From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues
Abstract
1. Introduction
2. The SLRP Family: Classification and Structure
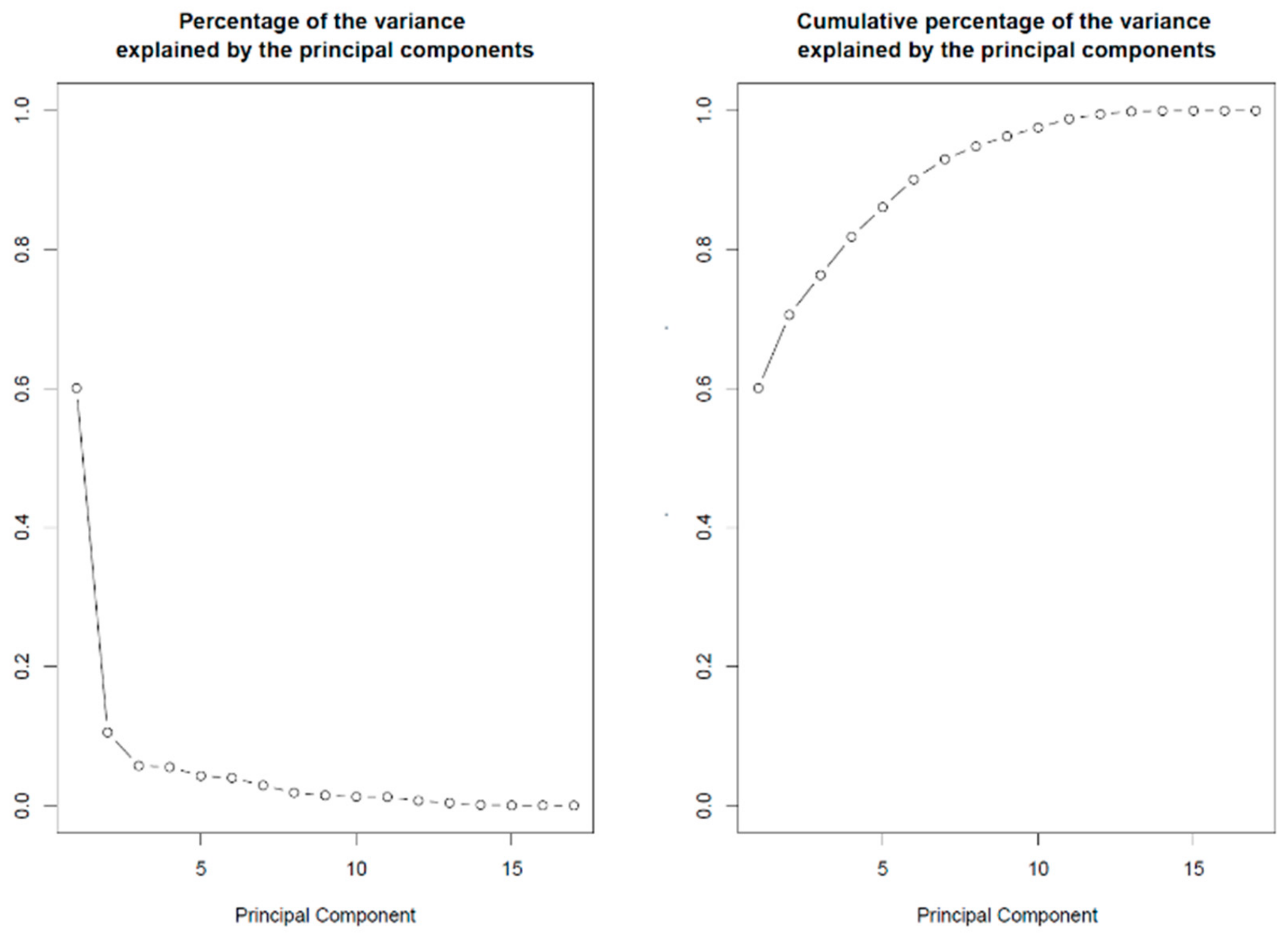
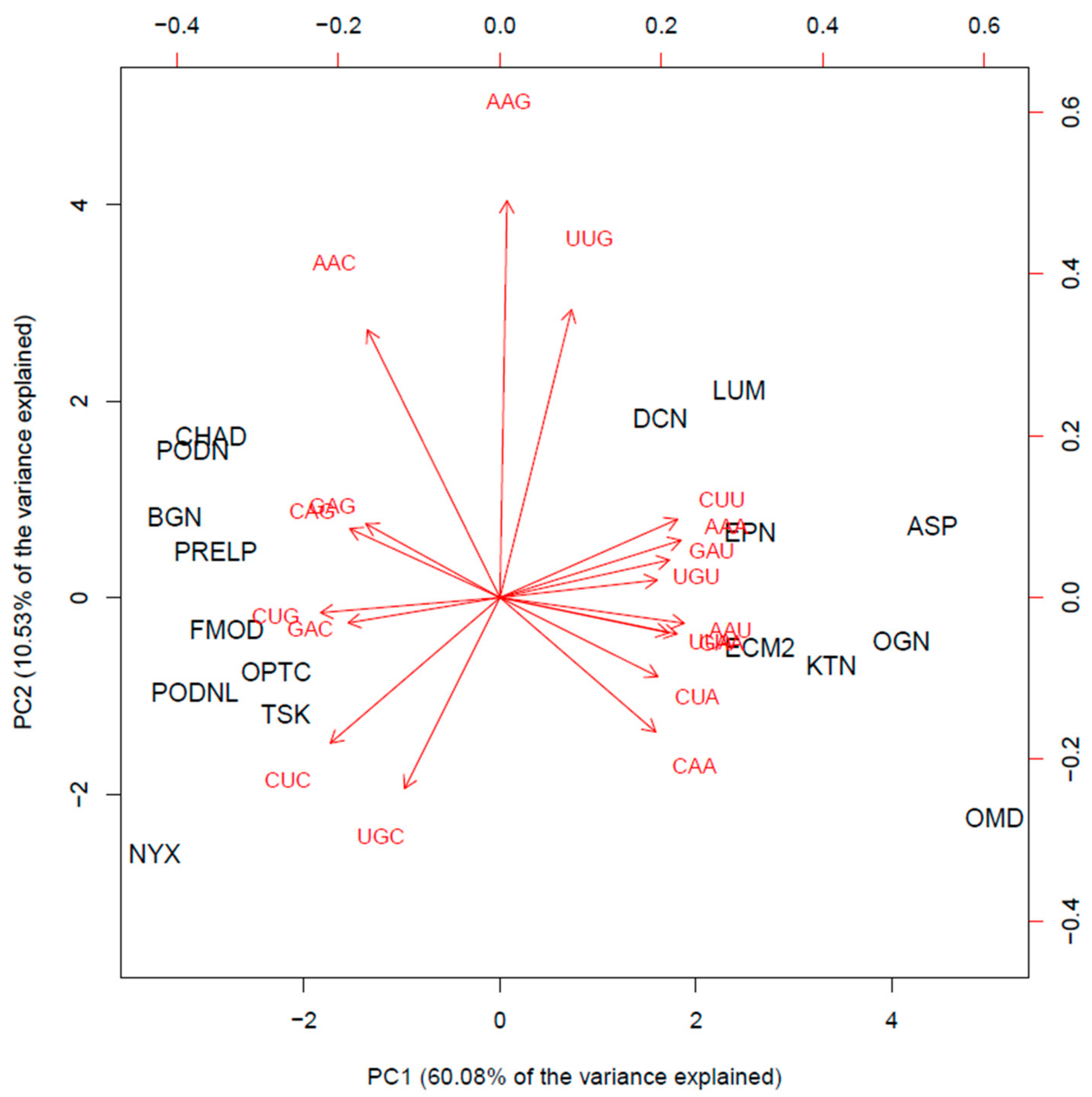
3. SLRP Codon Usage Patterns May Fine-Tune Selective Translation Pathways during Cellular Stress Conditions
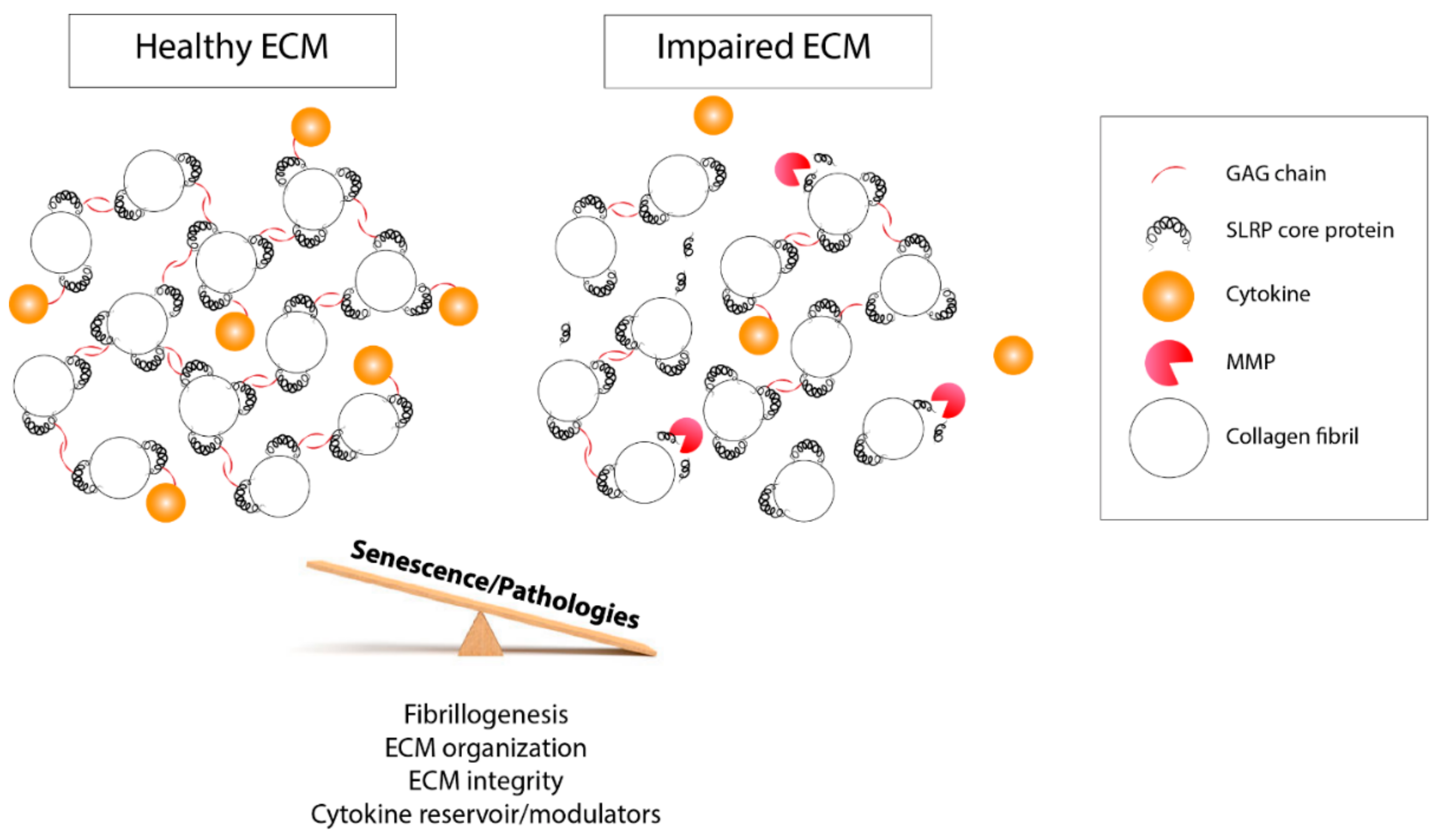
4. Roles of SRLP GAG Moieties in Fibrillogenesis
5. SLRP GAG Moieties: Fingerprints of the Tissue Status and Active Players
6. Other SLRP Post-Translational Events in Skeletal Tissues
6.1. Sulfation
6.2. SLRP Degradation and Cleavage in Skeletal Tissues
6.3. SLRP Intracellular Degradation Pathways
7. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Hynes, R.O.; Naba, A. Overview of the matrisome-An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, 4903. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Boil. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kalamajski, S.; Oldberg, A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Boil. 2010, 29, 248–253. [Google Scholar] [CrossRef]
- Nikitovic, A.; Aggelidakis, J.; Young, M.F.; Iozzo, R.V.; Karamanos, N.K.; Tzanakakis, G.N. The Biology of Small Leucine-rich Proteoglycans in Bone Pathophysiology. J. Boil. Chem. 2012, 287, 33926–33933. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef]
- Chen, X.-D.; Fisher, L.W.; Robey, P.G.; Young, M.F. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004, 18, 948–958. [Google Scholar] [CrossRef]
- Bi, Y.; Stuelten, C.H.; Kilts, T.; Wadhwa, S.; Iozzo, R.V.; Robey, P.G.; Chen, X.-D.; Young, M.F. Extracellular Matrix Proteoglycans Control the Fate of Bone Marrow Stromal Cells. J. Boil. Chem. 2005, 280, 30481–30489. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R. V Biological Functions of the Small Leucine-rich Proteoglycans: From Genetics to Signal Transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]
- Kram, V.; Kilts, T.M.; Bhattacharyya, N.; Li, L.; Young, M.F. Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci. Rep. 2017, 7, 12627. [Google Scholar] [CrossRef]
- Marín, M. Folding at the rhythm of the rare codon beat. Biotechnol. J. 2008, 3, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.B.; Kudla, G. Synonymous but not the same: The causes and consequences of codon bias. Nat. Rev. Genet. 2011, 12, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.Y.; Qian, S.-B. Less is more: Improving proteostasis by translation slow down. Trends Biochem. Sci. 2013, 38, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Brule, C.E.; Grayhack, E.J. Synonymous Codons: Choose Wisely for Expression. Trends Genet. 2017, 33, 283–297. [Google Scholar] [CrossRef]
- Chan, C.; Pham, P.; Dedon, P.C.; Begley, T.J. Lifestyle modifications: Coordinating the tRNA epitranscriptome with codon bias to adapt translation during stress responses. Genome Boil. 2018, 19, 228. [Google Scholar] [CrossRef]
- Duan, G.; Walther, D. The Roles of Post-translational Modifications in the Context of Protein Interaction Networks. PLoS Comput. Boil. 2015, 11, e1004049. [Google Scholar] [CrossRef]
- Schaefer, L.; Schaefer, R.M. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 2010, 339, 237–246. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Goldoni, S.; Berendsen, A.D.; Young, M.F. Small Leucine-Rich Proteoglycans. In The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 197–231. [Google Scholar]
- McEwan, P.A.; Scott, P.G.; Bishop, P.N.; Bella, J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J. Struct. Boil. 2006, 155, 294–305. [Google Scholar] [CrossRef]
- Kobe, B. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Boil. 2001, 11, 725–732. [Google Scholar] [CrossRef]
- Chen, S.; Sun, M.; Iozzo, R.V.; Kao, W.W.-Y.; Birk, D.E. Intracellularly-retained decorin lacking the C-terminal ear repeat causes ER stress: A cell-based etiological mechanism for congenital stromal corneal dystrophy. Am. J. Pathol. 2013, 183, 247–256. [Google Scholar] [CrossRef]
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113, 193–205. [Google Scholar]
- Funderburgh, J.L. Keratan Sulfate Biosynthesis. IUBMB Life 2002, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Meikle, P.J.; Hopwood, J.J. Glycosaminoglycan degradation fragments in mucopolysaccharidosis I. Glycobiology 2004, 14, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Langer, R.; Cooney, C.L.; Sasisekharan, R. Enzymatic Degradation of GlycosaminogIycans. Crit. Rev. Biochem. Mol. Boil. 1995, 30, 387–444. [Google Scholar] [CrossRef]
- Krishnan, P.; Hocking, A.M.; Scholtz, J.M.; Pace, C.N.; Holik, K.K.; McQuillan, D.J. Distinct secondary structures of the leucine-rich repeat proteoglycans decorin and biglycan. Glycosylation-dependent conformational stability. J. Boil. Chem. 1999, 274, 10945–10950. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.-S.; Hocking, A.M.; Höök, M.; McQuillan, D.J. Decorin Core Protein Secretion Is Regulated byN-Linked Oligosaccharide and Glycosaminoglycan Additions. J. Boil. Chem. 2005, 280, 42774–42784. [Google Scholar] [CrossRef]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 28, 32. [Google Scholar]
- Fisher, L.W.; Termine, J.D.; Young, M.F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J. Boil. Chem. 1989, 264, 4571–4576. [Google Scholar]
