Seed Priming with Jasmonic Acid Counteracts Root Knot Nematode Infection in Tomato by Modulating the Activity and Expression of Antioxidative Enzymes
Abstract
1. Introduction
2. Materials and Methods
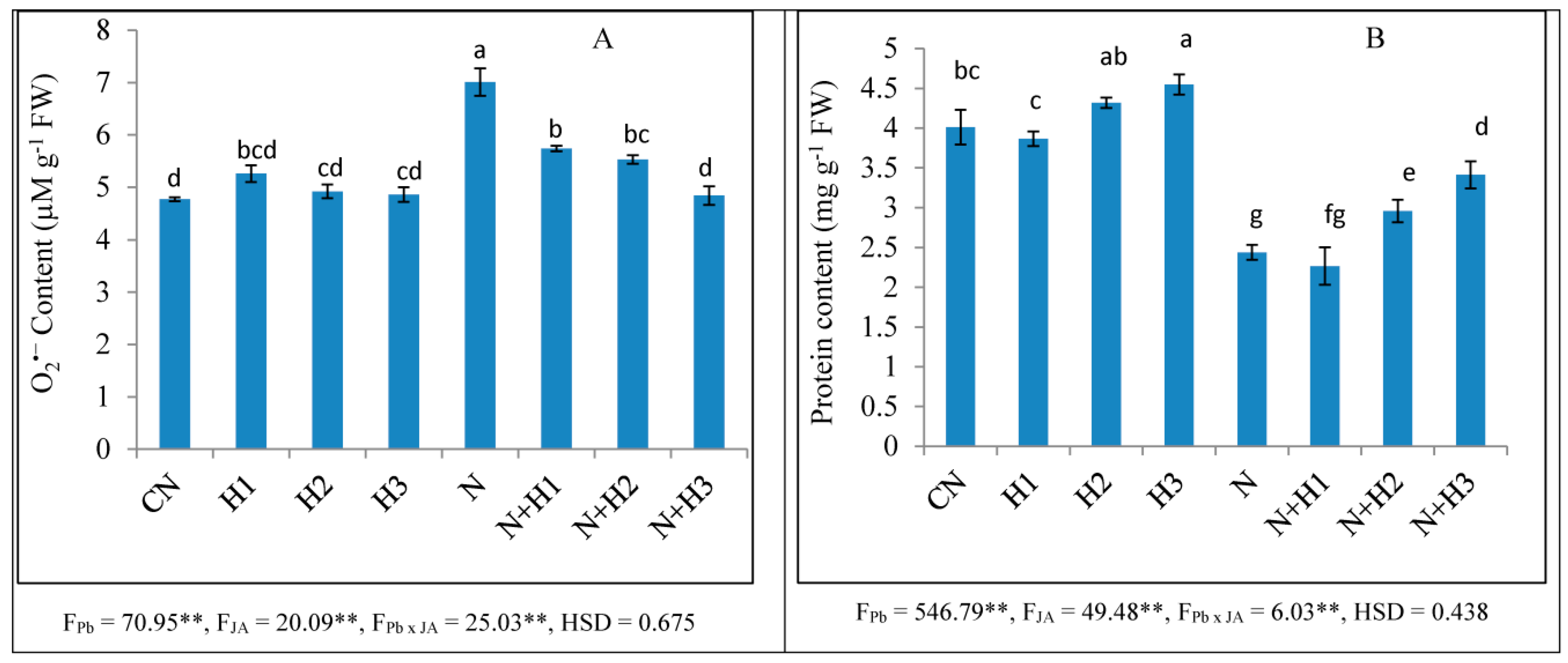
2.1. O2•− Content
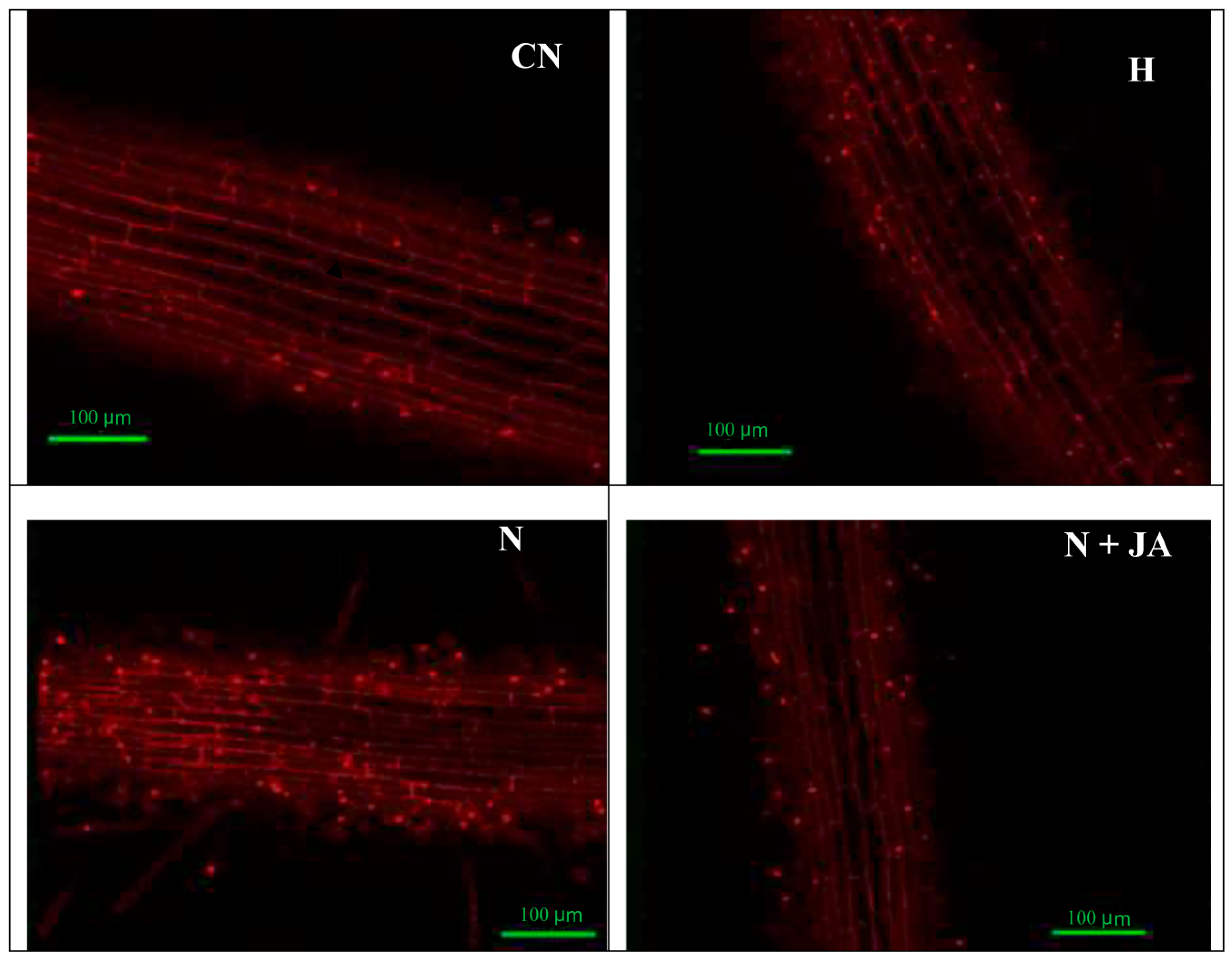
2.2. Membrane Damage
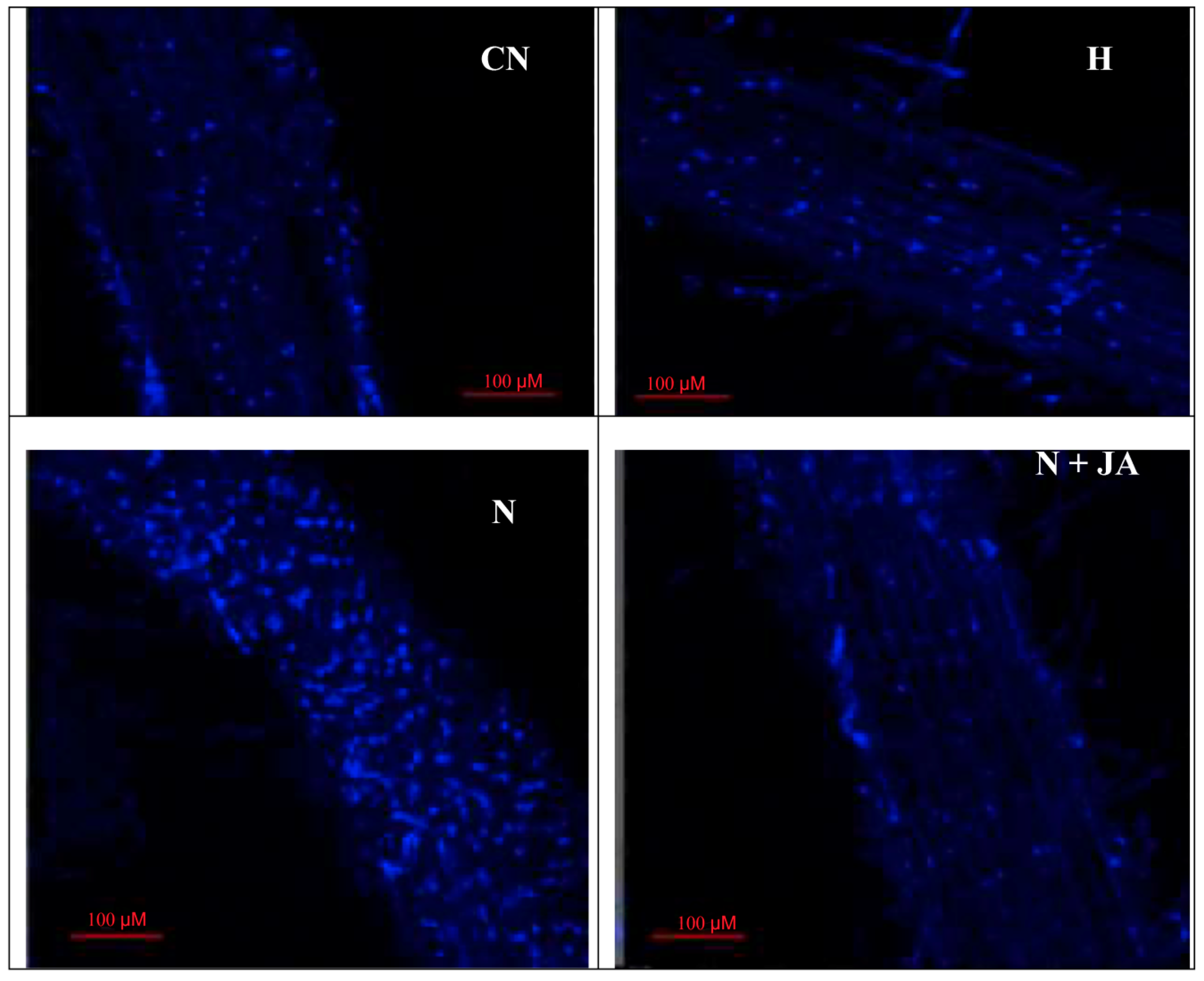
2.3. Nuclear Damage
2.4. Protein Content and Antioxidative Enzymes
2.5. Amino Acid Profiling
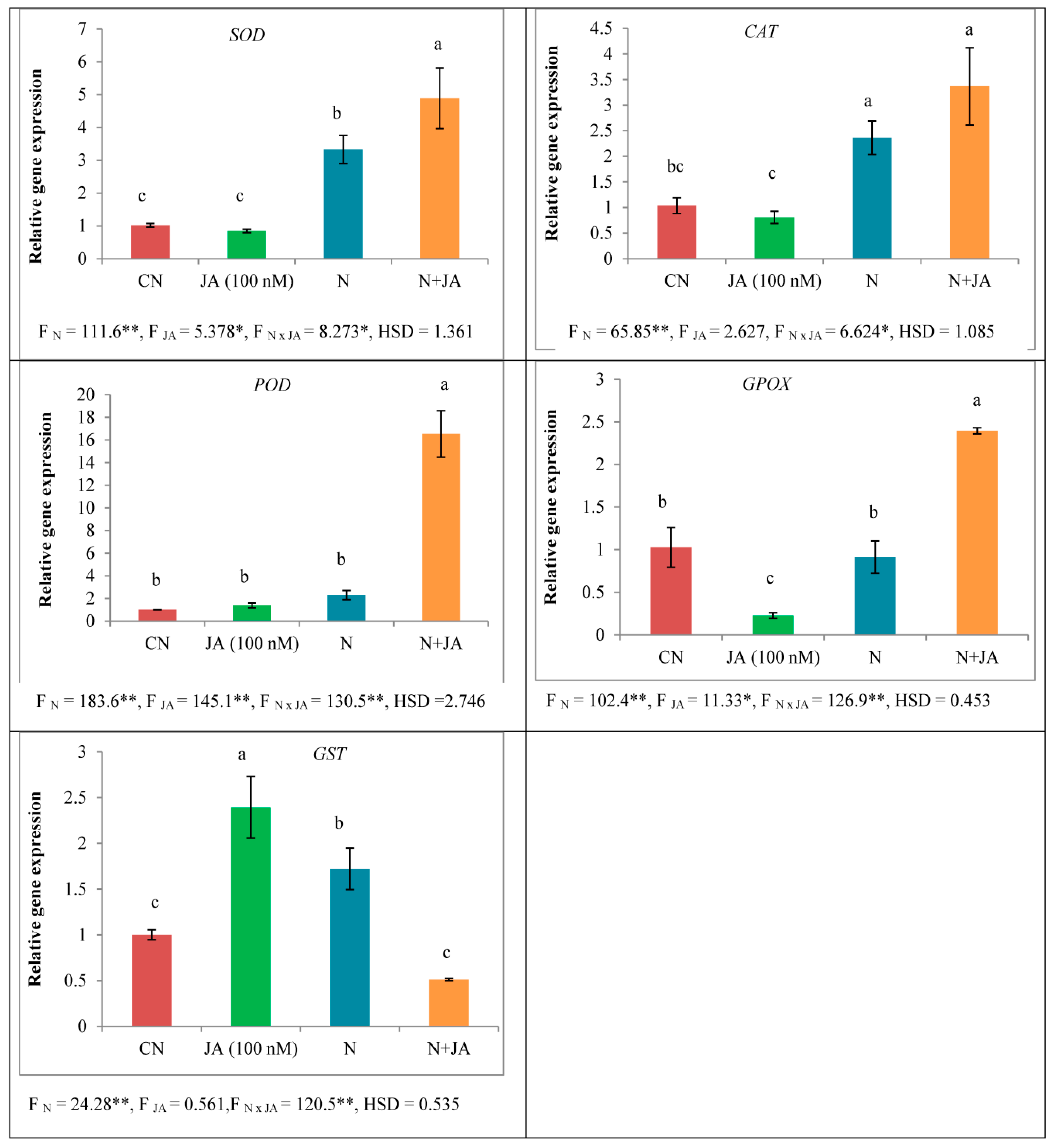
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Root Fresh Weight
3.2. O2•− Content
3.3. Membrane and Nuclear Damage
3.4. Protein Content and Antioxidative Enzymes Activities
3.5. Amino Acid Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kayani, M.Z.; Mukhtar, T.; Hussain, M.A.; Ul-Haque, M.I. Infestation assessment of root-knot nematodes (Meloidogyne spp.) associated with cucumber in the Pothowar region of Pakistan. Crop Prot. 2013, 47, 49–54. [Google Scholar] [CrossRef]
- Abad, P.; Favery, B.; Rosso, M.-N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Haseeb, A.; Srivastava, N.; Pandey, R. The influence of Meloidogyne incognita on growth, physiology, nutrient concentration and alkaloid yield of muHyoscyas niger. Nematol. Mediterr. 1990, 18, 127–129. [Google Scholar]
- Ahmed, N.; Abbasi, M.W.; Shaukat, S.S.; Zaki, M.J. Physiological changes in leaves of mungbean plants infected with Meloidogyne javanica. Phytopathol. Mediterr. 2009, 48, 262–268. [Google Scholar]
- Boia, B.; Neog, P. Effect of oil cakes for management of Meloidogyne incognita in tea. Ann. Plant Prot. Sci. 2006, 14, 522–523. [Google Scholar]
- Vovlas, N.; Troccoli, A.; Minuto, A.; Bruzzone, C.; Sasanelli, N.; Castillo, P. Pathogenicity and Host–Parasite Relationships of Meloidogyne arenaria in Sweet Basil. Plant Dis. 2008, 92, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Azam, T.; Hisamuddin; Singh, S.; Robab, M.I. Effect of different inoculum levels of Meloidogyne incognita on growth and yield of Lycopersicon esculentum, and internal structure of infected root. Arch. Phytopathol. Plant Prot. 2011, 44, 1829–1839. [Google Scholar] [CrossRef]
