Effects of Allium mongolicum Regel and Its Flavonoids on Constipation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Loperamide-Induced Mouse Constipation Model
2.4. Measurement of Gastrointestinal Transit
2.5. Measurement of Serum Motilin Level in Mice
2.6. Histopathology of Colon Tissues
2.7. RNA Isolation and Real-Time PCR Analysis
2.8. Western Blot Analysis
2.9. Isolated Intestinal Tissue Preparation and Isometric Measurements
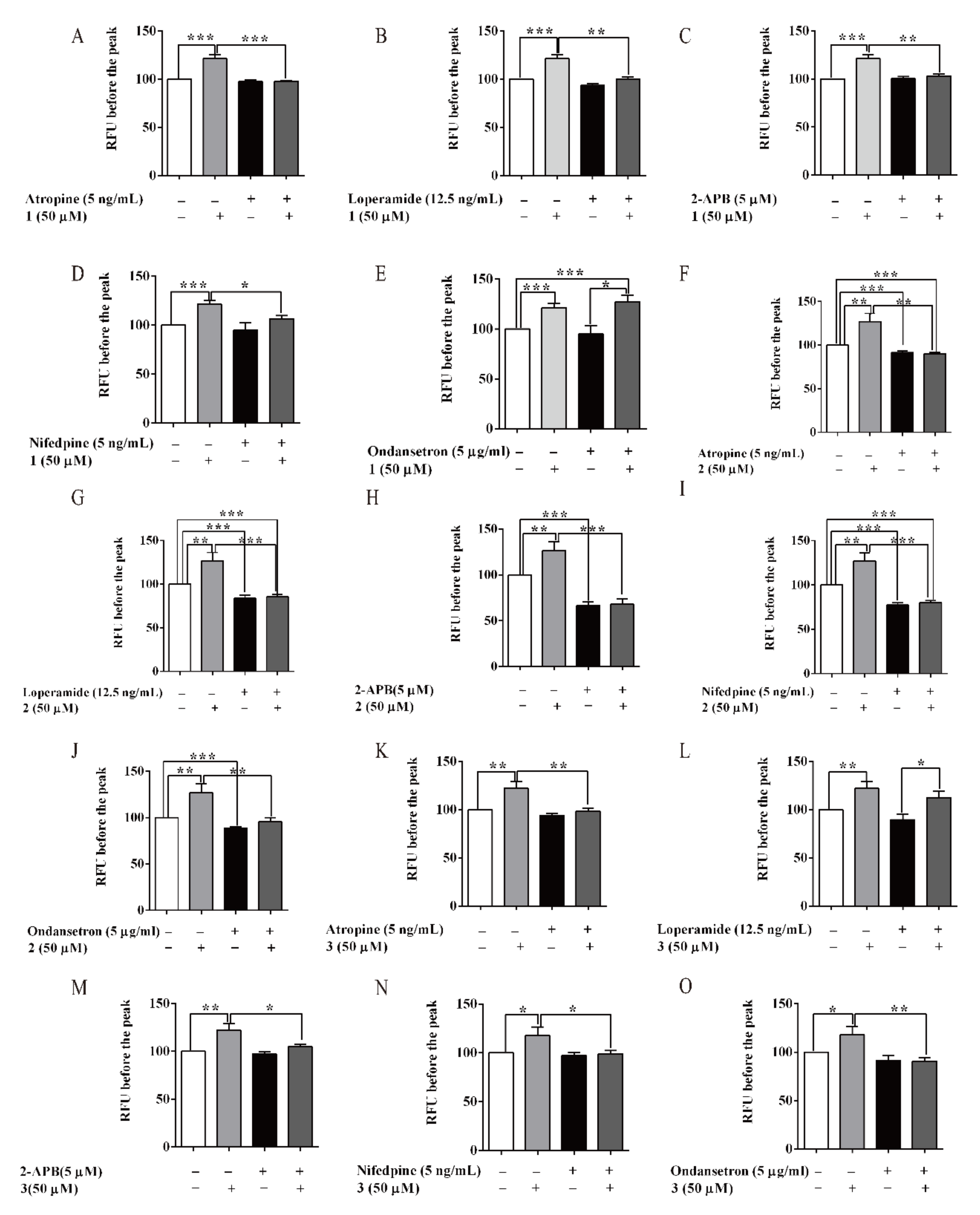
2.10. Effects of AM and its Compounds on Cellular Calcium Flux in HCT 116 Cells
2.11. Statistical Analysis
3. Results
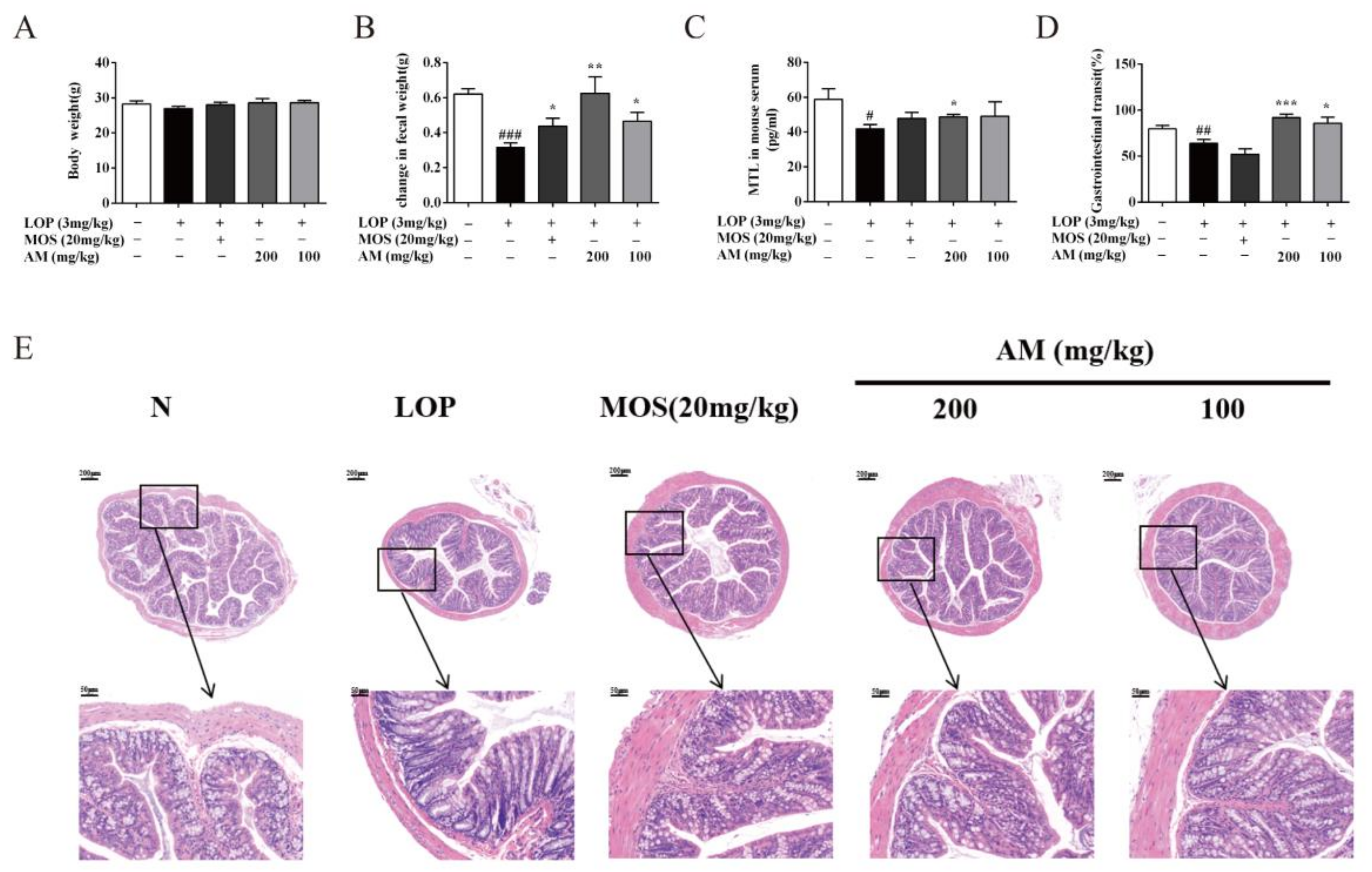
3.1. AM Alleviate Symptoms of LOP-Induced Constipation in Mice
3.2. AM Improved Pathological Symptoms of LOP-Induced Constipation in Mice
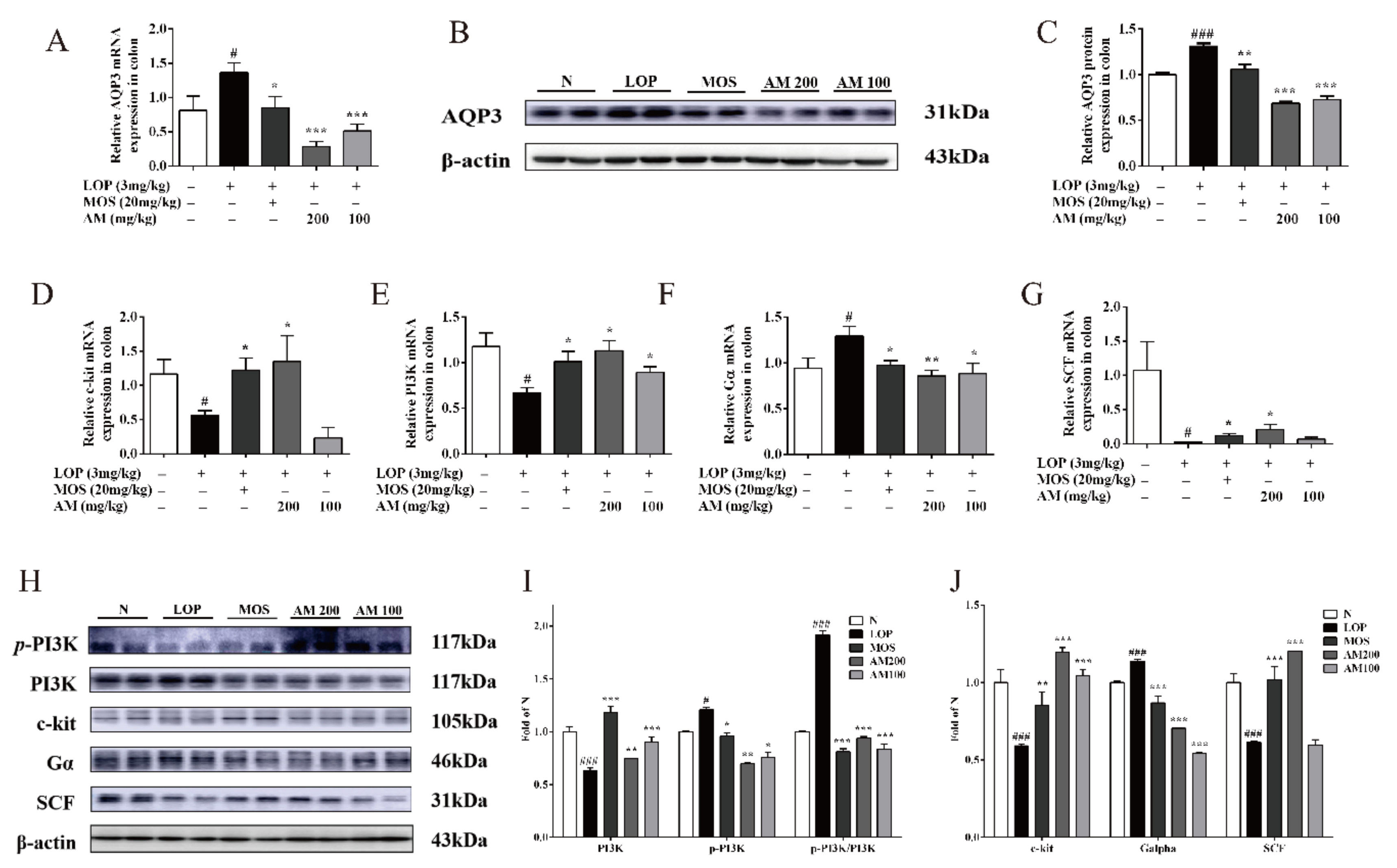
3.3. AM Regulate the Expressions of AQP3 and Intestinal-Motility-Related Protein Expression in LOP-Induced Constipation in Mice
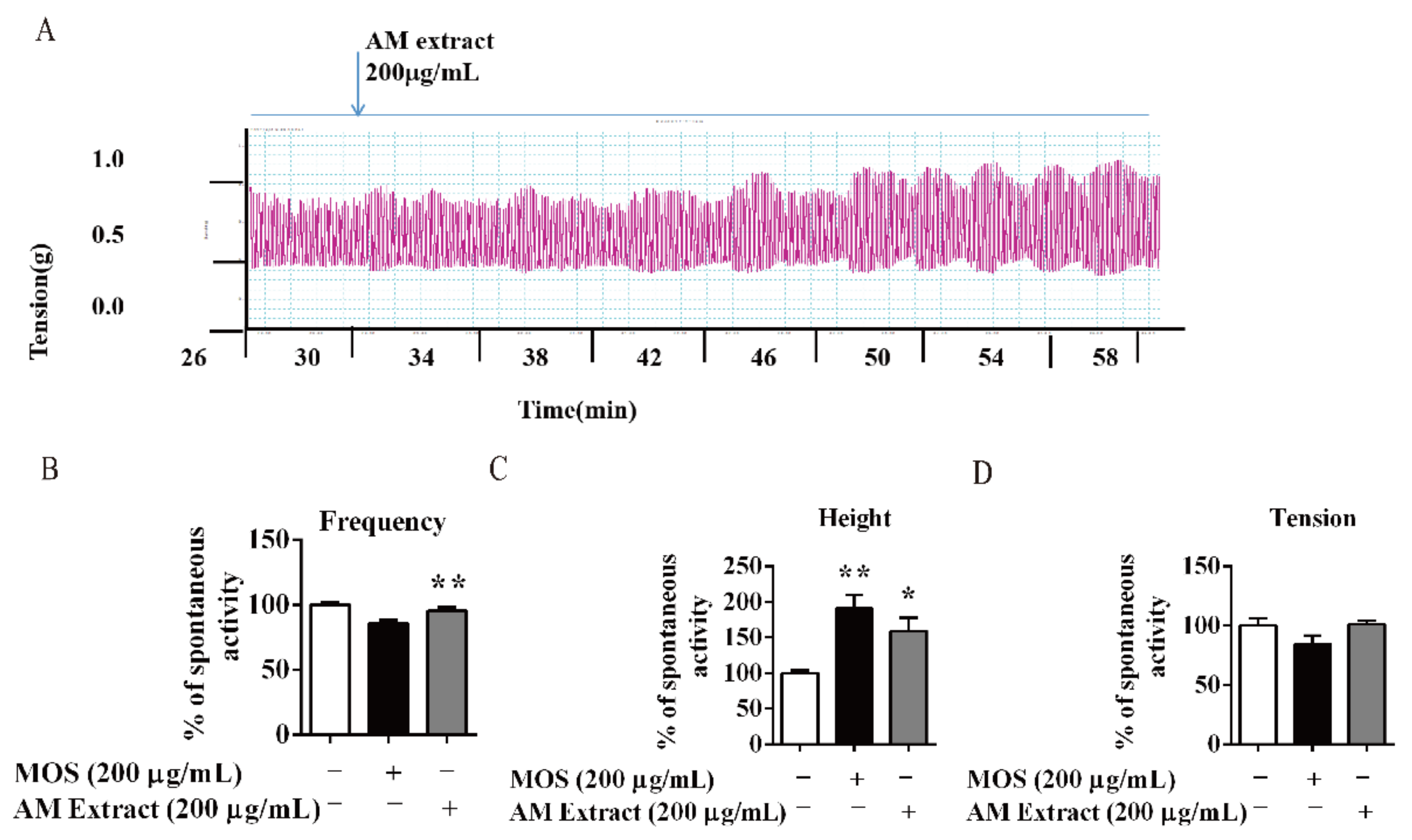
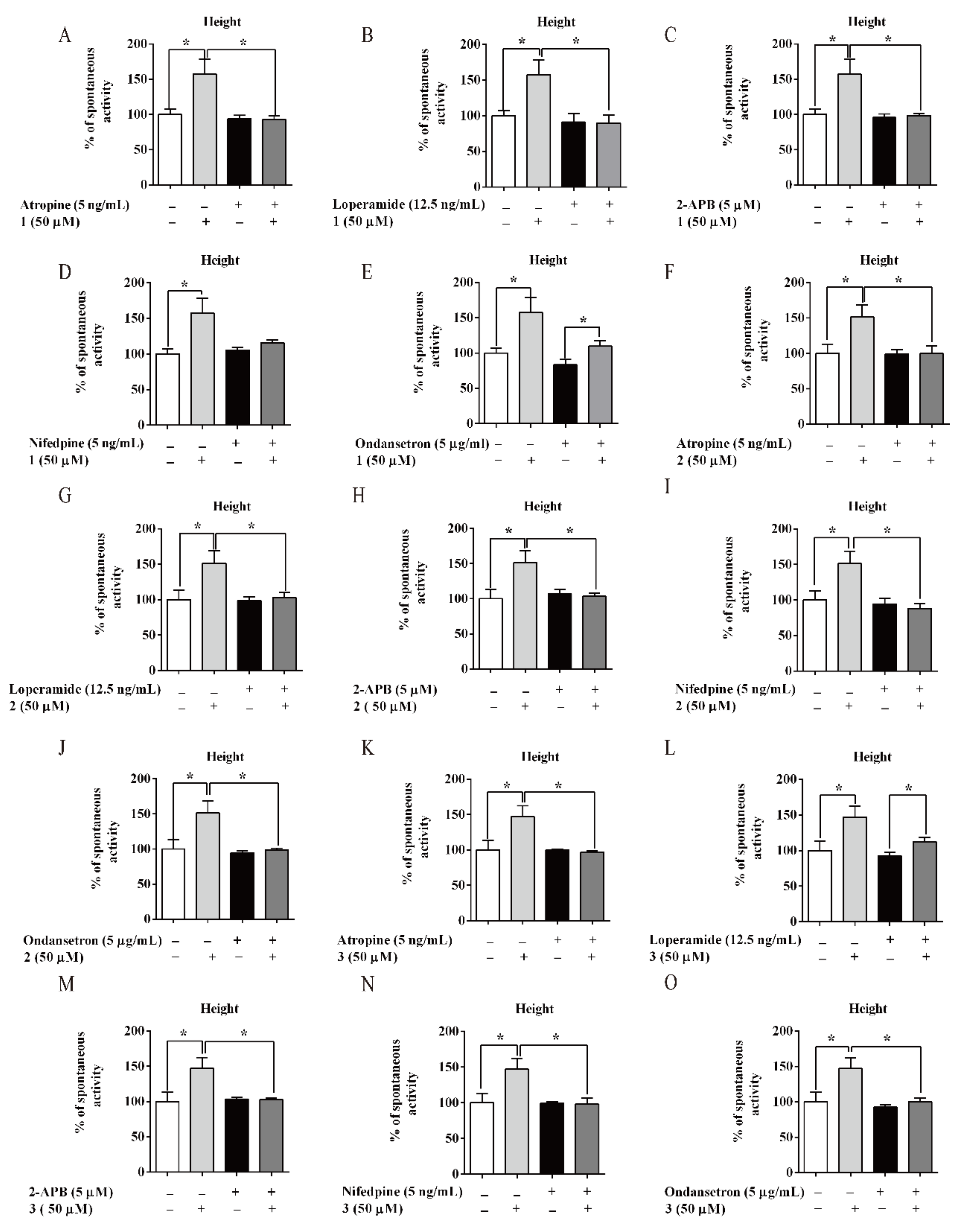
3.4. AM and its Flavonoids-Promoted Mice Intestine Smooth Muscle Contraction
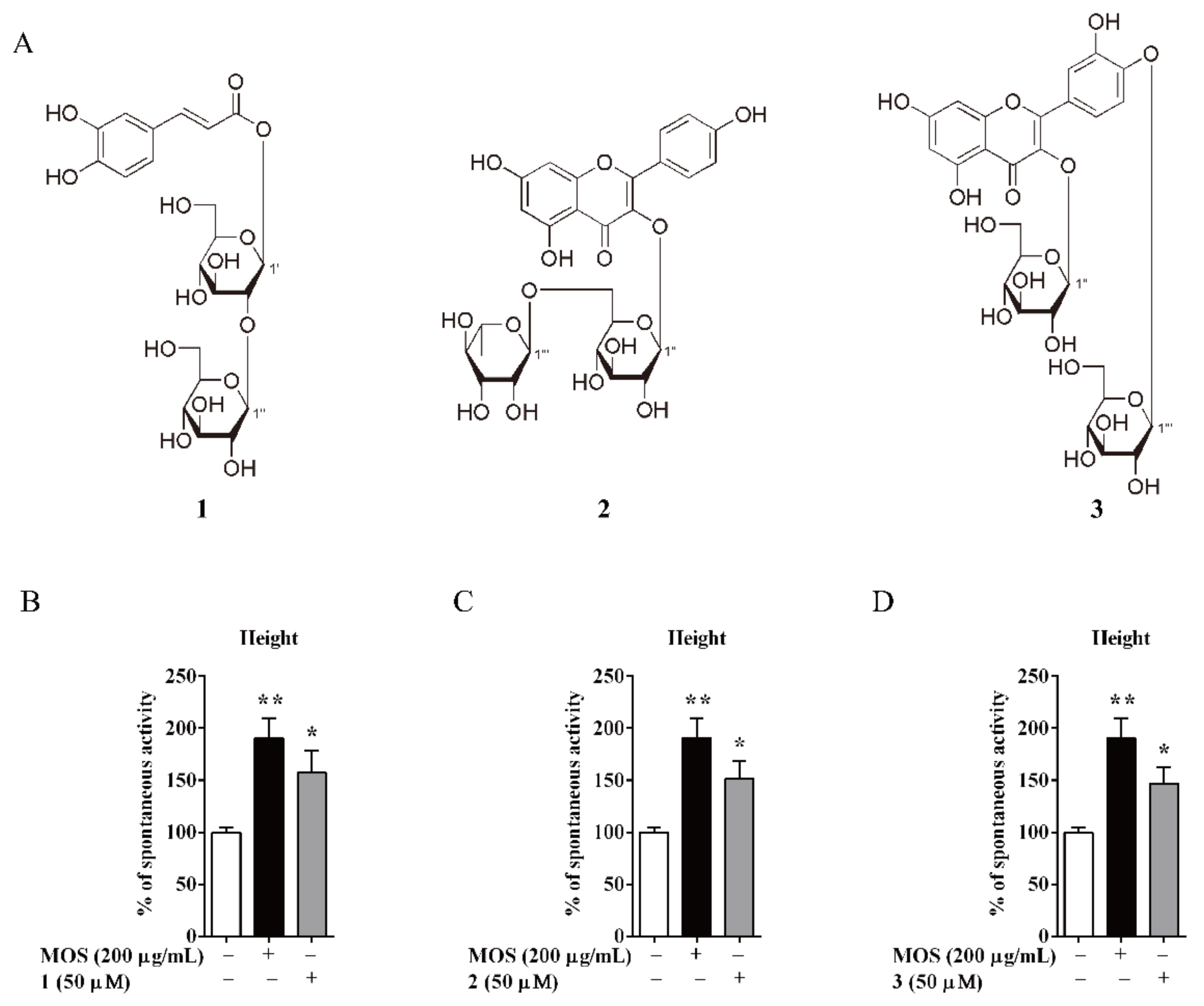
3.5. Compounds of AM-Stimulated Calcium Influx
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation: A review of literature. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef] [PubMed]
- Thürmann, P.A. Adverse drugs reactions: Diagnosis and assessment. Pathologe 2006, 27, 6–12. [Google Scholar]