- Johnson, H.J.; Rosenberg, L.; Choi, H.U.; Garza, S.; Höök, M.; Neame, P.J. Characterization of epiphycan, a small proteoglycan with a leucine-rich repeat core protein. J. Boil. Chem. 1997, 272, 18709–18717. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Boil. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Roughley, P.J.; White, R.J. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem. J. 1989, 262, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Krusius, T.; Ruoslahti, E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc. Natl. Acad. Sci. USA 1986, 83, 7683–7687. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Meng, L.; Zaia, J. Deep sequencing of complex proteoglycans: A novel strategy for high coverage and sitespecific identification of glycosaminoglycanlinked peptides. Mol. Cell. Proteom. 2018, 17, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Aspberg, A.; Önnerfjord, P.; Bayliss, M.T.; Neame, P.J.; Heinegård, D. Identification and Characterization of Asporin. J. Boil. Chem. 2001, 276, 12201–12211. [Google Scholar] [CrossRef] [PubMed]
- Blochberger, T.C.; Vergnes, J.P.; Hempel, J.; Hassell, J.R. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J. Boil. Chem. 1992, 267, 347–352. [Google Scholar]
- Cornuet, P.K.; Blochberger, T.C.; Hassell, J.R. Molecular polymorphism of lumican during corneal development. Investig. Ophthalmol. Vis. Sci. 1994, 35, 870–877. [Google Scholar]
- Önnerfjord, P.; Heathfield, T.F.; Heinegård, D. Identification of Tyrosine Sulfation in Extracellular Leucine-rich Repeat Proteins Using Mass Spectrometry. J. Biol. Chem. 2004, 279, 26–33. [Google Scholar] [CrossRef]
- Corpuz, L.M.; Funderburgh, J.L.; Funderburgh, M.L.; Bottomley, G.S.; Prakash, S.; Conrad, G.W. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J. Boil. Chem. 1996, 271, 9759–9763. [Google Scholar] [CrossRef]
- Oldberg, A.; Antonsson, P.; Lindblom, K.; Heinegård, D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 1989, 8, 2601–2604. [Google Scholar] [CrossRef]
- Antonsson, P.; Heinegird, D.; Oldberg, A. Posttranslational Modifications of Fibromodulin. J. Biol. Chem. 1991, 267, 6132–6136. [Google Scholar]
- Plaas, A.H.; Wong-Palms, S. Biosynthetic mechanisms for the addition of polylactosamine to chondrocyte fibromodulin. J. Boil. Chem. 1993, 268, 26634–26644. [Google Scholar]
- Wendel, M.; Sommarin, Y.; Heinegård, D. Characterization of osteoadherin—a novel, cell binding keratan sulfate proteoglycan from bone. Acta Orthop. Scand. 1995, 66, 77. [Google Scholar] [CrossRef][Green Version]
- Wendel, M.; Sommarin, Y.; Heinegård, D. Bone Matrix Proteins: Isolation and Characterization of a Novel Cell-binding Keratan Sulfate Proteoglycan (Osteoadherin) from Bovine Bone. J. Cell Boil. 1998, 141, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Sommarin, Y. Osteoadherin, a Cell-binding Keratan Sulfate Proteoglycan in Bone, Belongs to the Family of Leucine-rich Repeat Proteins of the Extracellular Matrix. J. Boil. Chem. 1998, 273, 16723–16729. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, E.; Neame, P.J.; Heinegård, D.; Sommarin, Y. The Primary Structure of a Basic Leucine-rich Repeat Protein, PRELP, Found in Connective Tissues. J. Biol. Chem. 1995, 270, 25639–25644. [Google Scholar] [CrossRef]
- Bengtsson, E.; Aspberg, A.; Heinegãrd, D.; Sommarin, Y.; Spillmann, D. The Amino-terminal Part of PRELP Binds to Heparin and Heparan Sulfate. J. Boil. Chem. 2000, 275, 40695–40702. [Google Scholar] [CrossRef]
- Bentz, H.; Nathan, R.M.; Rosen, D.M.; Armstrong, R.M.; Thompson, A.Y.; Segarini, P.R.; Mathews, M.C.; Dasch, J.R.; Piez, K.A.; Seyedin, S.M. Purification and characterization of a unique osteoinductive factor from bovine bone. J. Boil. Chem. 1989, 264, 20805–20810. [Google Scholar]
- Funderburgh, J.L.; Corpuz, L.M.; Roth, M.R.; Funderburgh, M.L.; Tasheva, E.S.; Conrad, G.W. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J. Boil. Chem. 1997, 272, 28089–28095. [Google Scholar] [CrossRef]
- Kampmann, A.; Fernández, B.; Deindl, E.; Kubin, T.; Pipp, F.; Eitenmüller, I.; Hoefer, I.E.; Schaper, W.; Zimmermann, R. The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis. Mol. Cell. Biochem. 2009, 322, 15–23. [Google Scholar] [CrossRef]
- Rienks, M.; Papageorgiou, A.; Wouters, K.; Verhesen, W.; van Leeuwen, R.; Carai, P.; Summer, G.; Westermann, D.; Heymans, S. A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis. Cell. Mol. Life Sci. 2017, 74, 1511–1525. [Google Scholar] [CrossRef]
- Madisen, L.; Neubauer, M.; Plowman, G.; Rosen, D.; Segarini, P.; Dasch, J.; Thompson, A.; Ziman, J.; Bentz, H.; Purchio, A. Molecular Cloning of a Novel Bone-Forming Compound: Osteoinductive Factor. DNA Cell Boil. 1990, 9, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.J.; Le Goff, M.; Briggs, M.D.; McLeod, D.; Sheehan, J.K.; Thornton, D.J.; Bishop, P.N. Identification in vitreous and molecular cloning of opticin, a novel member of the family of leucine-rich repeat proteins of the extracellular matrix. J. Boil. Chem. 2000, 275, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Hobby, P.; Wyatt, M.K.; Gan, W.; Bernstein, S.; Tomarev, S.; Slingsby, C.; Wistow, G. Cloning, modeling, and chromosomal localization for a small leucine-rich repeat proteoglycan (SLRP) family member expressed in human eye. Mol. Vis. 2000, 6, 72–78. [Google Scholar] [PubMed]
