates high glucose-induced TGF-beta1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit. Dial. Int. 2005, 25, 38–47. [Google Scholar]
- Nessim, S.J.; Perl, J.; Bargman, J.M. The renin-angiotensin-aldosterone system in peritoneal dialysis: Is what is good for the kidney also good for the peritoneum. Kidney Int. 2010, 78, 23–28. [Google Scholar] [CrossRef]
- Nakamoto, H.; Imai, H.; Fukushima, R.; Ishida, Y.; Yamanouchi, Y.; Suzuki, H. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit. Dial. Int. 2008, 28 (Suppl. 3), S83–S87. [Google Scholar]
- Kyuden, Y.; Ito, T.; Masaki, T.; Yorioka, N.; Kohno, N. Tgf-beta1 induced by high glucose is controlled by angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker on cultured human peritoneal mesothelial cells. Perit. Dial. Int. 2005, 25, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Duman, S.; Gunal, A.I.; Sen, S.; Asci, G.; Ozkahya, M.; Terzioglu, E.; Akcicek, F.; Atabay, G. Does enalapril prevent peritoneal fibrosis induced by hypertonic (3.86%) peritoneal dialysis solution? Perit. Dial. Int. 2001, 21, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Duman, S.; Sen, S.; Duman, C.; Oreopoulos, D.G. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int. J. Artif. Organs 2005, 28, 156–163. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Dekker, F.W.; Noordzij, M.; le Cessie, S.; Struijk, D.G.; Krediet, R.T. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit. Dial. Int. 2007, 27, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, X.; Fu, P.; Wu, H.M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst. Rev. 2014, 2014, CD009120. [Google Scholar] [CrossRef]
- Phatthanasobhon, S.; Nochaiwong, S.; Thavorn, K.; Noppakun, K.; Panyathong, S.; Suteeka, Y.; Hutton, B.; Sood, M.M.; Knoll, G.A.; Ruengorn, C. Effectiveness of Renin-Angiotensin-Aldosterone System Blockade on Residual Kidney Function and Peritoneal Membrane Function in Peritoneal Dialysis Patients: A Network Meta-Analysis. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Platten, M.; Youssef, S.; Eun, M.H.; Ho, P.P.; Han, M.H.; Lanz, T.V.; Phillips, L.K.; Goldstein, M.J.; Bhat, R.; Raine, C.S.; et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl. Acad. Sci. USA 2009, 106, 14948–14953. [Google Scholar] [CrossRef]
- Uzawa, A.; Mori, M.; Taniguchi, J.; Kuwabara, S. Modulation of the kallikrein/kinin system by the angiotensin-converting enzyme inhibitor alleviates experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2014, 178, 245–252. [Google Scholar] [CrossRef]
- Weber, J.; Tiriveedhi, V.; Takenaka, M.; Lu, W.; Hachem, R.; Trulock, E.; Patterson, G.A.; Mohanakumar, T. Inhibition of renin angiotensin aldosterone system causes abrogation of obliterative airways disease through inhibition of tumor necrosis factor-αdependant interleukin-17. J. Heart Lung Transplant. 2012, 31, 419–426. [Google Scholar] [CrossRef]
