Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Estimation of Photosynthetic Pigments, Photosynthesis, and Relative Water Content (RWC)
2.2. Estimation of Electrolyte Leakage, Hydrogen Peroxide (H2O2) and Superoxide (O2–)
2.3. Lipid Peroxidation and Lipoxygenase Activity
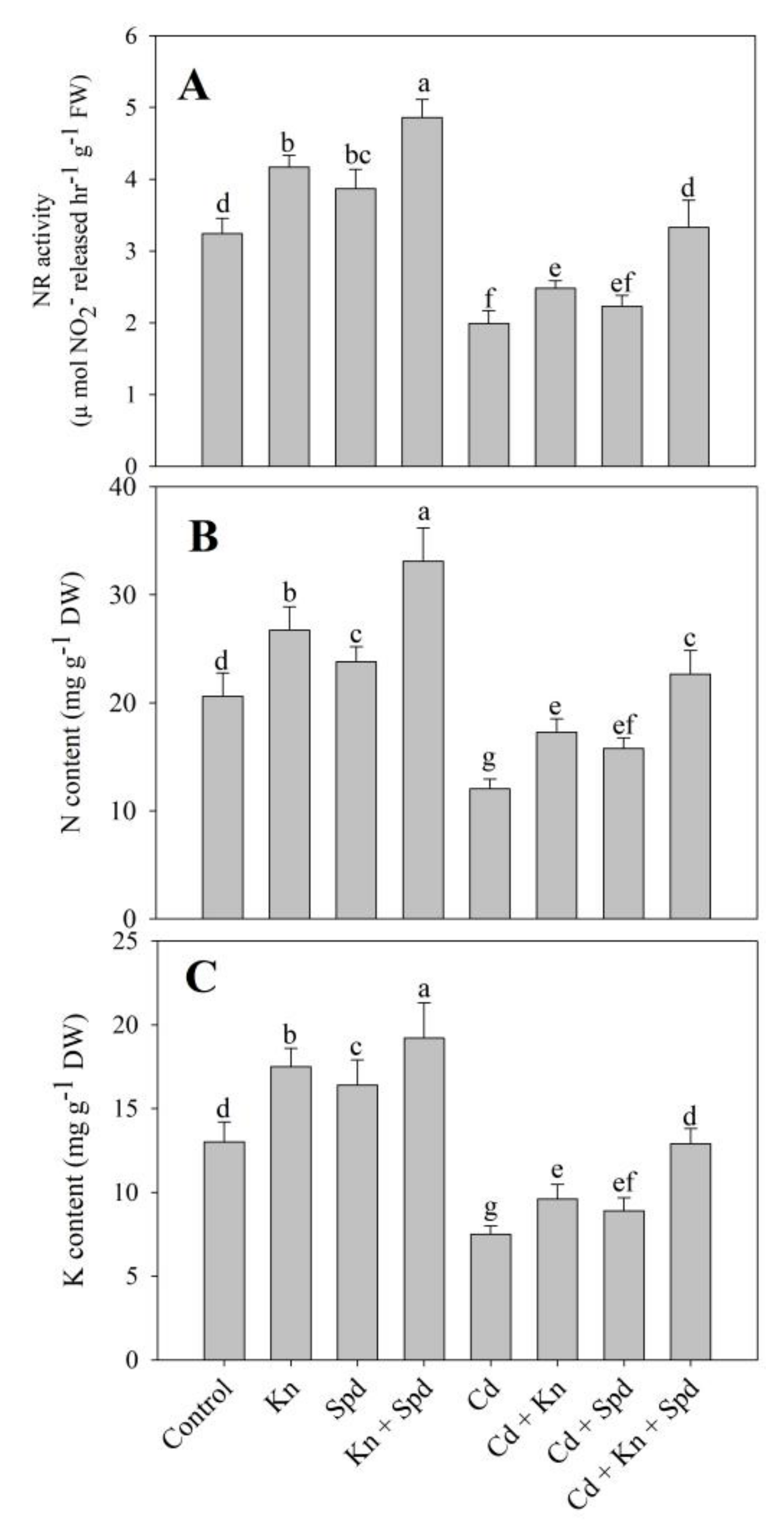
2.4. Estimation of Nitrate Reductase Activity
2.5. Estimation of Proline, Glycine Betaine, and Sugars
2.6. Determination of Antioxidant Enzyme Activities
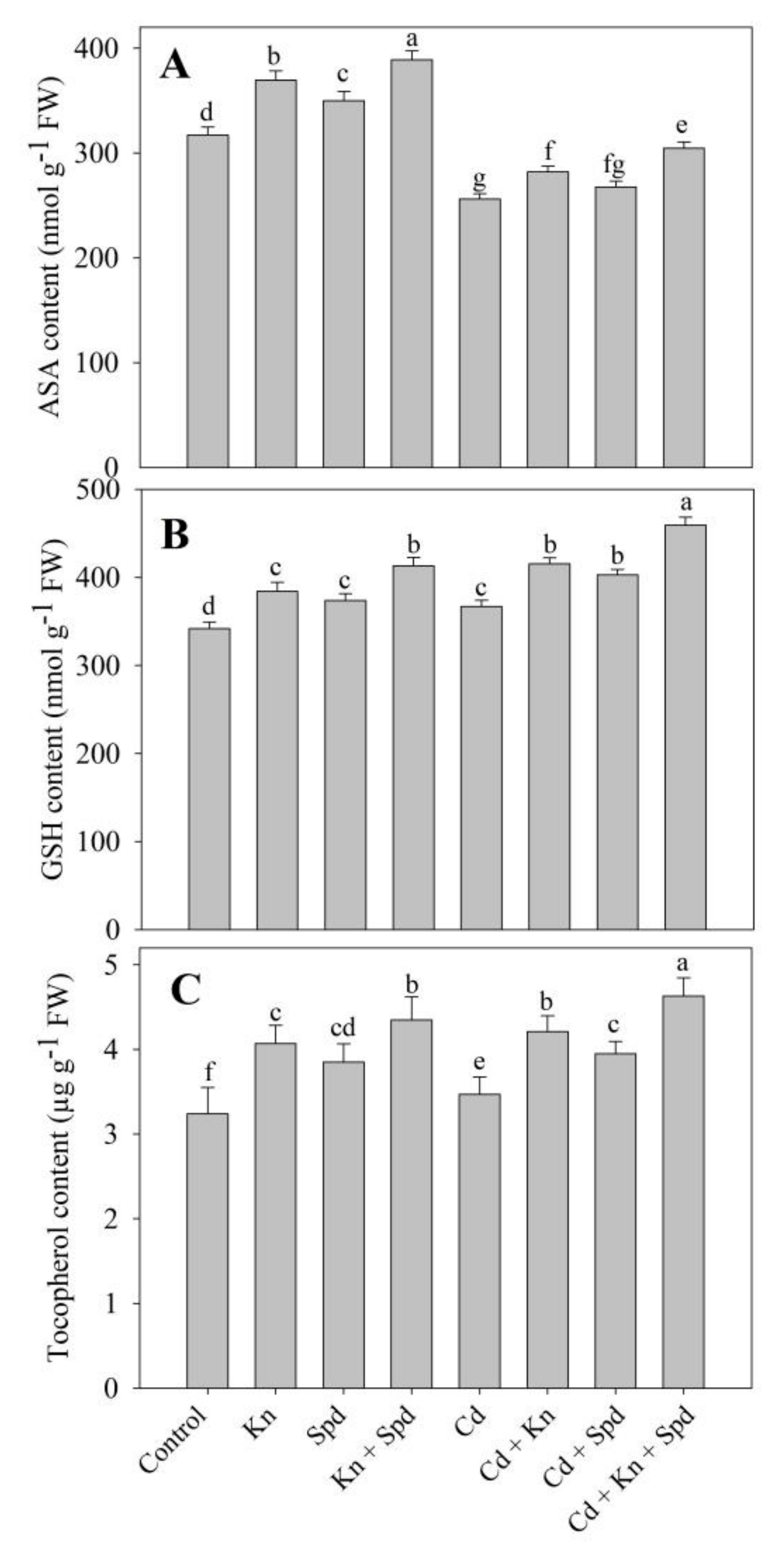
2.7. Estimation of Ascorbate, Reduced Glutathione, and Tocopherol
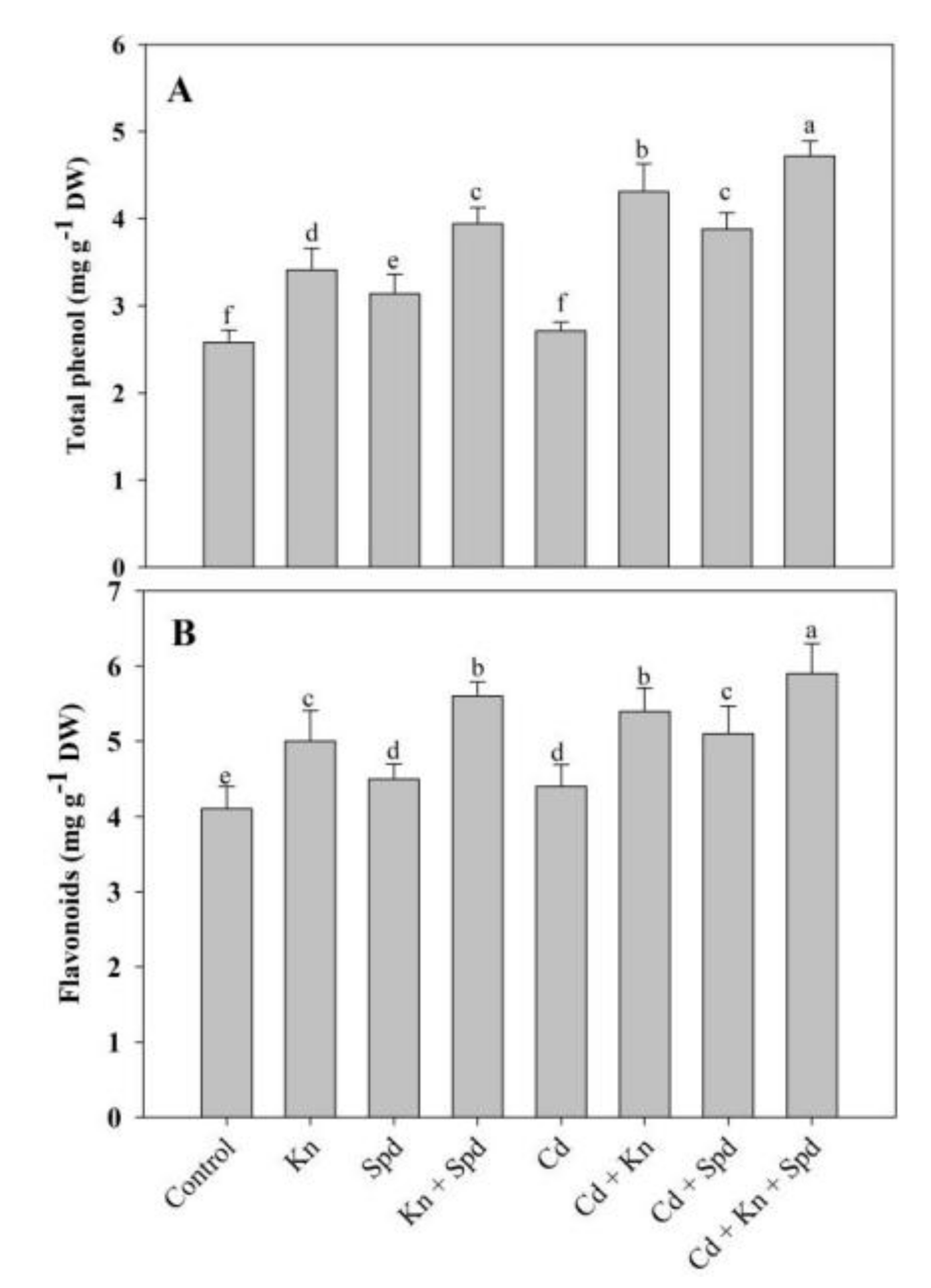
2.8. Total Phenols and Flavonoids
2.9. Estimation of K, N, and Cd
2.10. Statistical Analysis
3. Results
3.1. Kn and Spd Application Reduced the Cd Uptake and Improved Growth
3.2. Application of Kn and Spd Improves Pigment Synthesis and Photosynthesis
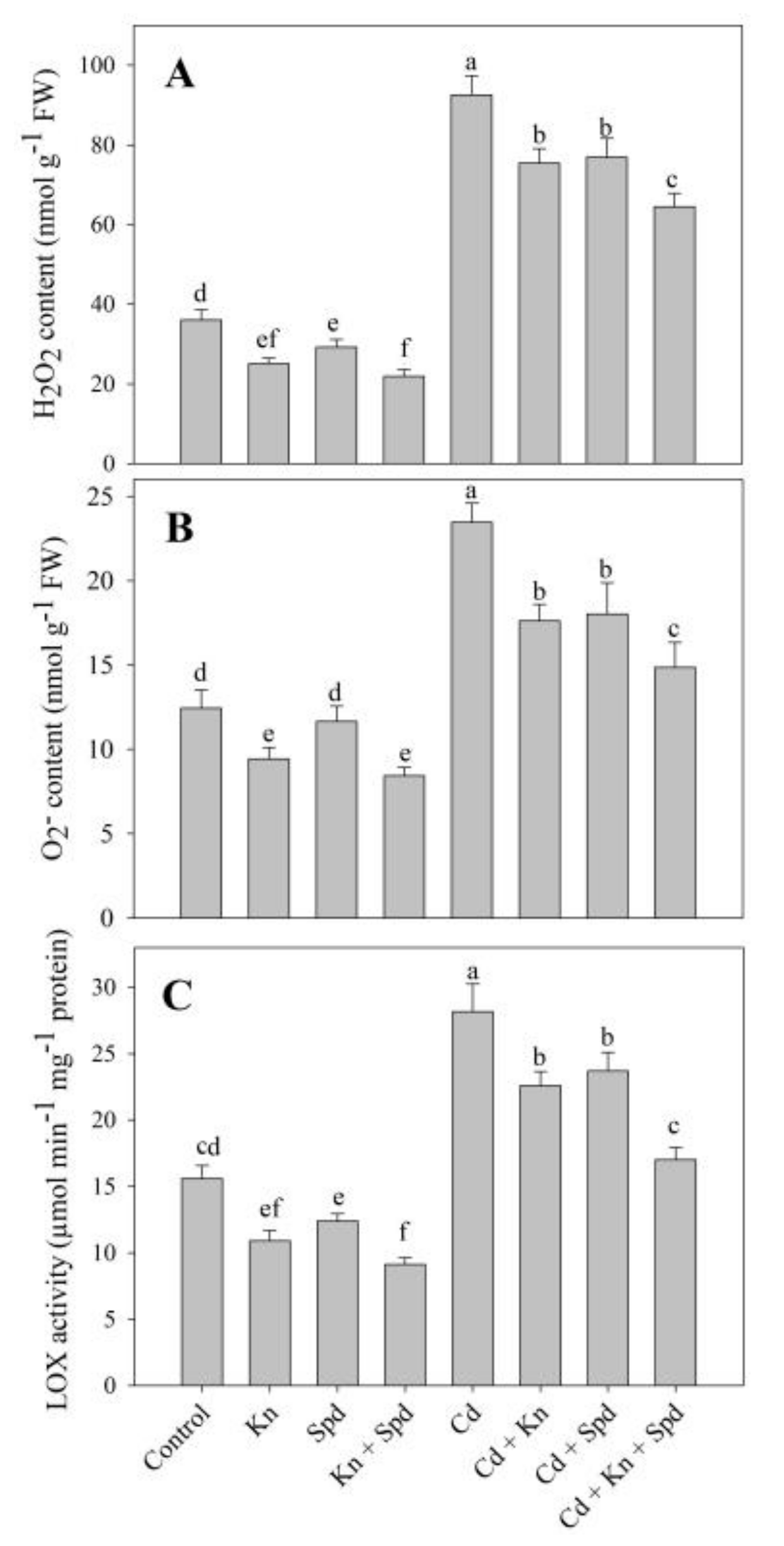
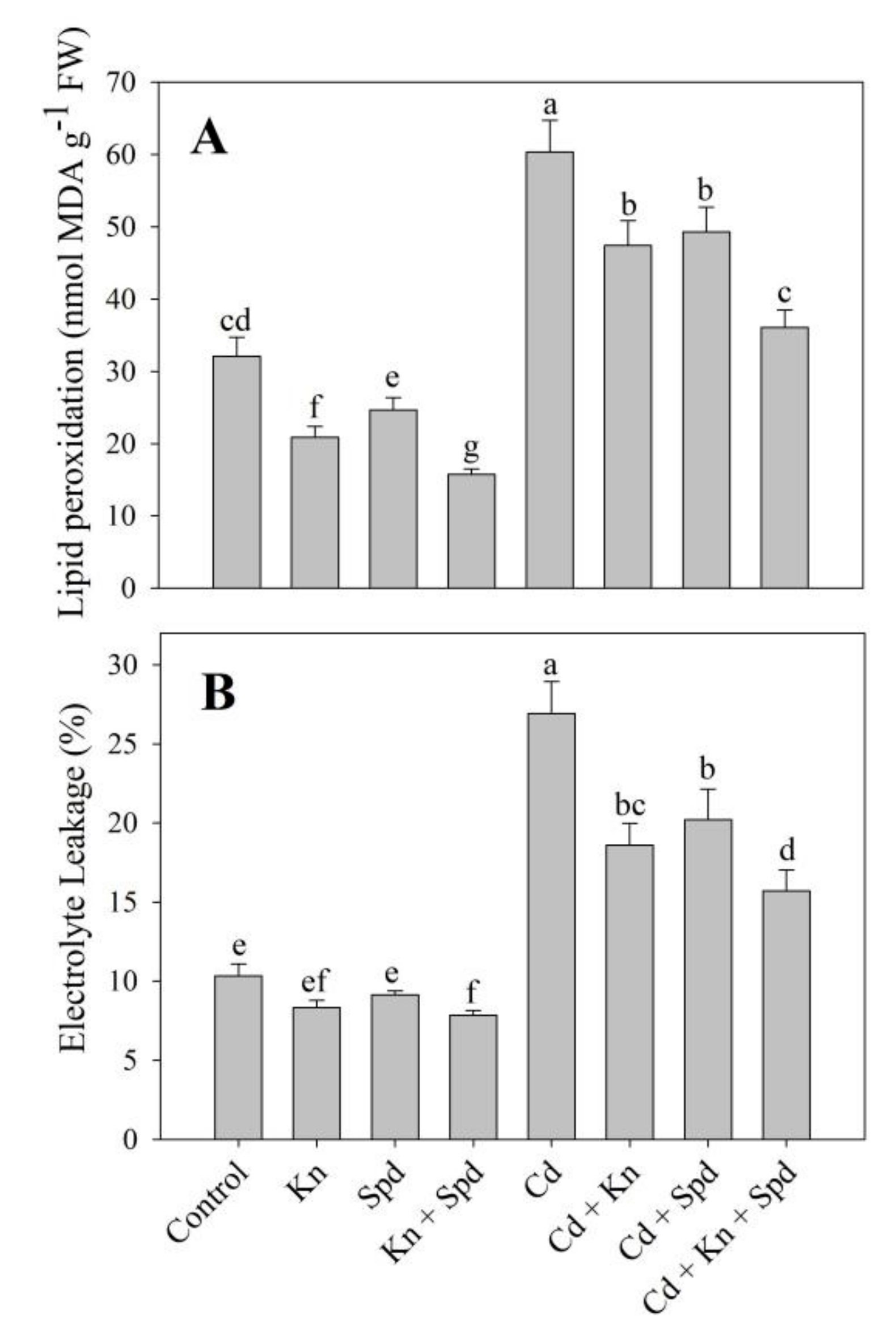
3.3. Kn and Spd Application Reduced Cd-Induced Oxidative Stress
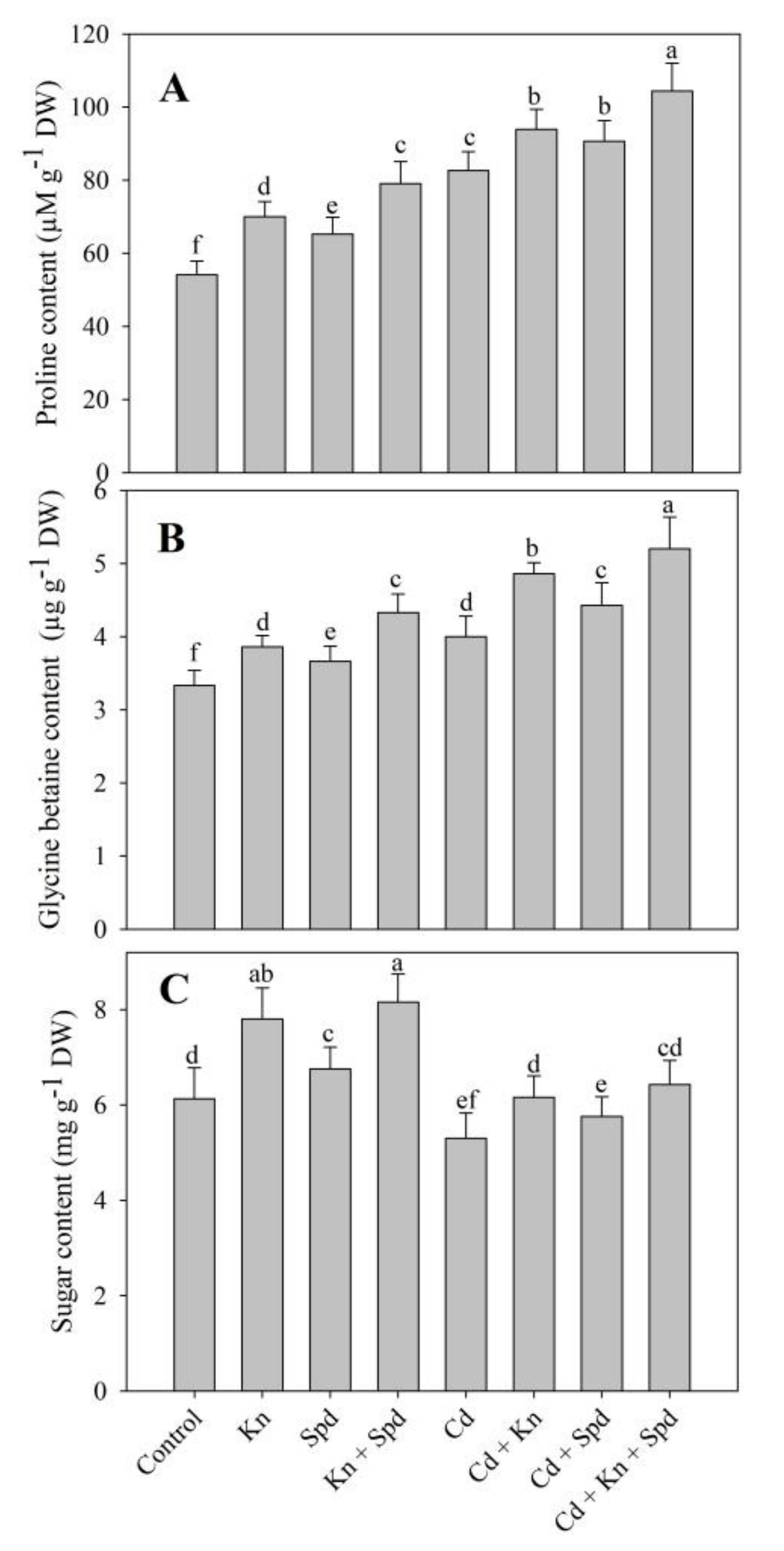
3.4. Effect of Kn and Spd on Accumulation of Sugars, Proline, and Glycine Betaine, and RWC
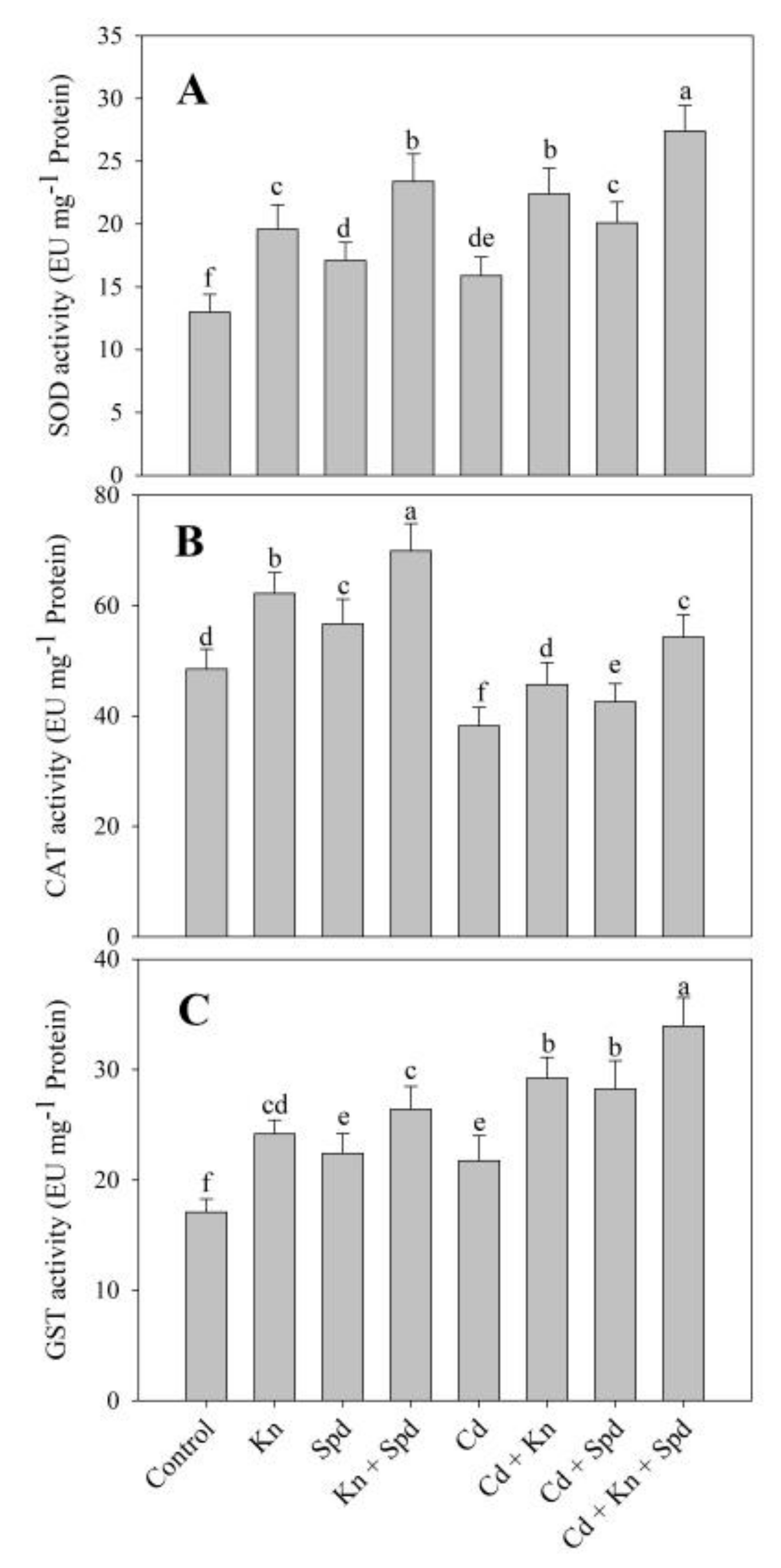
3.5. Kn and Spd Application Influences the Antioxidant System
3.6. Effect of Kn and Spd on Phenol and Flavonoid Accumulation under Cd Stress