- Neame, P.J.; Sommarin, Y.; Boynton, R.E.; Heinegård, D. The structure of a 38-kDa leucine-rich protein (chondroadherin) isolated from bovine cartilage. J. Boil. Chem. 1994, 269, 21547–21554. [Google Scholar]
- Shimizu-Hirota, R.; Sasamura, H.; Kuroda, M.; Kobayashi, E.; Saruta, T. Functional characterization of podocan, a member of a new class in the small leucine-rich repeat protein family. FEBS Lett. 2004, 563, 69–74. [Google Scholar] [CrossRef]
- Ross, M.D.; Bruggeman, L.A.; Hanss, B.; Marras, D.; Klotman, M.E.; Sunamoto, M.; Klotman, P.E. Podocan, a Novel Small Leucine-rich Repeat Protein Expressed in the Sclerotic Glomerular Lesion of Experimental HIV-associated Nephropathy. J. Boil. Chem. 2003, 278, 33248–33255. [Google Scholar] [CrossRef]
- Mochida, Y.; Kaku, M.; Yoshida, K.; Katafuchi, M.; Atsawasuwan, P.; Yamauchi, M. Podocan-like protein: A novel small leucine-rich repeat matrix protein in bone. Biochem. Biophys. Res. Commun. 2011, 410, 333–338. [Google Scholar]
- Endres, L.; Dedon, P.C.; Begley, T.J. Codon-biased translation can be regulated by wobble-base tRNA modification systems during cellular stress responses. RNA Biol. 2015, 12, 603–614. [Google Scholar] [CrossRef]
- Rapino, F.; Delaunay, S.; Zhou, Z.; Chariot, A.; Close, P. tRNA Modification: Is Cancer Having a Wobble? Trends Cancer 2017, 3, 249–252. [Google Scholar] [CrossRef]
- Hou, Y.-M.; Gamper, H.; Yang, W. Post-transcriptional modifications to tRNA--a response to the genetic code degeneracy. RNA 2015, 21, 642–644. [Google Scholar] [CrossRef]
- Rapino, F.; Delaunay, S.; Rambow, F.; Zhou, Z.; Tharun, L.; De Tullio, P.; Sin, O.; Shostak, K.; Schmitz, S.; Piepers, J.; et al. Codon-specific translation reprogramming promotes resistance to targeted therapy. Nat. 2018, 558, 605–609. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Volume 112, p. 18. [Google Scholar]
- Raspanti, M.; Viola, M.; Forlino, A.; Tenni, R.; Gruppi, C.; Tira, M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Boil. 2008, 164, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Tatara, Y.; Kakizaki, I.; Suto, S.; Ishioka, H.; Negishi, M.; Endo, M. Chondroitin sulfate cluster of epiphycan from salmon nasal cartilage defines binding specificity to collagens. Glycobiology 2015, 25, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.N.; Pinali, C.; Young, R.D.; Meek, K.M.; Quantock, A.J.; Knupp, C. Structural Interactions between Collagen and Proteoglycans Are Elucidated by Three-Dimensional Electron Tomography of Bovine Cornea. Structure 2010, 18, 239–245. [Google Scholar] [CrossRef]
- Parfitt, G.J.; Pinali, C.; Young, R.D.; Quantock, A.J.; Knupp, C. Three-dimensional reconstruction of collagen–proteoglycan interactions in the mouse corneal stroma by electron tomography. J. Struct. Boil. 2010, 170, 392–397. [Google Scholar] [CrossRef]
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M.; Müller, A. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Boil. 2013, 183, 394–403. [Google Scholar] [CrossRef]
- Scott, J.E. Extracellular matrix, supramolecular organisation and shape. J. Anat. 1995, 187, 259–269. [Google Scholar]
- Scott, J.E.; Thomlinson, A.M. The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J. Anat. 1998, 192, 391–405. [Google Scholar] [CrossRef]
- Lujan, T.J.; Underwood, C.J.; Henninger, H.B.; Thompson, B.M.; Weiss, J.A. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J. Orthop. Res. 2007, 25, 894–903. [Google Scholar] [CrossRef]
- Seidler, D.G.; Faiyaz-Ul-Haque, M.; Hansen, U.; Yip, G.W.; Zaidi, S.H.E.; Teebi, A.S.; Kiesel, L.; Götte, M. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers–Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (β4GalT-7). J. Mol. Med. 2006, 84, 583–594. [Google Scholar] [CrossRef]
- Rühland, C.; Schönherr, E.; Robenek, H.; Hansen, U.; Iozzo, R.V.; Bruckner, P.; Seidler, D.G. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007, 274, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Kariminejad, A.; Van Damme, T.; Gauche, C.; Syx, D.; Merhi-Soussi, F.; Gulberti, S.; Symoens, S.; Vanhauwaert, S.; Willaert, A.; et al. Defective initiation of glycosaminoglycan synthesis due to B3GALT6 mutations causes a pleiotropic Ehlers-Danlos-syndrome-like connective tissue disorder. Am. J. Hum. Genet. 2013, 92, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, P.; Geng, Y.; Lamplugh, L.; Nanci, A.; Roughley, P.J. Absence of the dermatan sulfate chain of decorin does not affect mouse development. J. Negat. Results Biomed. 2017, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.P.; Underwood, C.J.; Weiss, J.A. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Boil. 2013, 32, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J. Physiol. 2003, 553, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, A.; Vesentini, S.; Soncini, M.; Vena, P.; Mantero, S.; Montevecchi, F. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—A computational study from molecular to microstructural level. J. Biomech. 2003, 36, 1555–1569. [Google Scholar] [CrossRef]
- Vesentini, S.; Redaelli, A.; Montevecchi, F.M. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J. Biomech. 2005, 38, 433–443. [Google Scholar] [CrossRef]