- Suda, N.; Moriyama, K.; Ganburgedc, G. Effect of angiotensin II receptor blocker on experimental periodontitis in a mouse model of marfan syndrome. Infect. Immun. 2013, 81, 182–188. [Google Scholar] [CrossRef]
- Coelho dos Santos, J.S.; Menezes, C.A.S.; Villani, F.N.A.; Magalhães, L.M.D.; Scharfstein, J.; Gollob, K.J.; Dutra, W.O. Captopril increases the intensity of monocyte infection by Trypanosoma cruzi and induces human T helper type 17 cells. Clin. Exp. Immunol. 2010, 162, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Nigwekar, S.U.; Perkovic, V.; Johnson, D.W.; Craig, J.C.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst. Rev. 2013, 9, CD004289. [Google Scholar]
- Obialo, C.I.; Ofili, E.O.; Norris, K.C. Statins and cardiovascular disease outcomes in chronic kidney disease: Reaffirmation vs. repudiation. Int. J. Environ. Res. Public Health 2018, 15, 2733. [Google Scholar] [CrossRef]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Barany, P.; Jaminon, A.M.; Schurgers, L.J.; Heimbürger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef]
- Kumar, S.; Raftery, M.; Yaqoob, M.; Fan, S.L.-S. Anti-inflammatory effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors (statins) in peritoneal dialysis patients. Perit. Dial. Int. 2007, 27, 283–287. [Google Scholar] [CrossRef]
- Carrión, B.; Pérez-Martínez, F.C.; Monteagudo, S.; Pérez-Carrión, M.D.; Gómez-Roldán, C.; Ceña, V.; Pérez-Martínez, J. Atorvastatin reduces high glucose toxicity in rat peritoneal mesothelial cells. Perit. Dial. Int. 2011, 31, 325–331. [Google Scholar] [CrossRef]
- Chang, T.I.; Kang, H.Y.; Kim, K.S.; Lee, S.H.; Nam, B.Y.; Paeng, J.; Kim, S.; Park, J.T.; Yoo, T.H.; Kang, S.W.; et al. The effect of statin on epithelial-mesenchymal transition in peritoneal mesothelial cells. PLoS ONE 2014, 9, e109628. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Liu, Y.; Xu, Y.; Zhao, X.; Qian, J.; Sun, B.; Xing, C. Fluvastatin inhibits the expression of fibronectin in human peritoneal mesothelial cells induced by high-glucose peritoneal dialysis solution via SGK1 pathway. Clin. Exp. Nephrol. 2015, 19, 336–342. [Google Scholar] [CrossRef]
- Duman, S.; Sen, S.; Sözmen, E.Y.; Oreopoulos, D.G. Atorvastatin improves peritoneal sclerosis induced by hypertonic PD solution in rats. Int. J. Artif. Organs 2005, 28, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Markovic-Plese, S. Statins’ immunomodulatory potential against Th17 cell-mediated autoimmune response. Immunol. Res. 2008, 41, 165–174. [Google Scholar]