3.7. Activity of NR and N Content Increased Due to Kn and Spd Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.-S.P. Alleviation of Cadmium Toxicity in Brassica juncea L. (Czern. & Coss.) by Calcium Application Involves Various Physiological and Biochemical Strategies. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl Jasmonate Alleviates Cadmium-Induced Photosynthetic Damages through Increased S-Assimilation and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Groppa, M.D.; Ianuzzo, M.P.; Rosales, E.P.; Vázquez, S.C.; Benavides, M.P. Cadmium modulates NADPH oxidase activity and expression in sunflower leaves. Biol. Plant. 2012, 56, 167–171. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Sun, L.; Chen, W.; Sheng, G.D.; Liu, W.; Fu, Z. Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat. Toxicol. 2009, 94, 56–61. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. Growth, photosynthesis and oxidative responses of Solanum melongena L. seedlings to cadmium stress: Mechanism of toxicity amelioration by kinetin. Sci. Hortic. 2014, 176, 1–10. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ashraf, M.; Bajguz, A.; Ahmad, P. Brassinosteroids Regulate Growth in Plants Under Stressful Environments and Crosstalk with Other Potential Phytohormones. J. Plant Growth Regul. 2018, 37, 1007–1024. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zalabák, D.; Pospíšilová, H.; Šmehilová, M.; Mrízová, K.; Frébort, I.; Galuszka, P. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants. Biotechnol. Adv. 2013, 31, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Brugière, N.; Humbert, S.; Rizzo, N.; Bohn, J.; Habben, J.E. A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development. Plant Mol. Biol. 2008, 67, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Brzobohaty, B.; Moore, I.; Kristoffersen, P.; Bako, L.; Campos, N.; Schell, J.; Palme, K. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science 1993, 262, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Vyroubalová, Š.; Václavíková, K.; Turečková, V.; Novák, O.; Šmehilová, M.; Hluska, T.; Ohnoutková, L.; Frébort, I.; Galuszka, P. Characterization of New Maize Genes Putatively Involved in Cytokinin Metabolism and Their Expression during Osmotic Stress in Relation to Cytokinin Levels. Plant Physiol. 2009, 151, 433–447. [Google Scholar] [CrossRef]
- Younis, M.E.; El-Shahaby, O.A.; Nemat Alla, M.N.; El-Bastawisy, Z.M. Kinetin alleviates the influence of waterlogging and salinity on growth and affects the production of plant growth regulators in Vigna sinensis and Zea mays. Agronomie 2003, 23, 277–285. [Google Scholar] [CrossRef]
- Aldesuquy, H.; Baka, Z.; Mickky, B. Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egypt. J. Basic Appl. Sci. 2014, 1, 77–87. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Z.; Li, X.; Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 2012, 34, 2105–2114. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Du, H.; Yu, W.; Liu, Y.; Gianfagna, T.; Huang, B. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J. Exp. Bot. 2012, 63, 1315–1328. [Google Scholar] [CrossRef]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2017, 255, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Kumar, A.; Gupta, A.; Hu, X.; Azooz, M.M.; Sharma, S. Polyamines: Role in plants under abiotic stress. In Crop Production for Agricultural Improvement; Springer: Dordrecht, The Netherlands, 2012; pp. 491–512. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity–alkalinity mixed stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Puyang, X.; An, M.; Xu, L.; Han, L.; Zhang, X. Protective effect of exogenous spermidine on ion and polyamine metabolism in Kentucky bluegrass under salinity stress. Hortic. Environ. Biotechnol. 2016, 57, 11–19. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Zhang, L.; Pan, X.; Hu, X. Exogenous spermidine is enhancing tomato tolerance to salinity–alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid Estimates of Relative Water Content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Doderer, A.; Kokkelink, I.; van der Veen, S.; Valk, B.E.; Schram, A.; Douma, A.C. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1992, 1120, 97–104. [Google Scholar] [CrossRef]
- Srivastava, H. In vivo activity of nitrate reductase in maize seedlings. Indian J. Biochem. 1974, 11, 230. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S.; Shrivastava, M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J. Plant Interact. 2015, 10, 211–223. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: New York, NY, USA, 1984; pp. 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Baker, H.; Frank, O.; DeAngelis, B.; Feingold, S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980, 21, 531–536. [Google Scholar]
- Malick, C.P.; Singh, M. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Li, Q.; Wang, H.; Wang, H.; Zheng, W.; Wu, D.; Wang, Z. Effects of kinetin on plant growth and chloroplast ultrastructure of two Pteris species under arsenate stress. Ecotoxicol. Environ. Saf. 2018, 158, 37–43. [Google Scholar] [CrossRef]
- Alsokari, S.S. Synergistic effect of kinetin and spermine on some physiological aspects of seawater stressed Vigna sinensis plants. Saudi J. Biol. Sci. 2011, 18, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, W.; Gao, X. Effects of Cadmium on Root Growth, Cell Division and Nucleoli in Root Tip Cells of Garlic. Biol. Plant. 2003, 47, 79–83. [Google Scholar] [CrossRef]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene Potentiates Sulfur-Mediated Reversal of Cadmium Inhibited Photosynthetic Responses in Mustard. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant. Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiang, L.; Li, S.; Zou, Z.; Hu, X.-H. Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiol. Plant. 2016, 156, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R.M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1953–1963. [Google Scholar] [CrossRef]
- Bashri, G.; Singh, M.; Mishra, R.K.; Kumar, J.; Singh, V.P.; Prasad, S.M. Kinetin Regulates UV-B-Induced Damage to Growth, Photosystem II Photochemistry, and Nitrogen Metabolism in Tomato Seedlings. J. Plant Growth Regul. 2018, 37, 233–245. [Google Scholar] [CrossRef]