- Liao, J.; Vesely, I. Skewness angle of interfibrillar proteoglycans increases with applied load on mitral valve chordae tendineae. J. Biomech. 2007, 40, 390–398. [Google Scholar] [CrossRef]
- Lujan, T.J.; Underwood, C.J.; Jacobs, N.T.; Weiss, J.A. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J. Appl. Physiol. 2009, 106, 423–431. [Google Scholar] [CrossRef]
- Fessel, G.; Snedeker, J.G. Equivalent stiffness after glycosaminoglycan depletion in tendon—An ultra-structural finite element model and corresponding experiments. J. Theor. Boil. 2011, 268, 77–83. [Google Scholar] [CrossRef]
- Svensson, R.B.; Hassenkam, T.; Hansen, P.; Kjaer, M.; Magnusson, S.P. Tensile Force Transmission in Human Patellar Tendon Fascicles Is Not Mediated by Glycosaminoglycans. Connect. Tissue Res. 2011, 52, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.K.; Smith, M.M.; Martin, J.H.; Clarke, J.L.; Dart, A.J.; Little, C.B.; Clarke, E.C. Chondroitin sulphate glycosaminoglycans contribute to widespread inferior biomechanics in tendon after focal injury. J. Biomech. 2016, 49, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Rigozzi, S.; Müller, R.; Stemmer, A.; Snedeker, J. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding—AFM observations at the nanoscale. J. Biomech. 2013, 46, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Tatara, Y.; Suto, S.; Itoh, K. Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology 2017, 27, 1089–1098. [Google Scholar] [CrossRef]
- Johnstone, B.; Markopoulos, M.; Neame, P.; Caterson, B. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem. J. 1993, 292, 661–666. [Google Scholar] [CrossRef]
- Roughley, P.J.; White, R.J.; Magny, M.C.; Liu, J.; Pearce, R.H.; Mort, J.S. Non-proteoglycan forms of biglycan increase with age in human articular cartilage. Biochem. J. 1993, 295, 421–426. [Google Scholar] [CrossRef]
- Waddington, R.; Hall, R.; Embery, G.; Lloyd, D. Changing profiles of proteoglycans in the transition of predentine to dentine. Matrix Boil. 2003, 22, 153–161. [Google Scholar] [CrossRef]
- Chan, W.L.; Steiner, M.; Witkos, T.; Egerer, J.; Busse, B.; Mizumoto, S.; Pestka, J.M.; Zhang, H.; Hausser, I.; Khayal, L.A.; et al. Impaired proteoglycan glycosylation, elevated TGF-β signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018, 14, e1007242. [Google Scholar] [CrossRef]
- Derwin, K.A.; Soslowsky, L.J.; Kimura, J.H.; Plaas, A.H. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J. Orthop. Res. 2001, 19, 269–277. [Google Scholar] [CrossRef]
- Roughley, P.J.; White, R.J.; Cs-Szabó, G.; Mort, J.S. Changes with age in the structure of fibromodulin in human articular cartilage. Osteoarthr. Cartil. 1996, 4, 153–161. [Google Scholar] [CrossRef]
- Santer, V.; White, R.J.; Roughley, P.J. O-linked oligosaccharides of human articular cartilage proteoglycan. BBA Gen. Subj. 1982, 716, 277–282. [Google Scholar] [CrossRef]
- Grover, J.; Chen, X.N.; Korenberg, J.R.; Roughley, P.J. The human lumican gene. Organization, chromosomal location, and expression in articular cartilage. J. Boil. Chem. 1995, 270, 21942–21949. [Google Scholar] [CrossRef] [PubMed]
- Melching, L.I.; Roughley, P.J. Modulation of keratan sulfate synthesis on lumican by the action of cytokines on human articular chondrocytes. Matrix Boil. 1999, 18, 381–390. [Google Scholar] [CrossRef]
- Sugars, R.V.; Olsson, M.L.; Marchner, S.; Hultenby, K.; Wendel, M. The glycosylation profile of osteoadherin alters during endochondral bone formation. Bone 2013, 53, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Waddington, R.J.; Roberts, H.C.; Sugars, R.V.; Schönherr, E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater 2003, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Rapoport, O.; Septier, D.; Palmier, K.; Hall, R.; Embery, G.; Young, M.; Ameye, L. Proteoglycans in predentin: The last 15 micrometers before mineralization. Connect. Tissue Res. 2003, 44, 184–188. [Google Scholar] [CrossRef]
- Ho, S.P.; Sulyanto, R.M.; Marshall, S.J.; Marshall, G.W. The cementum–dentin junction also contains glycosaminoglycans and collagen fibrils. J. Struct. Boil. 2005, 151, 69–78. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Stankoska, K.; Swain, M.V. Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron 2012, 43, 229–236. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Kury, M.; Rathsam, C.; Little, C.B.; Swain, M.V. The role of proteoglycans in the nanoindentation creep behavior of human dentin. J. Mech. Behav. Biomed. Mater. 2015, 55, 264–270. [Google Scholar] [CrossRef]
- Dorvee, J.R.; Gerkowicz, L.; Bahmanyar, S.; Deymier-Black, A.; Veis, A. Chondroitin sulfate is involved in the hypercalcification of the organic matrix of bovine peritubular dentin. Arch. Oral Biol. 2016, 62, 93–100. [Google Scholar] [CrossRef]
- Farina, A.P.; Vidal, C.M.P.; Cecchin, D.; Aguiar, T.R.; Bedran-Russo, A.K. Structural and biomechanical changes to dentin extracellular matrix following chemical removal of proteoglycans. Odontology 2019, 107, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Osses, N.; Brandan, E. Changes in secreted and cell associated proteoglycan synthesis during conversion of myoblasts to osteoblasts in response to bone morphogenetic protein-2: Role of decorin in cell response to BMP-2. J. Cell Physiol. 2006, 206, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Halper, J.; Kim, B.; Khan, A.; Yoon, J.H.; Mueller, P.O.E. Degenerative suspensory ligament desmitis as a systemic disorder characterized by proteoglycan accumulation. BMC Vet. Res. 2006, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yoon, J.H.; Zhang, J.; Mueller, P.E.; Halper, J. Glycan profiling of a defect in decorin glycosylation in equine systemic proteoglycan accumulation, a potential model of progeroid form of Ehlers-Danlos syndrome. Arch. Biochem. Biophys. 2010, 501, 221–231. [Google Scholar] [CrossRef]