- Zhang, X.; Jin, J.; Peng, X.; Ramgolam, V.S.; Markovic-Plese, S. Simvastatin Inhibits IL-17 Secretion by Targeting Multiple IL-17-Regulatory Cytokines and by Inhibiting the Expression of IL-17 Transcription Factor RORC in CD4+ Lymphocytes. J. Immunol. 2008, 180, 6988–6996. [Google Scholar] [CrossRef]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized Low-Density Lipoprotein (OxLDL)-Treated Dendritic Cells Promote Activation of T Cells in Human Atherosclerotic Plaque and Blood, Which Is Repressed by Statins: MicroRNA let-7c Is Integral to the Effect. J. Am. Heart Assoc. 2016, 5, e003976. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Niu, X.; Liu, J.; Ping, M.; Li, R.; Xie, X.; Guo, L. Immunomodulatory synergy by combining atorvastatin and rapamycin in the treatment of experimental autoimmune encephalomyelitis (EAE). J. Neuroimmunol. 2012, 250, 9–17. [Google Scholar] [CrossRef]
- Aktunc, E.; Kayhan, B.; Arasli, M.; Gun, B.D.; Barut, F. The effect of atorvastatin and its role on systemic cytokine network in treatment of acute experimental colitis. Immunopharmacol. Immunotoxicol. 2011, 33, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kang, E.W.; Yoon, S.-J.; Yoon, H.S.; Lee, H.C.; Yoo, T.H.; Choi, K.H.; Han, D.-S.; Kang, S.-W. Combined vascular effects of HMG-CoA reductase inhibitor and angiotensin receptor blocker in non-diabetic patients undergoing peritoneal dialysis. Nephrol. Dial. Transpl. 2011, 26, 3722–3728. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Wei, F.; Ye, L.; Lu, F.; Zhang, H.; Diao, Y.; Song, H.; Qi, Z. Treatment with telmisartan/rosuvastatin combination has a beneficial synergistic effect on ameliorating Th17/Treg functional imbalance in hypertensive patients with carotid atherosclerosis. Atherosclerosis 2014, 233, 291–299. [Google Scholar] [CrossRef]
- Ma, X.; Liu, S.; Li, T.; Yuan, H. Intensive statin treatment ameliorate the Th17/Treg functional imbalance in patients with non-ST elevation acute coronary syndrome underwent percutaneous coronary intervention. Clin. Cardiol. 2019, 43, 379–385. [Google Scholar] [CrossRef]
- Rostamzadeh, D.; Yousefi, M.; Haghshenas, M.R.; Ahmadi, M.; Dolati, S.; Babaloo, Z. mTOR Signaling pathway as a master regulator of memory CD8+ T-cells, Th17, and NK cells development and their functional properties. J. Cell. Physiol. 2019, 234, 12353–12368. [Google Scholar]
- Ikejiri, A.; Nagai, S.; Goda, N.; Kurebayashi, Y.; Osada-Oka, M.; Takubo, K.; Suda, T.; Koyasu, S. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int. Immunol. 2012, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Miao, J.; Cao, R.; Han, M.; Sun, Y.; Liu, X.; Guo, L. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the mTOR-STAT3 Pathway. Neurochem. Res. 2017, 42, 2831–2840. [Google Scholar] [CrossRef]