- Navrot, N.; Rouhier, N.; Gelhaye, E.; Jacquot, J.-P. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 2007, 129, 185–195. [Google Scholar] [CrossRef]
- Del Rio, L.A. Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, S.; Feussner, I. Oxylipins. In Plant Lipids; Murphy, D.J., Ed.; Blackwell: Oxford, UK, 2005; pp. 329–354. [Google Scholar]
- Nahar, K.; Kyndt, T.; De Vleesschauwer, D.; Höfte, M.; Gheysen, G. The Jasmonate Pathway is a Key Player in Systemically Induced Defense against Root Knot Nematodes in Rice. Plant Physiol. 2011, 157, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.W.; Hu, C.L.; Zhang, L.N.; Li, Z.L.; Zhao, F.K.; Wang, S.H. Jasmonic Acid Mediates Tomato’s Response to Root Knot Nematodes. J. Plant Growth Regul. 2014, 34, 196–205. [Google Scholar] [CrossRef]
- Gleason, C.; Leelarasamee, N.; Meldau, D.; Feussner, I. OPDA Has Key Role in Regulating Plant Susceptibility to the Root-Knot Nematode Meloidogyne hapla in Arabidopsis. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol. Entomol. 2001, 26, 312–324. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, F.; Shao, S.; Zhang, H.; Li, G.; Xia, X.; Zhou, Y.; Yu, J.; Shi, K. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front. Plant Sci. 2015, 6, 193. [Google Scholar] [CrossRef]
- Chitwood, B.G. Root-knot nematodes. Plant Soil 1951, 3, 47–50. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Liu, W.; Ye, X.F.; Zai, H.F.; Zhao, L.J. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: Changes in leaf antioxidant defense systems. Plant Soil Environ. 2010, 56, 470–475. [Google Scholar] [CrossRef]
- Gutierrez-Alcala, G.; Gotor, C.; Meyer, A.J.; Fricker, M.; Vega, J.M.; Romero, L.C. Glutathione biosynthesis in Arabidopsis trichome cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11108–11113. [Google Scholar] [CrossRef]
- Callard, D.; Axelos, M.; Mazzolini, L. Novel Molecular Markers for Late Phases of the Growth Cycle of Arabidopsis thaliana Cell-Suspension Cultures Are Expressed during Organ Senescence. Plant Physiol. 1996, 112, 705–715. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 685–690. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dalton, D.A.; Russell, S.A.; Hanus, F.J.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Kumar, K.; Khan, P. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of Resistance to Gray Mold with Benzothiadiazole Modifies Amino Acid Profile and Increases Proanthocyanidins in Grape: Primary versus Secondary Metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139. [Google Scholar] [CrossRef]