- Rao, S.S.C.; Rattanakovit, K.; Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ratz, P.H.; Flaim, S.F. Acetylcholine- and 5-hydroxytryptamine-stimulated contraction and calcium uptake in bovine coronary arteries: Evidence for two populations of receptor-operated calcium channels. J. Pharm. Exp. Ther. 1985, 234, 641–647. [Google Scholar]
- Drossman, D. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef]
- Din, A.T.U.; Khan, A.H.; Bajwa, H.; Maqood, M.H.; Malik, M.N. Clinical Efficacy and Safety Profile of Prucalopride in Chronic Idiopathic Constipation. Cureus. 2019, 11, e4382. [Google Scholar]
- Ford, A.; Moayyedi, P.; Lacy, B.; Lembo, A.; Saito, Y.; Schiller, L.; Soffer, E.; Spiegel, B.; Quigley, E.; Task Force on the Management of Functional Bowel, D. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109, 2–26. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.; Lee, Y.J.; Seo, S.; Hong, J.T.; Hwang, D.Y. Laxative Effect of Spicatoside A by Cholinergic Regulation of Enteric Nerve in Loperamide-Induced Constipation: ICR Mice Model. Molecules 2019, 24, 896. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Lee, M.R.; Park, J.J.; Choi, J.Y.; Song, B.R.; Son, H.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm Biol. 2018, 56, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Shimin, T.; Yaping, Z.; Jie, G.; Guoying, C.; Ying, A.; Shazhou, A. Changes of leaf’s carbohydrate and protein content during growth and development of Allium mongolicum Regel. Xinjiang Agric. Sci. 2010, 47, 512–516. [Google Scholar]
- Yongzhe, D.; Wenzhong, S.; Shengcai, Y.; Xiaoxia, L.; Yi, Z.; Tao, W. Isolation and structural identification of constituents from Allium mongolicm Regel II. J. Tianjin Univ. Tradit. Chin. Med. 2016, 35, 404–408. [Google Scholar]