- Hou, H.; Cao, R.; Quan, M.; Sun, Y.; Sun, H.; Zhang, J.; Li, B.; Guo, L.; Song, X. Rapamycin and fingolimod modulate Treg/Th17 cells in experimental autoimmune encephalomyelitis by regulating the Akt-mTOR and MAPK/ERK pathways. J. Neuroimmunol. 2018, 324, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Sekiguchi, Y.; Oh, K.H.; Patterson, S.E.; Kolb, M.R.J.; Margetts, P.J. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 2010, 77, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Zhang, J.; Patterson, S.; Liu, L.; Hamada, C.; Tomino, Y.; Margetts, P.J. Rapamycin inhibits transforming growth factor β-induced peritoneal angiogenesis by blocking the secondary hypoxic response. J. Cell. Mol. Med. 2012, 16, 1934–1945. [Google Scholar] [CrossRef]
- Aguilera, A.; Aroeira, L.S.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; Del Peso, G.; Valenzuela-Fernández, A.; Sánchez-Tomero, J.A.; López-Cabrera, M.; et al. Effects of rapamycin on the epithelial-to-mesenchymal transition of human peritoneal mesothelial cells. Int. J. Artif. Organs 2005, 28, 164–169. [Google Scholar] [CrossRef]
- Gonzalez-Mateo, G.T.; Aguirre, A.R.; Loureiro, J.; Abensur, H.; Sandoval, P.; Sanchez-Tomero, J.A.; del Peso, G.; Jimenez-Heffernan, J.A.; Ruiz-Carpio, V.; Selgas, R.; et al. Rapamycin Protects from Type-I Peritoneal Membrane Failure Inhibiting the Angiogenesis, Lymphangiogenesis, and Endo-MT. Biomed Res. Int. 2015, 2015, 989560. [Google Scholar] [CrossRef]
- Xiang, S.; Li, M.; Xie, X.; Xie, Z.; Zhou, Q.; Tian, Y.; Lin, W.; Zhang, X.; Jiang, H.; Shou, Z.; et al. Rapamycin inhibits epithelial-to-mesenchymal transition of peritoneal mesothelium cells through regulation of Rho GTPases. FEBS J. 2016, 283, 2309–2325. [Google Scholar] [CrossRef]
- Xu, T.; Xie, J.Y.; Wang, W.M.; Ren, H.; Chen, N. Impact of rapamycin on peritoneal fibrosis and transport function. Blood Purif. 2012, 34, 48–57. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, C.-M.; Feng, Y.; Zhu, W.; Jin, B.; Xia, Y.-Y.; Zhang, Q.-Y.; Xu, P.-F.; Zhang, M. Rapamycin inhibits peritoneal fibrosis by modifying lipid homeostasis in the peritoneum. Am. J. Transl. Res. 2019, 11, 1473–1485. [Google Scholar]
- Aroeira, L.S.; Lara-Pezzi, E.; Loureiro, J.; Aguilera, A.; Ramírez-Huesca, M.; González-Mateo, G.; Pérez-Lozano, M.L.; Albar-Vizcaíno, P.; Bajo, M.A.; Del Peso, G.; et al. Cyclooxygenase-2 mediates dialysate-Lnduced alterations of the peritoneal membrane. J. Am. Soc. Nephrol. 2009, 20, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, P.; Schilte, M.N.; Zareie, M.; ter Wee, P.M.; Keuning, E.D.; Beelen, R.H.J.; van den Born, J. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol. Dial. Transplant. 