- Awasthi, P.; Mahajan, V.; Jamwal, V.L.; Kapoor, N.; Rasool, S.; Bedi, Y.S.; Gandhi, S.G. Cloning and expression analysis of chalcone synthase gene from Coleus forskohlii. J. Genet. 2016, 95, 647–657. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Mahajan, V.; Rather, I.A.; Gupta, A.P.; Rasool, S.; Bedi, Y.S.; Vishwakarma, R.A.; Gandhi, S.G. Plant Omics: Isolation, Identification, and Expression Analysis of Cytochrome P450 Gene Sequences from Coleus forskohlii. Omics J. Integr. Biol. 2015, 19, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.R.; Ghani, M. The optimal concentrations of Purpureocillium lilacinum and jasmonic acid in controlling Meloidogyn javanica on tomato. Arch. Phytopathol. Plant Prot. 2019, 52, 582–600. [Google Scholar] [CrossRef]
- Lahari, Z.; Ullah, C.; Kyndt, T.; Gershenzon, J.; Gheysen, G. Strigolactones enhance root-knot nematode (Meloidogyne graminicola) infection in rice by antagonizing the jasmonate pathway. New Phytol. 2019, 224, 454–465. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, F.; Bao, A.; You, Q.; Li, Z.; Chen, J.; Cheng, Y.; Zhao, W.; Shen, X.; Zhou, X.; et al. The soybean Rhg1 amino acid transporter gene alters glutamate homeostasis and jasmonic acid-induced resistance to soybean cyst nematode. Mol. Plant Pathol. 2018, 20, 270–286. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, K.; Sun, S.; Zhou, J.; Yu, J.-Q. COP9 Signalosome CSN4 and CSN5 Subunits Are Involved in Jasmonate-Dependent Defense Against Root-Knot Nematode in Tomato. Front. Plant Sci. 2019, 10, 1223. [Google Scholar] [CrossRef]
- Clemens, S. Evolution and function of phytochelatin synthases. J. Plant Physiol. 2006, 163, 319–332. [Google Scholar] [CrossRef]