- Khalil, R.R.; Moustafa, A.N.; Bassuony, F.M.; Haroun, S.A. Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J. Environ. Sci 2017, 46, 103–120. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant. Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—from gene to biotechnology. Aob Plants 2017, 9. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- de Moura, F.B.; Vieira, M.R.d.S.; SimÕEs, A.d.N.; Ferreira-Silva, S.L.; de Souza, C.A.V.; de Souza, E.S.; da Rocha, A.T.; da Silva, L.F.; JÚNior, M.A. Physiological Effect of Kinetin on the Photosynthetic Apparatus and Antioxidant Enzymes Activities During Production of Anthurium. Hortic. Plant. J. 2018, 4, 182–192. [Google Scholar] [CrossRef]
- Hashem, H.A. Cadmium toxicity induces lipid peroxidation and alters cytokinin content and antioxidant enzyme activities in soybean. Botany 2014, 92, 1–7. [Google Scholar] [CrossRef]
- Mehri, S. Effect of gibberellic acid foliar and kinetin on the antioxidant catalase anzymes and peroxidase in maize under drought stress. Cumhur. Üniversitesi Fen-Edeb. Fakültesi Fen Bilimleri Derg. 2015, 36, 595–603. [Google Scholar]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Signal transduction and biotechnology in response to environmental stresses. Biol. Plant. 2017, 61, 401–416. [Google Scholar] [CrossRef]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Ye, Y.R.; Wang, W.L.; Zheng, C.S.; Fu, D.J.; Liu, H.W.; Shen, X. Foliar-application of α-tocopherol enhanced salt tolerance of Carex leucochlora. Biol. Plant. 2017, 61, 565–570. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Ibrahim, M.; Chee Kong, Y.; Mohd Zain, N. Effect of Cadmium and Copper Exposure on Growth, Secondary Metabolites and Antioxidant Activity in the Medicinal Plant Sambung Nyawa (Gynura procumbens (Lour.) Merr). Molecules 2017, 22, 1623. [Google Scholar] [CrossRef]