- Young, M.; Moshood, O.; Zhang, J.; Sarbacher, C.A.; Mueller, P.O.E.; Halper, J. Does BMP2 play a role in the pathogenesis of equine degenerative suspensory ligament desmitis? BMC Res. Notes 2018, 11, 672. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, W.; Guo, F.; Zhang, W.; Wang, J.; Chen, A. Glycosaminoglycan chains of biglycan promote bone morphogenetic protein-4-induced osteoblast differentiation. Int. J. Mol. Med. 2012, 30, 1075–1080. [Google Scholar] [CrossRef]
- Wang, X.; Harimoto, K.; Xie, S.; Cheng, H.; Liu, J.; Wang, Z. Matrix protein biglycan induces osteoblast differentiation through extracellular signal-regulated kinase and Smad pathways. Boil. Pharm. Bull. 2010, 33, 1891–1897. [Google Scholar] [CrossRef]
- Miguez, P.; Terajima, M.; Nagaoka, H.; Mochida, Y.; Yamauchi, M. Role of Glycosaminoglycans of Biglycan in BMP-2 Signaling. Biochem. Biophys. Res. Commun. 2011, 405, 262–266. [Google Scholar] [CrossRef]
- Miguez, P.; Terajima, M.; Nagaoka, H.; Ferreira, J.; Braswell, K.; Ko, C.; Yamauchi, M. Recombinant Biglycan Promotes Bone Morphogenetic Protein-induced Osteogenesis. J. Dent. Res. 2014, 93, 406–411. [Google Scholar] [CrossRef]
- Kanan, Y.; Siefert, J.C.; Kinter, M.; Al-Ubaidi, M.R. Complement factor H, vitronectin, and opticin are tyrosine-sulfated proteins of the retinal pigment epithelium. PLoS ONE 2014, 9, e105409. [Google Scholar] [CrossRef]
- Heathfield, T.F.; Önnerfjord, P.; Dahlberg, L.; Heinegård, D. Cleavage of Fibromodulin in Cartilage Explants Involves Removal of the N-terminal Tyrosine Sulfate-rich Region by Proteolysis at a Site That Is Sensitive to Matrix Metalloproteinase-13. J. Biol. Chem. 2004, 279, 6286–6295. [Google Scholar] [CrossRef] [PubMed]
- Tillgren, V.; Önnerfjord, P.; Haglund, L.; Heinegård, D. The Tyrosine Sulfate-rich Domains of the LRR Proteins Fibromodulin and Osteoadherin Bind Motifs of Basic Clusters in a Variety of Heparin-binding Proteins, Including Bioactive Factors. J. Boil. Chem. 2009, 284, 28543–28553. [Google Scholar] [CrossRef] [PubMed]
- Tillgren, V.; Mörgelin, M.; Önnerfjord, P.; Kalamajski, S.; Aspberg, A. The Tyrosine Sulfate Domain of Fibromodulin Binds Collagen and Enhances Fibril Formation. J. Boil. Chem. 2016, 291, 23744–23755. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Hiramatsu, A.; Fukushima, D.; Pierschbacher, M.D.; Okada, Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-β1 release. Biochem. J. 1997, 322, 809–814. [Google Scholar] [CrossRef]
- Li, Y.; Aoki, T.; Mori, Y.; Ahmad, M.; Miyamori, H.; Takino, T.; Sato, H. Cleavage of Lumican by Membrane-Type Matrix Metalloproteinase-1 Abrogates This Proteoglycan-Mediated Suppression of Tumor Cell Colony Formation in Soft Agar. Cancer Res. 2004, 64, 7058–7064. [Google Scholar] [CrossRef]
- Geng, Y.; McQuillan, D.; Roughley, P.J. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Boil. 2006, 25, 484–491. [Google Scholar] [CrossRef]
- Monfort, J.; Tardif, G.; Reboul, P.; Mineau, F.; Roughley, P.; Pelletier, J.-P.; Martel-Pelletier, J. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: Identification of a new biglycan cleavage site. Arthritis Res. Ther. 2006, 8, 26. [Google Scholar] [CrossRef]
- Monfort, J.; Tardif, G.; Roughley, P.; Reboul, P.; Boileau, C.; Bishop, P.; Pelletier, J.-P.; Martel-Pelletier, J. Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: A novel target for MMP-13 in osteoarthritis. Osteoarthr. Cartil. 2008, 16, 749–755. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Yang, N.; Cavey, G.; Davidson, P.; Gibson, G. Molecular Interactions of MMP-13 C-Terminal Domain with Chondrocyte Proteins. Connect. Tissue Res. 2010, 51, 230–239. [Google Scholar] [CrossRef]
- Tío, L.; Martel-Pelletier, J.; Pelletier, J.-P.; Bishop, P.N.; Roughley, P.; Farran, A.; Benito, P.; Monfort, J. Characterization of opticin digestion by proteases involved in osteoarthritis development. Jt. Bone Spine 2014, 81, 137–141. [Google Scholar] [CrossRef]
- Genovese, F.; Barascuk, N.; Larsen, L.; Larsen, M.R.; Nawrocki, A.; Li, Y.; Zheng, Q.; Wang, J.; Veidal, S.S.; Leeming, D.J.; et al. Biglycan fragmentation in pathologies associated with extracellular matrix remodeling by matrix metalloproteinases. Fibrogenesis Tissue Repair 2013, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Melching, L.; Fisher, W.; Lee, E.; Mort, J.; Roughley, P. The cleavage of biglycan by aggrecanases. Osteoarthr. Cartil. 2006, 14, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Boivin, W.A.; Shackleford, M.; Vanden Hoek, A.; Zhao, H.; Hackett, T.L.; Knight, D.A.; Granville, D.J. Granzyme B Cleaves Decorin, Biglycan and Soluble Betaglycan, Releasing Active Transforming Growth Factor-β1. PLoS ONE 2012, 7, 33163. [Google Scholar] [CrossRef]