- Yongzhe, D.; Lu, Q.; Xiaoxia, L.; Lifeng, H.; Tao, W.; Yi, Z. Isolation and identification of constituents from Allium mongolicum Regel I. Chin. J. Med. Chem. 2015, 25, 298–302. [Google Scholar]
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel. Food Sci Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Guo, W.; Guo, G.; Zhu, X.; Niu, X.; Shan, X.; Tian, J.; Wng, G.; Zhang, D. Effect of sub-chronic exposure to selenium and Allium mongolicum Regel flavonoids on Channa argus: Bioaccumulation, oxidative stress, immune responses and immune-related signaling molecules. Fish. Shellfish Immunol. 2019, 91, 122–129. [Google Scholar] [CrossRef]
- Sechenbater; Liu, X. Nutritional components and ethnobotanics of Allium mongolicum Regel. Grassl. China 2002, 24, 52–54. [Google Scholar]
- Kim, J.E.; Yun, W.B.; Sung, J.E.; Lee, H.A.; Choi, J.Y.; Choi, Y.S.; Jung, Y.S.; Kim, K.S.; Hwang, D.Y. Characterization the response of Korl:ICR mice to loperamide induced constipation. Lab. Anim Res. 2016, 32, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Fen, P.; Shouxiang, Y.; Yaping, L.; Xiaorong, C.; Yiwen, L.; Hongguo, Z. Effect of moxibustion on motility, absorption and content of ATP in small intestine of spleen-deficiency rats. Zhongguo Zhen Jiu. 2012, 32, 246–250. [Google Scholar]