- Pham, H.N.; Michalet, S.; Bodillis, J.; Nguyen, T.D.; Nguyen, T.K.O.; Le, T.P.Q.; Haddad, M.; Nazaret, S.; Dijoux-Franca, M.-G. Impact of metal stress on the production of secondary metabolites in Pteris vittata L. and associated rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 16735–16750. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Mustafavi, S.H.; Naghdi Badi, H.; Sękara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 2018, 40. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Control | Kn | Spd | Kn + Spd | Cd | Cd + Kn | Cd + Spd | Cd + Kn + Spd |
|---|---|---|---|---|---|---|---|---|
| Total chlorophyll | 1.13 ± 0.15 d | 1.41 ± 0.10 b | 1.23 ± 0.05 c | 1.58 ± 0.03 a | 0.772 ± 0.031 f | 0.9306 ± 0.082 e | 0.9172 ± 0.08 e | 1.166 ± 0.16 cd |
| Carotenoids | 0.4072 ± 0.01 c | 0.5083 ± 0.01 b | 0.4985 ± 0.02 b | 0.5822 ± 0.015 a | 0.2539 ± 0.01 f | 0.3311 ± 0.016 d | 0.3096 ± 0.01 e | 0.3961 ± 0.02 c |
| Photosynthesis (Pn) | 15.89 ± 1.0 c | 18.28 ± 1.3 b | 17.63 ± 1.1 b | 23.70 ± 1.9 a | 8.86 ± 0.7 f | 11.03 ± 0.45 d | 9.76 ± 0.51 e | 16.2 ± 1.0 c |
| Transpiration rate | 3.26 ± 0.05 c | 3.96 ± 0.20 b | 3.86 ± 0.15 b | 4.20 ± 0.26 a | 2.09 ± 0.30 f | 2.93 ± 0.14 d | 2.87 ± 0.20 de | 3.26 ± 0.25 c |
| Intercellular CO2 | 238.3 ± 7.6 d | 274.3 ± 6.6 b | 268.0 ± 6.5 b | 301.6 ± 9.0 a | 160.6 ± 5.1 f | 234.3 ± 3.5 d | 228.6 ± 2.5 de | 255.3 ± 4.0 c |