- Hu, J.Z.; Shi, G.X.; Xu, Q.S.; Wang, X.; Yuan, Q.H.; Du, K.H. Effects of Pb2+ on the active oxygen-scavenging enzyme activities and ultrastructure in Potamogeton crispus leaves. Russ. J. Plant Physiol. 2007, 54, 414–419. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Afify, A.; Farahat, A.; Al-Sayed, A.; Mahfoud, N. Antioxidant enzymes as well as oxidant activities involved in defense mechanisms against the root-knot, reniform and citrus nematodes in their host plants. Int. J. Biotechnol. Food Sci. 2014, 2, 102–111. [Google Scholar]
- Singh, I.; Shah, K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 2014, 108, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl Jasmonate Regulates Antioxidant Defense and Suppresses Arsenic Uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Pradhan, A. Quantitative estimation of free aminoacids and amides in resistant and susceptible greengram varieties inoculated with root-knot nematode, Meloidogyne incognita. Indian J. Nematol. 1989, 19, 74–76. [Google Scholar]
- Gautam, S.K.; Poddar, A.N. Study on protein and sugar content in Meloidogyne incognita infested roots of bitter gourd. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 470–478. [Google Scholar]
- Ruiz-Lozano, J.M.; Azcon, R.; Palma, J.M. Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol. 1996, 134, 327–333. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000, 30, 151–155. [Google Scholar] [CrossRef]
- Gaspar, T.; Penel, C.; Castillo, F.J.; Greppin, H. A two-step control of basic and acidic peroxidases and its significance for growth and development. Physiol. Plant. 1985, 64, 418–423. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Grant, C.M.; Collinson, L.P.; Roe, J.-H.; Dawes, I.W. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol. Microbiol. 1996, 21, 171–179. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.F.; Melillo, M.T.; Delibes, A.; Romero, M.D.; Bleve-Zacheo, T. Changes in wheat root enzymes correlated with resistance to cereal cyst nematodes. New Phytol. 2001, 152, 343–354. [Google Scholar] [CrossRef]
- Lobna, H.; Aymen, E.M.; Hajer, R.; Naima, M.H.-B.; Najet, H.-R. Biochemical and plant nutrient alterations induced by Meloidogyne javanica and Fusarium oxysporum f.Sp.radicis lycopersici co-infection on tomato cultivars with differing level of resistance to M. javanica. Eur. J. Plant Pathol. 2017, 148, 463–472. [Google Scholar] [CrossRef]