2009, 24, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Kim, S.H.; Chang, J.W.; Lee, S.K.; Min, W.K.; Chi, H.S.; Park, J.S. Effects of celecoxib on high-sensitivity C-reactive protein in chronic peritoneal dialysis patients. Ren. Fail. 2004, 26, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Alyce Bradbury, J.; Dackor, R.T.; Edin, M.L.; Graves, J.P.; DeGraff, L.M.; Wang, P.M.; Bortner, C.D.; Maruoka, S.; Lih, F.B.; et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am. J. Respir. Crit. Care Med. 2011, 184, 37–49. [Google Scholar] [CrossRef]
- Paulissen, S.M.J.; van Hamburg, J.P.; Davelaar, N.; Asmawidjaja, P.S.; Hazes, J.M.W.; Lubberts, E. Synovial Fibroblasts Directly Induce Th17 Pathogenicity via the Cyclooxygenase/Prostaglandin E2 Pathway, Independent of IL-23. J. Immunol. 2013, 191, 1364–1372. [Google Scholar] [CrossRef]
- Perazella, M.A. COX-2 selective inhibitors: Analysis of the renal effects. Expert Opin. Drug Saf. 2002, 1, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Novak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.S.; Kim, S.R.; Min, K.H.; Choe, Y.H.; Moon, H.; Chae, H.J.; Yoo, W.H.; Lee, Y.C. Peroxisome Proliferator-Activated Receptor γ Agonist Down-Regulates IL-17 Expression in a Murine Model of Allergic Airway Inflammation. J. Immunol. 2009, 183, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; He, J.; Li, C.; Deng, W.; Ran, X.; Wang, D. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates airway inflammation by inhibiting the proliferation of effector T cells in a murine model of neutrophilic asthma. Immunol. Lett. 2014, 157, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Farnesi-de-Assunção, T.S.; Alves, C.F.; Carregaro, V.; de Oliveira, J.R.; da Silva, C.A.T.; Cheraim, A.B.; Cunha, F.Q.; Napimoga, M.H. PPAR-γ agonists, mainly 15d-PGJ 2, reduce eosinophil recruitment following allergen challenge. Cell. Immunol. 2012, 273, 23–29. [Google Scholar] [CrossRef]
- Sandoval, P.; Loureiro, J.; González-Mateo, G.; Pérez-Lozano, M.L.; Maldonado-Rodríguez, A.; Sánchez-Tomero, J.A.; Mendoza, L.; Santamaría, B.; Ortiz, A.; Ruíz-Ortega, M.; et al. PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Lab. Investig. 2010, 90, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wang, Q.; Su, Y.Y.; Wang, J.L.; Hua, B.J.; Yang, S.; Feng, J.X.; Li, H.Y. PPAR-γ agonist rosiglitazone protects rat peritoneal mesothelial cells against peritoneal dialysis solution-induced damage. Mol. Med. Rep. 2017, 15, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, J.; Wang, Q.; Zhao, S.; Xu, J.; Li, H. PPAR-γ agonist rosiglitazone ameliorates peritoneal deterioration in peritoneal dialysis rats with LPS-induced peritonitis through up-regulation of AQP-1 and ZO-1. Biosci. Rep. 2018, 38, BSR20180009. [Google Scholar] [CrossRef] [PubMed]
- Pioglitazone Actavis | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pioglitazone-actavis (accessed on 27 April 2020).