| RWC | 81.63 ± 4.3 bc | 86.88 ± 4.4 b | 85.38 ± 5.5 b | 90.80 ± 5.9 a | 56.88 ± 3.2 f | 69.67 ± 4.4 e | 66.88 ± 4.3 e | 73.66 ± 4.8 d |
| Plant height | 19.6 ± 2.1 d | 25.5 ± 2.1 b | 22.30 ± 1.4 c | 27.8 ± 2.1 a | 13.4 ± 0.55 f | 16.8 ± 0.71 e | 15.7 ± 0.66 e | 20.1 ±1.21 d |
| Plant dry weight | 2.2 ± 0.11 d | 3.4 ± 0.28 ab | 2.98 ± 0.21 c | 3.90 ± 0.24 a | 1.43 ± 0.09 f | 1.90 ± 0.11 e | 1.70 ± 0.14 e | 2.30 ± 0.24 d |
| Shoot Cd | nd | nd | nd | nd | 55.23 ± 4.1 a | 33.61 ± 3.0 b | 35.23 ± 2.9 b | 21.03 ± 2.3 c |
| Root Cd | nd | nd | nd | nd | 107.26 ± 6.1 a | 85.63 ± 3.6 b | 77.96 ± 3.8 b | 67.23 ± 3.2 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahanger, M.A.; Aziz, U.; Alsahli, A.; Alyemeni, M.N.; Ahmad, P. Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress. Biomolecules 2020, 10, 147. https://doi.org/10.3390/biom10010147
Ahanger MA, Aziz U, Alsahli A, Alyemeni MN, Ahmad P. Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress. Biomolecules. 2020; 10(1):147. https://doi.org/10.3390/biom10010147
Chicago/Turabian StyleAhanger, Mohammad Abass, Usman Aziz, Abdulaziz Alsahli, Mohammed Nasser Alyemeni, and Parvaiz Ahmad. 2020. "Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress" Biomolecules 10, no. 1: 147. https://doi.org/10.3390/biom10010147
APA StyleAhanger, M. A., Aziz, U., Alsahli, A., Alyemeni, M. N., & Ahmad, P. (2020). Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress. Biomolecules, 10(1), 147. https://doi.org/10.3390/biom10010147