- Kathiresan, T.; Mehta, U.K. Changes of catalase activity in sugarcane due to early and post infection of Pratylenchus zeae. Indian J. Nematol. 2003, 33, 116–119. [Google Scholar]
- Xu, X.; Xu, K.; Yu, Q.; Zhang, X. The relationship between resistance to Meloidogyne incognita and phenylpropanes metabolism in roots of eggplant root-stock. Acta Phytophylacica Sin. 2008, 35, 43–46. [Google Scholar]
- Kalaiarasan, P. Biochemical markers for identification of root knot nematode (Meloidogyne incognita) resistance in tomato. Karnataka J. Agric. Sci. 2009, 22, 471–475. [Google Scholar]
- Kesba, H.H.; El-Beltagi, H.S. Biochemical changes in grape rootstocks resulted from humic acid treatments in relation to nematode infection. Asian Pac. J. Trop. Biomed. 2012, 2, 287–293. [Google Scholar] [CrossRef]
- Kaur, R.; Ohri, P.; Bhardwaj, R. Effect of 28-homobrassinolide on susceptible and resistant cultivars of tomato after nematode inoculation. Plant Growth Regul. 2013, 71, 199–205. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic Acid Modulates the Physio-Biochemical Attributes, Antioxidant Enzyme Activity, and Gene Expression in Glycine max under Nickel Toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef]
- Kaur, H.; Geetika, S.; Poonam, S. Effect of jasmonic acid on some biochemical and physiological parameters in salt-stressed Brassica napus seedlings. Int. J. Plant Physiol. Biochem. 2017, 9, 36–42. [Google Scholar] [CrossRef][Green Version]
- Anjum, S.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J. Agron. Crop. Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Liang, Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010, 178, 130–139. [Google Scholar] [CrossRef]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Alyemeni, M.N.; Hasan, S.A. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J. Biol. Sci. 2012, 19, 325–335. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.-Y.; Liu, X.-J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Kerkeb, L.; Krämer, U. The Role of Free Histidine in Xylem Loading of Nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiol. 2003, 131, 716–724. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Tamogami, S.; Rakwal, R.; Kodama, O. Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett. 1997, 412, 61–64. [Google Scholar] [CrossRef]