- Aufricht, C.; Endemann, M.; Bidmon, B.; Arbeiter, K.; Mueller, T.; Regele, H.; Herkner, K.; Eickelberg, O. Peritoneal dialysis fluids induce the stress response in human mesothelial cells. Perit. Dial. Int. 2001, 21, 85–88. [Google Scholar] [CrossRef]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Lichtenauer, A.M.; Salzer, E.; Lechner, M.; Kuster, L.; Bergmeister, K.; Rizzi, A.; Mayer, B.; et al. Alanyl-glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids. Nephrol. Dial. Transplant. 2012, 27, 937–946. [Google Scholar] [CrossRef]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2515S–2522S; discussion 2523S–2524S. [Google Scholar] [CrossRef]
- Bender, T.O.; Böhm, M.; Kratochwill, K.; Lederhuber, H.; Endemann, M.; Bidmon, B.; Aufricht, C. HSP-mediated cytoprotection of mesothelial cells in experimental acute peritoneal dialysis. Perit. Dial. Int. 2010, 30, 294–299. [Google Scholar] [CrossRef]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C.; et al. Addition of alanyl-glutamine to dialysis fluid restores peritoneal cellular stress responses ± a first-in-man trial. PLoS ONE 2016, 11, e0165045. [Google Scholar] [CrossRef]
- Herzog, R.; Kuster, L.; Becker, J.; Gluexam, T.; Pils, D.; Spittler, A.; Bhasin, M.K.; Alper, S.L.; Vychytil, A.; Aufricht, C.; et al. Functional and Transcriptomic Characterization of Peritoneal Immune-Modulation by Addition of Alanyl-Glutamine to Dialysis Fluid. Sci. Rep. 2017, 7, 6229. [Google Scholar] [CrossRef]
- Ferrantelli, E.; Liappas, G.; Vila Cuenca, M.; Keuning, E.D.; Foster, T.L.; Vervloet, M.G.; Lopéz-Cabrera, M.; Beelen, R.H.J. The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis. Kidney Int. 2016, 89, 625–635. [Google Scholar] [CrossRef]
- González-Mateo, G.T.; Fernández-Míllara, V.; Bellón, T.; Liappas, G.; Ruiz-Ortega, M.; López-Cabrera, M.; Selgas, R.; Aroeira, L.S. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef]
- Stavenuiter, A.W.D.; Farhat, K.; Vila Cuenca, M.; Schilte, M.N.; Keuning, E.D.; Paauw, N.J.; ter Wee, P.M.; Beelen, R.H.J.; Vervloet, M.G. Protective Effects of Paricalcitol on Peritoneal Remodeling during Peritoneal Dialysis. Biomed Res. Int. 2015, 2015, 468574. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, S.O.; Cho, K.H.; Park, J.W.; Yoon, K.W.; Do, J.Y. Paricalcitol ameliorates epithelial-to-mesenchymal transition in the peritoneal mesothelium. Nephron Exp. Nephrol. 2014, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kang, H.J.; Kim, D.A.; Ryu, E.S.; Yu, M.; Lee, H.; Lee, H.K.; Ryu, H.M.; Park, S.H.; Kim, Y.L.; et al. Paricalcitol attenuates TGF-b1-induced phenotype transition of human peritoneal mesothelial cells (HPMCs) via modulation of oxidative stress and NLRP3 inflammasome. FASEB J. 2019, 33, 3035–3050. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, T.; Malho Guedes, A.; Del Peso, G.; Silva, A.P.; Selgas, R.; Bajo, M.A.; Neves, P.L. Paricalcitol and Peritoneal Protein Loss in Peritoneal Dialysis: A Double-Center Study. Blood Purif. 2018, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Pascual, J.L.; Marchant, V.; Rodrigues-Diez, R.; Dolade, N.; Suarez-Alvarez, B.; Kerr, B.; Valdivielso, J.M.; Ruiz-Ortega, M.; Rayego-Mateos, S. Epigenetic modification mechanisms involved in inflammation and fibrosis in renal pathology. Mediat. Inflamm. 2018, 2018, 2931049. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Yanai, K.; Ishii, H.; Aomatsu, A.; Ishibashi, K.; Morishita, Y. MicroRNAs in peritoneal fibrosis: A systematic review. Discov. Med. 2018, 26, 271–280. [Google Scholar]
- Chen, J.; Kam-Tao, P.; Kwan, B.C.H.; Chow, K.M.; Lai, K.B.; Luk, C.C.W.; Szeto, C.C. Relation between microRNA expression in peritoneal dialysis effluent and peritoneal transport characteristics. Dis. Markers 2012, 33, 35–42. [Google Scholar] [CrossRef]
- Khan, D.; Ahmed, S.A. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Front. Genet. 2015, 6, 236. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Maley, E.; Tran, T.; Yin, Y.; Meng, S.; Pansuria, M.; Jiang, X.; Wang, H.; Yang, X.F. MicroRNAs and other mechanisms regulate interleukin-17 cytokines and receptors. Front. Biosci. (Elite Ed.) 2012, 4, 1478–1495. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Da Cunha, A.P.; Ajay, A.K.; Joller, N.; Garo, L.P.; Kumaradevan, S.; Yosef, N.; Vaidya, V.S.; Weiner, H.L. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J. Clin. Investig. 2015, 125, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.X.; Zhang, G.S.; Cao, H.X.; Song, G.; Li, Z.T.; Zhang, W.T. Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma. Asian Pac. J. Trop. Med. 2015, 8, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, X.; Tan, J.; Li, H.; Qian, W.; Chen, J.; Chen, Q.; Wang, J.; Xu, W.; Tao, C.; et al. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J. Cell. Mol. Med. 2014, 18, 2213–2224. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Zoccali, C.; Goldsmith, D.; Agarwal, R.; Blankestijn, P.J.; Fliser, D.; Wiecek, A.; Suleymanlar, G.; Ortiz, A.; Massy, Z.; Covic, A.; et al. The complexity of the cardio-renal link: Taxonomy, syndromes, and diseases. Kidney Int. Suppl. 2011, 1, 2–5. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dent, H.; Hawley, C.M.; McDonald, S.P.; Rosman, J.B.; Brown, F.G.; Bannister, K.; Wiggins, K.J. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1620–1628. [Google Scholar] [CrossRef]