- Kim, J.E.; Park, J.W.; Park, J.W.; Choi, H.J.; Bae, S.J.; Choi, Y.S.; Lee, Y.J.; Lee, H.S.; Hong, J.T.; Hwang, D.Y. Anti-Inflammatory Response and Muscarinic Cholinergic Regulation during the Laxative Effect of Asparagus cochinchinensis in Loperamide-Induced Constipation of SD Rats. Int J. Mol. Sci. 2019, 20, 946. [Google Scholar] [CrossRef] [Green Version]
- Gallo-Oller, G.; Ordoñez-Ciriza, R.; Dotor, J. A new background subtraction method for Western blot densitometry band quantification through image analysis software. J. Immunol Methods 2018, 457, 1–5. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Huang, X.; Zhang, C.; Liu, D.; Lu, H.; Kim, Y.; Xu, W. Hydrogen sulfide-induced enhancement of gastric fundus smooth muscle tone is mediated by voltage-dependent potassium and calcium channels in mice. World J. Gastroenterol. 2015, 21, 4840–4851. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Sudo, H.; Muramatsu, H.; Yogo, K.; Kamei, K.; Koga, H.; Itoh, Z.; Omura, S.; Takanashi, H. Mitemcinal (GM-611), an orally active motilin receptor agonist, accelerates colonic motility and bowel movement in conscious dogs. Inflammopharmacology 2007, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Yun, W.B.; Lee, M.L.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Hong, J.T.; Song, H.K.; Hwang, D.Y. Synergic Laxative Effects of an Herbal Mixture of Liriope platyphylla, Glycyrrhiza uralensis, and Cinnamomum cassia in Loperamide-Induced Constipation of Sprague Dawley Rats. J. Med. Food. 2019, 22, 294–304. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef]
- Yongxue, Z.; Yujin, W.; Hong, Z.; Shuguang, Y.; Bin, W.; Pei, X. Effect of vasoactive intestinal peptide on defecation and VIP-cAMP-PKA-AQP3 signaling pathway in rats with constipation. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016, 41, 1175–1180. [Google Scholar]