- Fuller, E.; Little, C.B.; Melrose, J. Interleukin-1α induces focal degradation of biglycan and tissue degeneration in an in-vitro ovine meniscal model. Exp. Mol. Pathol. 2016, 101, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Akhatib, B.; Onnerfjord, P.; Gawri, R.; Ouellet, J.; Jarzem, P.; Heinegård, D.; Mort, J.; Roughley, P.; Haglund, L. Chondroadherin Fragmentation Mediated by the Protease HTRA1 Distinguishes Human Intervertebral Disc Degeneration from Normal Aging. J. Boil. Chem. 2013, 288, 19280–19287. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.C.; Imamura, Y.; Pappano, W.N.; Troedel, J.M.; Recklies, A.D.; Roughley, P.J.; Greenspan, D.S. Bone Morphogenetic Protein-1 Processes Probiglycan. J. Boil. Chem. 2000, 275, 30504–30511. [Google Scholar] [CrossRef]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef]
- Rees, S.G.; Flannery, C.R.; Little, C.B.; Hughes, C.E.; Caterson, B.; Dent, C.M. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem. J. 2000, 350, 181–188. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.M.; Fuller, E.S.; Young, A.A.; Roughley, P.J.; Dart, A.; Little, C.B. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur. Spine J. 2007, 16, 2193–2205. [Google Scholar] [CrossRef]
- Zhen, E.Y.; Brittain, I.J.; Laska, D.A.; Mitchell, P.G.; Sumer, E.U.; Karsdal, M.A.; Duffin, K.L. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008, 58, 2420–2431. [Google Scholar] [CrossRef]
- Melrose, J.; Fuller, E.S.; Roughley, P.J.; Smith, M.M.; Kerr, B.; Hughes, C.E.; Caterson, B.; Little, C.B. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res. Ther. 2008, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.C.; Flannery, C.R.; Little, C.B.; Melrose, J. Catabolism of Fibromodulin in Developmental Rudiment and Pathologic Articular Cartilage Demonstrates Novel Roles for MMP-13 and ADAMTS-4 in C-terminal Processing of SLRPs. Int. J. Mol. Sci. 2019, 20, 579. [Google Scholar] [CrossRef] [PubMed]
- Cs-Szabo, G.; Roughley, P.J.; Plaas, A.H.K.; Glant, T.T. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995, 38, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Young, A.A.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Ghosh, P.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Roughley, P.J.; Little, C.B. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res. Ther. 2005, 7, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Haglund, L.; Ouellet, J.; Roughley, P. Variation in Chondroadherin Abundance and Fragmentation in the Human Scoliotic Disc. Spine 2009, 34, 1513–1518. [Google Scholar] [CrossRef]
- Brown, S.; Melrose, J.; Caterson, B.; Roughley, P.; Eisenstein, S.M.; Roberts, S. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Spine J. 2012, 21, 154–159. [Google Scholar]
- Erwin, W.M.; DeSouza, L.; Funabashi, M.; Kawchuk, G.; Karim, M.Z.; Kim, S.; Mädler, S.; Matta, A.; Wang, X.; Mehrkens, K.A. The biological basis of degenerative disc disease: Proteomic and biomechanical analysis of the canine intervertebral disc. Arthritis Res. 2015, 17, 240. [Google Scholar] [CrossRef]
- Bisson, D.G.; Lama, P.; Abduljabbar, F.; Rosenzweig, D.H.; Saran, N.; Ouellet, J.A.; Haglund, L. Facet joint degeneration in adolescent idiopathic scoliosis. JOR Spine 2018, 1, 1016. [Google Scholar] [CrossRef]
- Bock, H.; Michaeli, P.; Bode, C.; Schultz, W.; Kresse, H.; Herken, R.; Miosge, N. The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthr. Cartil. 2001, 9, 654–663. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, R.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Cells Mater. 2011, 21, 202. [Google Scholar]
- Fuller, E.; Smith, M.; Little, C.; Melrose, J. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthr. Cartil. 2012, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Billinghurst, R.C.; Dahlberg, L.; Ionescu, M.; Reiner, A.; Bourne, R.; Rorabeck, C.; Mitchell, P.; Hambor, J.; Diekmann, O.; Tschesche, H.; et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Investig. 1997, 99, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Schroeder, N.; Manon-Jensen, T.; Iozzo, R.V.; Schaefer, L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013, 280, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Soininen, A.; Ylinen, P.; Sandelin, J.; Konttinen, Y.T.; Nordström, D.C.; Eklund, K.K. Soluble biglycan: A potential mediator of cartilage degradation in osteoarthritis. Arthritis Res. 2015, 17, 379. [Google Scholar] [CrossRef]
- Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Calatroni, A.; Saitta, A.; Campo, S.; Campo, G.M. The proteoglycan biglycan mediates inflammatory response by activating TLR-4 in human chondrocytes: Inhibition by specific siRNA and high polymerized Hyaluronan. Arch. Biochem. Biophys. 2018, 640, 75–82. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef]
- Le Goff, M.M.; Sutton, M.J.; Slevin, M.; Latif, A.; Humphries, M.J.; Bishop, P.N. Opticin exerts its anti-angiogenic activity by regulating extracellular matrix adhesiveness. J. Boil. Chem. 2012, 287, 28027–28036. [Google Scholar] [CrossRef]
- Merline, R.; Schaefer, R.M.; Schaefer, L. The matricellular functions of small leucine-rich proteoglycans (SLRPs). J. Cell Commun. Signal. 2009, 3, 323–335. [Google Scholar] [CrossRef]
- Hildebrand, A.; Romaris, M.; Rasmussen, L.M.; Heinegård, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem. J. 1994, 302, 527–534. [Google Scholar] [CrossRef]