- Bali, S.; Kaur, P.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Jasmonic acid-induced tolerance to root-knot nematodes in tomato plants through altered photosynthetic and antioxidative defense mechanisms. Protoplasma 2017, 255, 471–484. [Google Scholar] [CrossRef]
| Gene Name | Primer Sequence |
|---|---|
| Ubiquitin | Forward primer 5′ GAGGAATGCAGATCTTCGTG 3′ Reverse primer 5′ TCCTTGTCCTGGATCTTAGC 3′ |
| SOD (Superoxide dismutase) | Forward primer 5′ CAAGATGATGATGGTCCAAC 3′ Reverse primer 5′ CTCCATGTGTCAATTTATTCGG 3′ |
| POD (Guaiacol peroxidase) | Forward primer 5′ TGCCCAATGTCGTGTATTC 3′ Reverse primer 5′ CATCAGATGTGGTTGGGT 3′ |
| CAT (Catalase) | Forward primer 5′ ACATGGTCCATGCTCTG 3′ Reverse primer 5′ CCCGTCCATATGCCTGTA 3′ |
| GPOX (Glutathione peroxidase) | Forward primer 5′ GAGATAATATTCAGTGGAATTTCGCTAA 3′ Reverse primer 5′ GTTGAGGGCTCAACCTT 3′ |
| GST (Glutathione-S-transferase) | Forward primer 5′ CATTTGTTATGAATTTATTGAGCAAGAT 3′ Reverse primer 5′ TAAGTGGCCATGTTTCTTCAATATAC 3′ |
| Treatments | Root Fresh Weight (g) | SOD (Unit Activity mg−1 Protein) | POD (Unit Activity mg−1 Protein) | CAT (Unit Activity mg−1 Rotein) | APOX (Unit Activity mg−1 Protein) | |
|---|---|---|---|---|---|---|
| N (Nematode) | JA (nM) | |||||
| 0 | 0 | 0.137 ± 0.003ef | 14.54 ± 1.39d | 34.79 ± 3.37e | 10.51 ± 1.40de | 31.24 ± 0.62cd |
| 0 | 0.01 | 0.125 ± 0.004g | 14.60 ± 2.09cd | 35.35 ± 2.843e | 7.86 ± 0.09e | 27.83 ± 1.56d |
| 0 | 1 | 0.133 ± 0.004f | 11.48 ± 1.55de | 41.15 ± 1.63de | 8.72 ± 0.75e | 28.0 ± 1.67d |
| 0 | 100 | 0.141 ± 0.002de | 8.23 ± 1.16e | 43.79 ± 0.75de | 9.82 ± 0.29e | 29.67 ± 1.16d |
| N | 0 | 0.181 ± 0.003a | 23.95 ± 3.24b | 50.53 ± 228cd | 16.61 ± 2.55c | 48.51 ± 4.04b |
| N | 0.01 | 0.165 ± 0.003b | 28.58 ± 3.31ab | 58.31 ± 3.01bc | 19.02 ± 2.02bc | 49.21 ± 4.31b |
| N | 1 | 0.156 ± 0.002c | 29.61 ± 0.248ab | 66.94 ± 4.23ab | 23.34 ± 1.48ab | 51.49 ± 1.23b |
| N | 100 | 0.147 ± 0.002d | 34.25 ± 3.41a | 75.11 ± 5.32a | 25.33 ± 2.12a | 62.82 ± 3.88a |
| FN | 615.6 ** | 370.6 ** | 298.1 ** | 295.5 ** | 458.5 ** | |