- Lu, Z.; Jiang, Y.-P.; Ballou, L.M.; Cohen, I.S.; Lin, R.Z. Galpha q inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J. Biol Chem. 2005, 280, 40347–40354. [Google Scholar] [CrossRef] [Green Version]
- MBBS, A.E.B.; Wald, A. Chronic Constipation. Mayo Clin. Proc. 2019, 94, 2340–2357. [Google Scholar]
- Xin, H.; Fang, X.; Zhu, L.; Xu, T.; Fei, G.; Wang, Z.; Chang, M.; Wang, L.; Sun, X.; Ke, M. Diagnosis of functional constipation: Agreement between Rome III and Rome II criteria and evaluation for the practicality. J. Dig. Dis. 2014, 15, 314–320. [Google Scholar] [CrossRef]
- Talley, N.; Jones, M.; Nuyts, G.; Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 2003, 98, 1107–1111. [Google Scholar] [CrossRef]
- Riemann, J.F.; Schmidt, H.; Zimmermann, W. The fine structure of colonic submucosal nerves in patients with chronic laxative abuse. Scand J. Gastroenterol. 1980, 15, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.L.; Haskvitz, E.M. Managing a patient’s constipation with physical therapy. Phys. Ther. 2006, 86, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Hayakawa, A.; Haga, Y.; Fueki, A.; Kusunoki, Y.; Tajima, M.; Ochiai, W.; Machida, Y.; Sugiyama, K. Morphine-Induced Constipation Develops With Increased Aquaporin-3 Expression in the Colon via Increased Serotonin Secretion. Toxicol. Sci. 2015, 145, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ge, T.; Xiang, P.; Mao, H.; Tang, S.; Li, A.; Li, A.; Wei, Y. Therapeutic effect of protease-activated receptor 2 agonist SLIGRL-NH2 on loperamide-induced Sprague-Dawley rat constipation model and the related mechanism. Drug Des. Devel Ther. 2018, 12, 2403–2411. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, R.D.; Ruggeri, E.; Stanghellini, V.; Eusebi, L.H.; Bazzoli, F.; Chiarioni, G. Chronic constipation in the elderly: A primer for the gastroenterologist. BMC Gastroenterol. 2015, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Yue, Y.; Wang, X.; Dong, H.; Zhen, S.; Wu, B.; Qian, H. Aqueous Extracts of Herba Cistanche Promoted Intestinal Motility in Loperamide-Induced Constipation Rats by Ameliorating the Interstitial Cells of Cajal. Evid Based Complement. Altern. Med. 2017, 2017, 13. [Google Scholar] [CrossRef]
- Hill-Eubanks, D.C.; Werner, M.E.; Heppner, T.J.; Nelson, M.T. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol. 2011, 3, a004549. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Zemskov, E.A.; Gonzales, J.; Gorshkov, B.A.; Sridhar, S.; Chakraborty, T.; Lucas, R.; Verin, A.D. Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J. Cell Physiol. 2010, 223, 215–223. [Google Scholar]
- Lock, J.T.; Alzayady, K.J.; Yule, D.I.; Parker, I. All three IP3 receptor isoforms generate Ca(2+) puffs that display similar characteristics. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, H.-L.; Lee, Y.-K.; Han, M.; Sacket, S.J.; Jo, J.-Y.; Kim, Y.-L.; Im, D.