- Kizawa, H.; Kou, I.; Iida, A.; Sudo, A.; Miyamoto, Y.; Fukuda, A.; Mabuchi, A.; Kotani, A.; Kawakami, A.; Yamamoto, S.; et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 2005, 37, 138–144. [Google Scholar] [CrossRef]
- Embree, M.C.; Kilts, T.M.; Ono, M.; Inkson, C.A.; Syed-Picard, F.; Karsdal, M.A.; Oldberg, A.; Bi, Y.; Young, M.F. Biglycan and Fibromodulin Have Essential Roles in Regulating Chondrogenesis and Extracellular Matrix Turnover in Temporomandibular Joint Osteoarthritis. Am. J. Pathol. 2010, 176, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Handley, C.J.; Hascall, V.C.; Campbell, R.A.; Lowther, D.A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch. Biochem. Biophys. 1984, 234, 275–289. [Google Scholar] [CrossRef]
- Campbell, M.A.; Winter, A.D.; Ilic, M.Z.; Handley, C.J. Catabolism and Loss of Proteoglycans from Cultures of Bovine Collateral Ligament. Arch. Biochem. Biophys. 1996, 328, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.D.; Campbell, M.A.; Robinson, H.; Handley, C.J. Catabolism of newly synthesized decorin by explant cultures of bovine ligament. Matrix Boil. 2000, 19, 129–138. [Google Scholar] [CrossRef]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Large aggregating and small leucine-rich proteoglycans are degraded by different pathways and at different rates in tendon. JBIC J. Boil. Inorg. Chem. 2004, 271, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.Z.; Carter, P.; Tyndall, A.; Dudhia, J.; Handley, C.J. Proteoglycans and catabolic products of proteoglycans present in ligament. Biochem. J. 2005, 385, 381–388. [Google Scholar] [CrossRef][Green Version]
- Hausser, H.; Hoppe, W.; Rauch, U.; Kresse, H. Endocytosis of a small dermatan sulphate proteoglycan. Identification of binding proteins. Biochem. J. 1989, 263, 137–142. [Google Scholar] [CrossRef]
- Hausser, H. Binding of heparin and of the small proteoglycan decorin to the same endocytosis receptor proteins leads to different metabolic consequences. J. Cell Boil. 1991, 114, 45–52. [Google Scholar] [CrossRef]
- Hausser, H.; Ober, B.; Quentin-Hoffmann, E.; Schmidt, B.; Kresse, H. Endocytosis of Different Members of the Small Chondroitin/Dermatan Sulfate Proteoglycan Family. J. Biol. Chem. 1992, 267, 11559–11564. [Google Scholar]
- Hausser, H.; Schönherr, E.; Müller, M.; Liszio, C.; Bin, Z.; Fisher, L.W.; Kresse, H. Receptor-Mediated Endocytosis of Decorin: Involvement of Leucine-Rich Repeat Structures. Arch. Biochem. Biophys. 1998, 349, 363–370. [Google Scholar] [CrossRef]
- Hausser, H.; Kresse, H. Decorin endocytosis: Structural features of heparin and heparan sulphate oligosaccharides interfering with receptor binding and endocytosis. Biochem. J. 1999, 344, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.; Hausser, H.; Kresse, H. Extracellular accumulation of small dermatan sulphate proteoglycan II by interference with the secretion-recapture pathway. Biochem. J. 1990, 266, 591–595. [Google Scholar] [PubMed]
- Feugaing, D.D.S.; Kresse, H.; Greb, R.R.; Götte, M. A Novel 110-kDa Receptor Protein is Involved in Endocytic Uptake of Decorin by Human Skin Fibroblasts. Sci. World J. 2006, 6, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Sunderkotter, C.; Iozzo, R.; Schaefer, L. Decorin, a Novel Player in the Insulin-like Growth Factor System. J. Boil. Chem. 2005, 280, 15767–15772. [Google Scholar] [CrossRef] [PubMed]
- Santiago-García, J.; Kodama, T.; Pitas, R.E. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J. Biol. Chem. 2003, 278, 6942–6946. [Google Scholar] [CrossRef] [PubMed]
- Feugaing, D.; Tammi, R.; Echtermeyer, F.; Stenmark, H.; Kresse, H.; Smollich, M.; Schönherr, E.; Kiesel, L.; Götte, M. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie 2007, 89, 637–657. [Google Scholar] [CrossRef]
- Townley, R.A.; Bülow, H.E. Deciphering functional glycosaminoglycan motifs in development. Curr. Opin. Struct. Boil. 2018, 50, 144–154. [Google Scholar] [CrossRef]
- Wang, M.; Xue, S.; Fang, Q.; Zhang, M.; He, Y.; Zhang, Y.; Lammi, M.J.; Cao, J.; Chen, J. Expression and localization of the small proteoglycans decorin and biglycan in articular cartilage of Kashin-Beck disease and rats induced by T-2 toxin and selenium deficiency. Glycoconj. J. 2019, 36, 1–9. [Google Scholar] [CrossRef]
- Cillero-Pastor, B.; Eijkel, G.B.; Kiss, A.; Blanco, F.J.; Heeren, R.M.A.; Garcia, F.J.B. Matrix-assisted laser desorption ionization-imaging mass spectrometry: A new methodology to study human osteoarthritic cartilage. Arthritis Rheum. 2013, 65, 710–720. [Google Scholar] [CrossRef]
- Balakrishnan, L.; Nirujogi, R.S.; Ahmad, S.; Bhattacharjee, M.; Manda, S.S.; Renuse, S.; Kelkar, D.S.; Subbannayya, Y.; Raju, R.; Goel, R.; et al. Poteomic analysis of human osteoarthritis synovial fluid. Clin. Proteom. 2014, 11, 6. [Google Scholar] [CrossRef]
- Peffers, M.J.; Cillero-Pastor, B.; Eijkel, G.B.; Clegg, P.D.; Heeren, R.M. Matrix assisted laser desorption ionization mass spectrometry imaging identifies markers of ageing and osteoarthritic cartilage. Arthritis Res. Ther. 2014, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.; McDermott, B.; Clegg, P.; Riggs, C. Comprehensive protein profiling of synovial fluid in osteoarthritis following protein equalization. Osteoarthr. Cartil. 2015, 23, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Thornton, D.J.; Clegg, P.D. Characterization of neopeptides in equine articular cartilage degradation. J. Orthop. Res. 2016, 34, 106–120. [Google Scholar] [CrossRef] [PubMed]