| FJA | 40.3 ** | 1.39 | 28.97 ** | 8.48 ** | 9.39 ** | |
| FN×JA | 56.4 ** | 16.17 ** | 5.46 ** | 9.58 ** | 8.26 ** | |
| HSD | 0.007 | 6.077 | 9.614 | 4.773 | 7.781 | |
| Treatments | DHAR (Unit Activity mg−1 Protein) | GPOX (Unit Activity mg−1 Protein) | GST (Unit Activity mg−1 Protein) | GR (Unit Activity mg−1 Protein) | PPO (Unit Activity mg−1 Protein) | |
|---|---|---|---|---|---|---|
| N (Nematode) | JA (nM) | |||||
| 0 | 0 | 16.98 ± 2.93d | 24.10 ± 1.70abc | 18.93 ± 0.89b | 11.31 ± 2.23d | 8.30 ± 1.49cd |
| 0 | 0.01 | 20.44 ± 0.24cd | 18.32 ± 0.44cde | 19.22 ± 1.32b | 9.14 ± 0.55d | 6.71 ± 0.11d |
| 0 | 1 | 20.96 ± 1.96cd | 16.89 ± 1.05de | 21.71 ± 1.16ab | 9.78 ± 0.64d | 6.86 ± 0.17d |
| 0 | 100 | 23.08 ± 2.52bcd | 11.47 ± 0.08e | 22.37 ± 1.64ab | 11.94 ± 0.33cd | 7.76 ± 0.66d |
| N | 0 | 23.58 ± 4.23bc | 20.07 ± 2.44bcd | 26.44 ± 3.81a | 18.06 ± 3.26bc | 15.49 ± 2.49b |
| N | 0.01 | 28.01 ± 2.79abc | 20.82 ± 3.64bcd | 19.96 ± 4.14b | 20.68 ± 2.36b | 13.79 ± 3.07b |
| N | 1 | 30.85 ± 1.45ab | 26.07 ± 0.95ab | 18.17 ± 2.37b | 23.16 ± 2.72b | 16.83 ± 2.01b |
| N | 100 | 35.81 ± 4.08a | 28.39 ± 4.72a | 17.05 ± 1.91b | 30.76 ± 3.15a | 22.28 ± 1.75a |
| FN | 63.18 ** | 37.04 ** | 0.221 | 173.5 ** | 162.58 ** | |
| FJA | 10.81 ** | 1.42 | 5.30 ** | 10.47 ** | 6.917 ** | |
| FN×JA | 1.39 | 19.79 ** | 14.44 ** | 6.78 ** | 5.26 * | |
| HSD | 8.032 | 6.996 | 6.146 | 6.640 | 5.266 | |
| Nematode (N) | 0 | 0 | N | N |
| JA (nM) | 0 | 100 | 0 | 100 |
| Aspartic Acid | 12.08 ± 0.1b | 20.27 ± 2.9a | 21.32 ± 1.4a | 26.24 ± 3.5a |
| Glutamic acid | 34.96 ± 4.6b | 52.93 ± 3.6a | 41.70 ± 1.9b | 55.97 ± 4.4a |
| Asparagine | 316.7 ± 28.8b | 355.68 ± 12.3b | 389.04 ± 67.4b | 514.5 ± 27.1a |
| Serine | 11.39 ± 1.4a | 11.14 ± 1.8a | 10.85 ± 0.9a | 14.22 ± 2.3a |
| Glutamine | 3242.5 ± 63.4b | 2770.9 ± 92.5c | 3157.4 ± 69.6b | 4454.4 ± 55.6a |
| Histidine | 26.2 ± 1.4b | 23.96 ± 4.6b | 19.58 ± 1.9b | 35.61 ± 4.9a |
| Glycine | 2.38 ± 0.3b | 2.02 ± 0.1b | 2.00 ± 0.1b | 3.29 ± 0.1a |
| Threonine | 21.06 ± 1.2b | 21.34 ± 2.0b | 22.19 ± 2.4b | 30.66 ± 4.2a |
| Arginine | 136.97 ± 9.5b | 4.61 ± 0.4c | 110.22 ± 10.3b | 215.88 ± 26.4a |
| B-Alanine | 25.91 ± 4.4c | 141.03 ± 2.7a | 32.86 ± 3.1c | 43.15 ± 3.6b |