- Caillon, A.; Schiffrin, E.L. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr. Hypertens. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Yao, W.; Sun, Y.; Wang, X.; Niu, K. Elevated Serum Level of Interleukin 17 in a Population with Prehypertension. J. Clin. Hypertens. 2015, 17, 770–774. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Hogg, J.P.; Scott, J.; Wallace, K.; Herse, F.; Moseley, J.; Wallukat, G.; Dechend, R.; La Marca, B. Administration of interleukin-17 soluble receptor c suppresses T H17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 2013, 62, 1068–1073. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Esmaeeli-Nadimi, A.; Nough, H.; Nemati, M.; Taghi Rezayati, M. Serum levels of interleukin (IL)-13, IL-17 and IL-18 in patients with ischemic heart disease. Anadolu Kardiyol. Derg. 2009, 9, 75–83. [Google Scholar]
- Allam, G.; Abdel-Moneim, A.; Gaber, A.M. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed. Pharmacother. 2018, 106, 1412–1418. [Google Scholar] [CrossRef]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; van der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339. [Google Scholar] [CrossRef]
- Silfvast-Kaiser, A.; Paek, S.Y.; Menter, A. Anti-IL17 therapies for psoriasis. Expert Opin. Biol. Ther. 2019, 19, 45–54. [Google Scholar] [CrossRef]
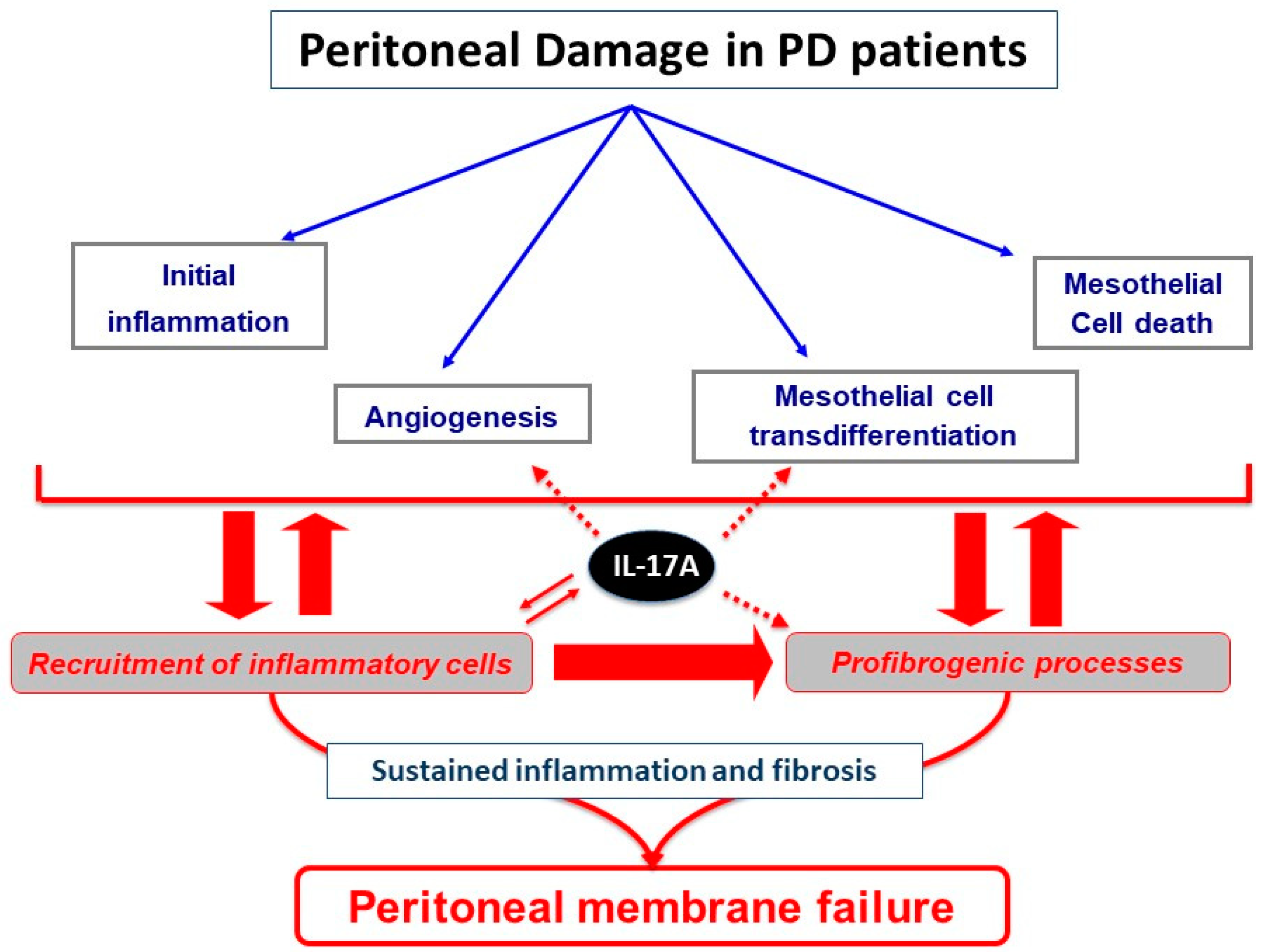

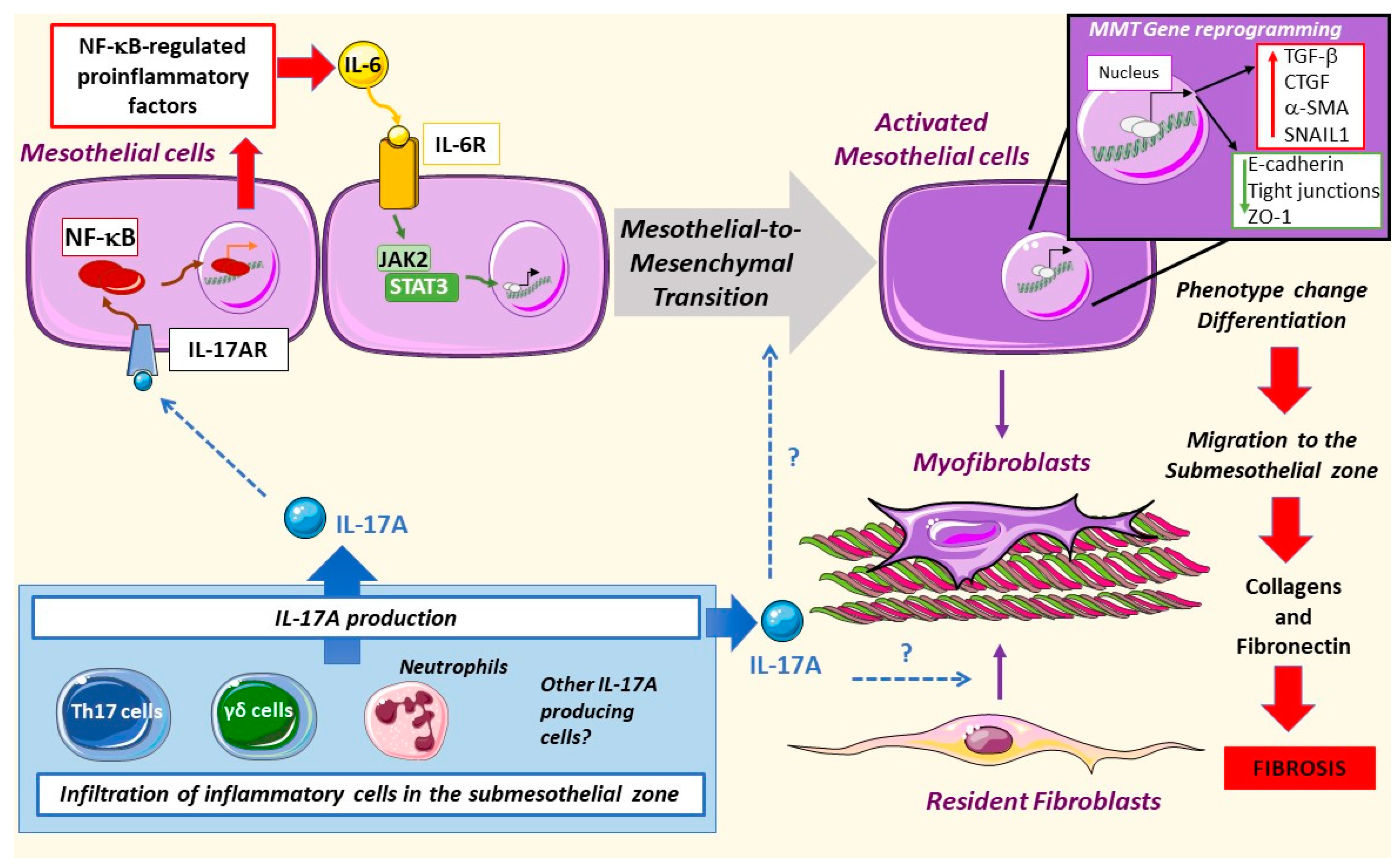
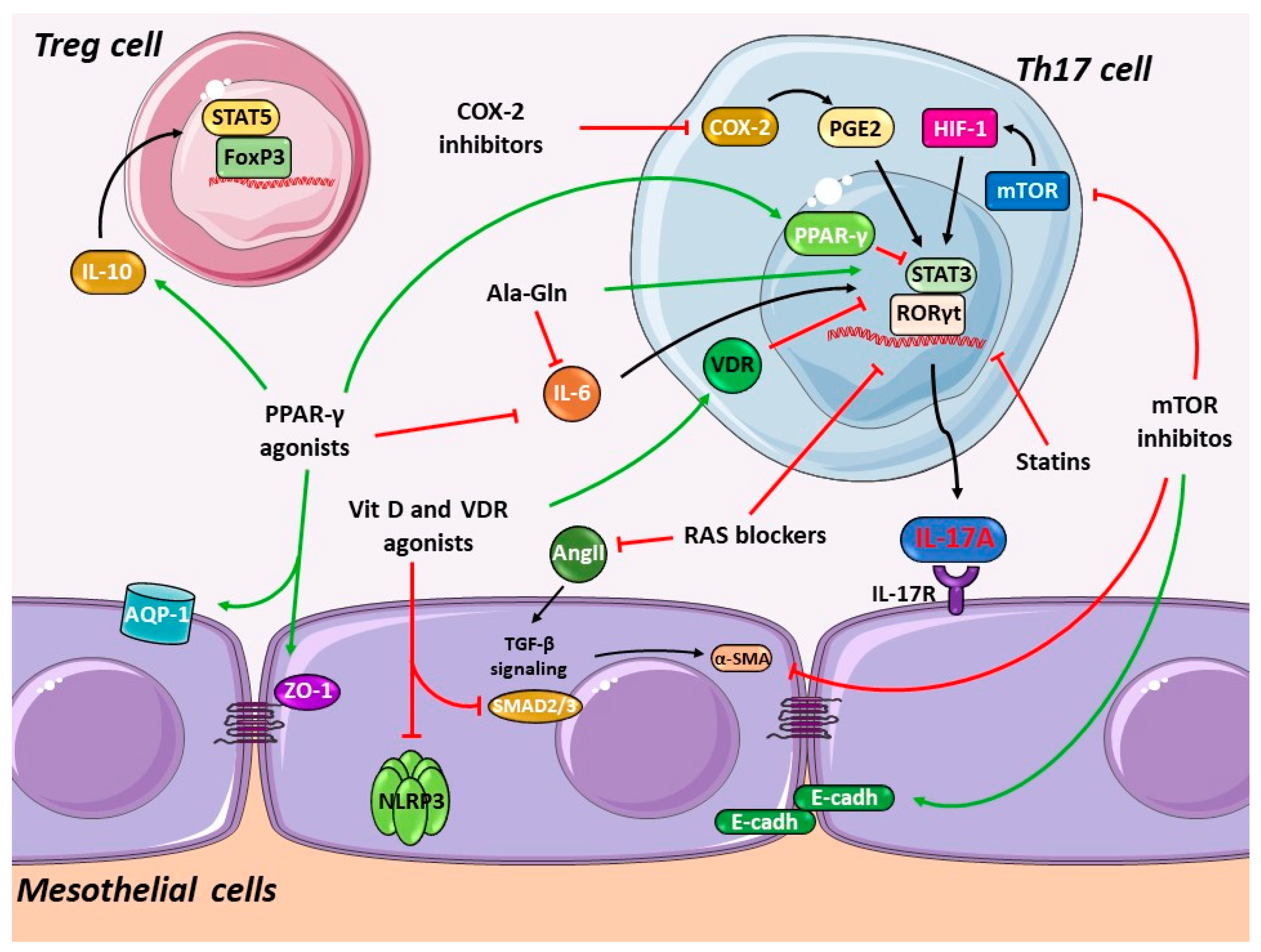
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchant, V.; Tejera-Muñoz, A.; Marquez-Expósito, L.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Tejedor, L.; Santos-Sanchez, L.; Egido, J.; Ortiz, A.; Valdivielso, J.M.; et al. IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis. Biomolecules 2020, 10, 1361. https://doi.org/10.3390/biom10101361
Marchant V, Tejera-Muñoz A, Marquez-Expósito L, Rayego-Mateos S, Rodrigues-Diez RR, Tejedor L, Santos-Sanchez L, Egido J, Ortiz A, Valdivielso JM, et al. IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis. Biomolecules. 2020; 10(10):1361. https://doi.org/10.3390/biom10101361
Chicago/Turabian StyleMarchant, Vanessa, Antonio Tejera-Muñoz, Laura Marquez-Expósito, Sandra Rayego-Mateos, Raul R. Rodrigues-Diez, Lucia Tejedor, Laura Santos-Sanchez, Jesús Egido, Alberto Ortiz, Jose M. Valdivielso, and et al. 2020. "IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis" Biomolecules 10, no. 10: 1361. https://doi.org/10.3390/biom10101361
APA StyleMarchant, V., Tejera-Muñoz, A., Marquez-Expósito, L., Rayego-Mateos, S., Rodrigues-Diez, R. R., Tejedor, L., Santos-Sanchez, L., Egido, J., Ortiz, A., Valdivielso, J. M., Fraser, D. J., López-Cabrera, M., Selgas, R., & Ruiz-Ortega, M. (2020). IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis. Biomolecules, 10(10), 1361. https://doi.org/10.3390/biom10101361