-S. Lysophosphatidylserine induces calcium signaling through Ki16425/VPC32183-sensitive GPCR in bone marrow-derived mast cells and in C6 glioma and colon cancer cells. Arch. Pharm Res. 2008, 31, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rameh, L.E.; Rhee, S.G.; Spokes, K.; Kazlauskas, A.; Cantley, L.C.; Cantley, L.G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J. Biol. Chem. 1998, 273, 23750–23757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, O.H.; Courjaret, R.; Machaca, K. Ca(2+) tunnelling through the ER lumen as a mechanism for delivering Ca(2+) entering via store-operated Ca(2+) channels to specific target sites. J. Physiol. 2017, 595, 2999–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, R.; Peng, P.; Zhang, J.; Du, M.; Lan, L.; Qian, Y.; Zhou, J.; Zhao, X. Lactobacillus plantarum CQPC02-Fermented Soybean Milk Improves Loperamide-Induced Constipation in Mice. J. Med. Food 2019, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fourbon, Y.; Guéguinou, M.; Félix, R.; Constantin, B.; Uguen, A.; Fromont, G.; Lajoie, L.; Magaud, C.; Lecomte, T.; Chamorey, E.; et al. Ca(2+) protein alpha 1D of CaV1.3 regulates intracellular calcium concentration and migration of colon cancer cells through a non-canonical activity. Sci. Rep. 2017, 7, 14199. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cheang, W.; Zhang, D.; Li, Y.; Lau, C.; Wang, G.; Huang, Y.; Ye, W. Antagonism of Ca2+ influx via L-type Ca2+ channels mediates the vasorelaxant effect of Catharanthus roseus-derived vindorosine in rat renal artery. Planta Med. 2014, 80, 1672–1677. [Google Scholar] [CrossRef]
| Gene | Sequence |
|---|---|