| Alanine | 29.25 ± 2.9a | 2.75 ± 0.6b | ||
| GABA | 4.36 ± 0.2a | 4.80 ± 2.2a | 4.60 ± 0.16a | 5.19 ± 0.4a |
| Tyrosine | 2.99 ± 0.3 | 4.46 ± 0.1 | 4.46 ± 0.14 | 7.52 ± 0.2 |
| Cysteine | 13.3 ± 0.4c | 2.54 ± 0.2d | 17.25 ± 0.4a | 16.2 ± 0.3b |
| Valine | 9.89 ± 0.6 | 115.69 ± 15.8 | 46.22 ± 4.8 | |
| Methionine | 242.5 ± 14.2a | 79.53 ± 5.4b | 73.99 ± 5.6b | |
| Phenylalanine | 68.21 ± 6.8c | 85.83 ± 3.5a | 71.14 ± 1.2bc | 95.46 ± 5.2a |
| Isoleucine | 7.16 ± 0.2b | 6.89 ± 0.7b | 13.01 ± 1.8a | |
| Leucine | 19.14 ± 1.8bc | 56.56 ± 4.1a | 13.20 ± 1.3c | 21.38 ± 3.2b |
| Lysine | 69.29 ± 6.8a | 0.82 ± 0.05c | 43.09 ± 1.6b | 69.07 ± 3.8a |
| Proline | 19.67 ± 2.8b | 22.29 ± 3.0b | 22.59 ± 2.9b | 30.33 ± 3.0a |
| Ornithine | 21.25 ± 2.9a | 15.64 ± 1.9b | 19.98 ± 1.5ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bali, S.; Kaur, P.; Jamwal, V.L.; Gandhi, S.G.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Ali, M.A.; Ahmad, P. Seed Priming with Jasmonic Acid Counteracts Root Knot Nematode Infection in Tomato by Modulating the Activity and Expression of Antioxidative Enzymes. Biomolecules 2020, 10, 98. https://doi.org/10.3390/biom10010098
Bali S, Kaur P, Jamwal VL, Gandhi SG, Sharma A, Ohri P, Bhardwaj R, Ali MA, Ahmad P. Seed Priming with Jasmonic Acid Counteracts Root Knot Nematode Infection in Tomato by Modulating the Activity and Expression of Antioxidative Enzymes. Biomolecules. 2020; 10(1):98. https://doi.org/10.3390/biom10010098
Chicago/Turabian StyleBali, Shagun, Parminder Kaur, Vijay Lakshmi Jamwal, Sumit G. Gandhi, Anket Sharma, Puja Ohri, Renu Bhardwaj, Mohammad Ajmal Ali, and Parvaiz Ahmad. 2020. "Seed Priming with Jasmonic Acid Counteracts Root Knot Nematode Infection in Tomato by Modulating the Activity and Expression of Antioxidative Enzymes" Biomolecules 10, no. 1: 98. https://doi.org/10.3390/biom10010098
APA StyleBali, S., Kaur, P., Jamwal, V. L., Gandhi, S. G., Sharma, A., Ohri, P., Bhardwaj, R., Ali, M. A., & Ahmad, P. (2020). Seed Priming with Jasmonic Acid Counteracts Root Knot Nematode Infection in Tomato by Modulating the Activity and Expression of Antioxidative Enzymes. Biomolecules, 10(1), 98. https://doi.org/10.3390/biom10010098