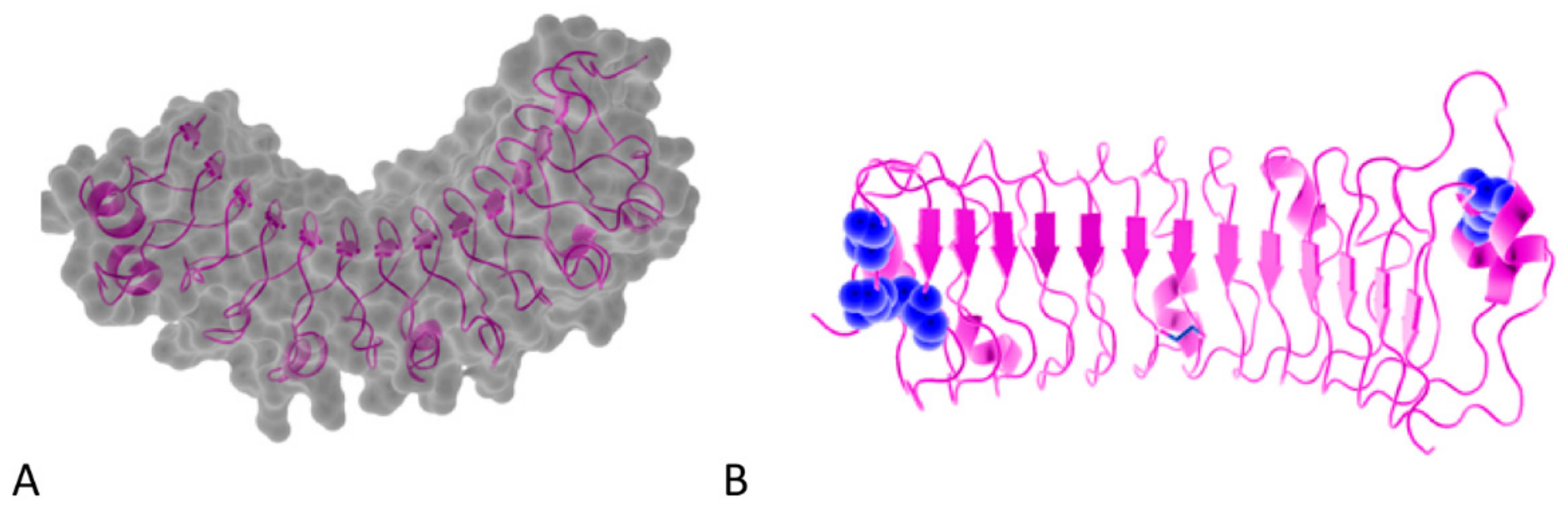
| Class | N-End Cysteine Motif | 3D Representation and PDB ID | Member | GAG Type/Glycosylation | Other | Ref. |
|---|---|---|---|---|---|---|
| I | CX3CXCX6C | 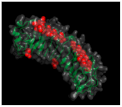 ID: 1XKU | BGN | Chondroitin sulfate Dermatan sulfate N-linked oligosaccharide O-linked oligosaccharide | [29,30,31,32] | |
 ID: 2FT3 | DCN | Chondroitin sulfate Dermatan sulfate N-linked oligosaccharide O-linked oligosaccharide | [30,31,32,33,34] | |||
| ASP | N-linked oligosaccharide O-linked oligosaccharide | [35] | ||||
| ECM2 | N-linked oligosaccharide No data on potential GAG | ECM2 has a peculiarity in its motif with only 2 conserved cysteines. | [19,31] | |||
| ECMX | No data on potential GAG or glycosylation | [31] | ||||
| II | CX3CXCX9C | LUM | Keratan sulfate Poly-lactosamine N-linked oligosaccharide | Tyrosine sulfation | [36,37,38] | |
| KTN | [39] | |||||
 ID: 5MX0 | FMOD | Keratan sulfate Poly-lactosamine N-linked oligosaccharide | Tyrosine sulfation Acidic patch | [38,40,41,42] | ||
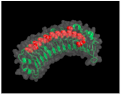 ID: 5YQ5 | OMD | Keratan sulfate N-linked oligosaccharide | [38,43,44,45] | |||
| PRELP | N-linked oligosaccharide | Basic patch | [46,47] | |||
| III | CX2CXCX6C | EPN | Chondroitin sulfate Dermatan sulfate N-linked oligosaccharide O-linked oligosaccharide | LRRs with only seven repeats Tyrosine sulfation Acidic patch | [30,31] | |
| OGN | Keratan sulfate Chondroitin sulfate Dermatan sulfate N-linked oligosaccharide | LRRs with only seven repeats Tyrosine sulfation | [31,48,49,50,51,52] | |||
| OPTC | N-linked oligosaccharide O-linked oligosaccharide | [31,53,54] | ||||
| IV | CX3CXCX6-17C |  ID: 5MX1 | CHAD | Keratan sulfate O-linked oligosaccharide | [31,55] | |
| NYX TSK | N-linked oligosaccharide | [31] | ||||
| V | CX3-4CXCX9C | PODN | N-linked oligosaccharide | High number of LRR with 20 repeats Acidic patch | [9,56,57] | |
| PODNL | High number of LRR with 21 repeats | [58] |
| Cluster 1 | Cluster 2 | ||
|---|---|---|---|
| Leucine | CUG | + | − |
| CUC | + | − | |
| CUU | − | + | |
| CUA | − | + | |
| UUG | − | + | |
| UUA | − | + | |
| Glutamate | GAG | + | − |
| GAA | − | + | |
| Glutamine | CAG | + | − |
| CAA | − | + | |
| Lysine | AAG | + | − |
| AAA | − | + | |
| Aspartate | GAC | + | − |
| GAU | − | + | |
| Asparagine | AAC | + | − |
| AAU | − | + | |
| Cysteine | UGC | + | − |
| UGU | − | + |
| SLRP | Species | Cleavage Site | Protease | Technique | In Vivo Data | Ref |
|---|---|---|---|---|---|---|
| Decorin | Human | |||||
| S241-L242 | MMP2 | N-terminal sequencing | - | [116] | ||
| S241-L242 | MMP3 | N-terminal sequencing | - | |||
| D31-A32 | MMP7 | N-terminal sequencing | - | |||
| E274-L273 | MMP7 | N-terminal sequencing | - | |||
| S240-L241 | MMP-13 | N-terminal sequencing | Comparison with WB on cartilage | [119] | ||
| Bovine | ||||||
| M200-K201 | - | N-terminal sequencing | Extracted from fresh matrix tendon | [129] | ||
| A209-D210 | - | |||||
| Q218-G219 | - | Extracted from medium of cultured tendon | ||||
| Biglycan | Human | |||||
| G177-V178 | MMP-13 | N-terminal sequencing | Comparison with WB on cartilage | [119] | ||
| Bovine | ||||||
| N187-C188 | ADAMTS-4 ADAMTS-5 | N-terminal sequencing | Comparison with WB on cartilage | [124] | ||
| Fibromodulin | Human | |||||
| Y63-T64 | MMP-13 | Data not shown | Data not shown | [113] | ||
| Bovine | ||||||
| Y63-A64 | MMP-13 | Mass spectrometry | Extracted from cartilage explant | [113] | ||
| Opticin | Human | |||||
| T87-S88 | MMP-2 MMP-7 | N-terminal sequencing | Comparison with WB on cartilage | [122] | ||
| E443-L444 | MMP-2 MMP-7 | |||||
| G114-L115 | MMP-2 MMP-7 | Prediction from [120] | ||||
| A20-S21 | MMP-7 | N-terminal sequencing | ||||
| E32-Q33 | MMP-7 | |||||
| Bovine | ||||||
| G104-L105 | MMP-13 | N-terminal sequencing | Comparison with WB on human cartilage; IHC on human cartilage and synovial membrane | [120] | ||
| P109-A110 | MMP-13 | |||||
| Chondroadherin | Human | |||||
| I80-Y81 | HTRA1 | Mass spectrometry | Comparison with WB on discs tissue | [127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappia, J.; Joiret, M.; Sanchez, C.; Lambert, C.; Geris, L.; Muller, M.; Henrotin, Y. From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues. Biomolecules 2020, 10, 80. https://doi.org/10.3390/biom10010080
Zappia J, Joiret M, Sanchez C, Lambert C, Geris L, Muller M, Henrotin Y. From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues. Biomolecules. 2020; 10(1):80. https://doi.org/10.3390/biom10010080
Chicago/Turabian StyleZappia, Jérémie, Marc Joiret, Christelle Sanchez, Cécile Lambert, Liesbet Geris, Marc Muller, and Yves Henrotin. 2020. "From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues" Biomolecules 10, no. 1: 80. https://doi.org/10.3390/biom10010080
APA StyleZappia, J., Joiret, M., Sanchez, C., Lambert, C., Geris, L., Muller, M., & Henrotin, Y. (2020). From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues. Biomolecules, 10(1), 80. https://doi.org/10.3390/biom10010080