| AQP3(mouse) | Forward: 5′- GCCAAGGTAGGATAGCAAATAA -3′ |
| Reverse: 5′- TTGAAAACTTGGTCCCTTGC -3′ | |
| c-kit(mouse) | Forward: 5′- CCGACGCAACTTCCTTATGAT -3′ |
| Reverse: 5′- TCAGGACCTTCAGTTCCGACA -3′ | |
| SCF(mouse) | Forward: 5′- ATAGTGGATGACCTCGTGTTA -3′ |
| Reverse: 5′- GAATCTTTCTCGGGACCTAAT -3′ | |
| PI3K(mouse) | Forward: 5′- TGTGTTCTCTGCTCGTCAGG -3′ |
| Reverse: 5′- TTCTGTAGTGTGGGGGTCCA -3′ | |
| Gα(mouse) | Forward: 5′- CCCCAGCAGGTTCCTAAGAC -3′ |
| Reverse: 5′- CGGTCAGGCAAGTAGGAAGG -3′ | |
| β-actin(mouse) | Forward: 5′- CTGTGCCCATCTACGAGGGCTAT -3′ |
| Reverse: 5′- TTTGATGTCACGCACGATTTCC -3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ding, Z.; Wu, Y.; Chen, Q.; Liu, M.; Yu, H.; Wang, D.; Zhang, Y.; Wang, T. Effects of Allium mongolicum Regel and Its Flavonoids on Constipation. Biomolecules 2020, 10, 14. https://doi.org/10.3390/biom10010014
Chen Y, Ding Z, Wu Y, Chen Q, Liu M, Yu H, Wang D, Zhang Y, Wang T. Effects of Allium mongolicum Regel and Its Flavonoids on Constipation. Biomolecules. 2020; 10(1):14. https://doi.org/10.3390/biom10010014
Chicago/Turabian StyleChen, Yue, Zhijuan Ding, Yuzheng Wu, Qian Chen, Mengyang Liu, Haiyang Yu, Dan Wang, Yi Zhang, and Tao Wang. 2020. "Effects of Allium mongolicum Regel and Its Flavonoids on Constipation" Biomolecules 10, no. 1: 14. https://doi.org/10.3390/biom10010014
APA StyleChen, Y., Ding, Z., Wu, Y., Chen, Q., Liu, M., Yu, H., Wang, D., Zhang, Y., & Wang, T. (2020). Effects of Allium mongolicum Regel and Its Flavonoids on Constipation. Biomolecules, 10(1), 14. https://doi.org/10.3390/biom10010014




