Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Endometrial Tissue Collection
2.2. Three-Dimensional (3D) Human Tissue Slice Culture System
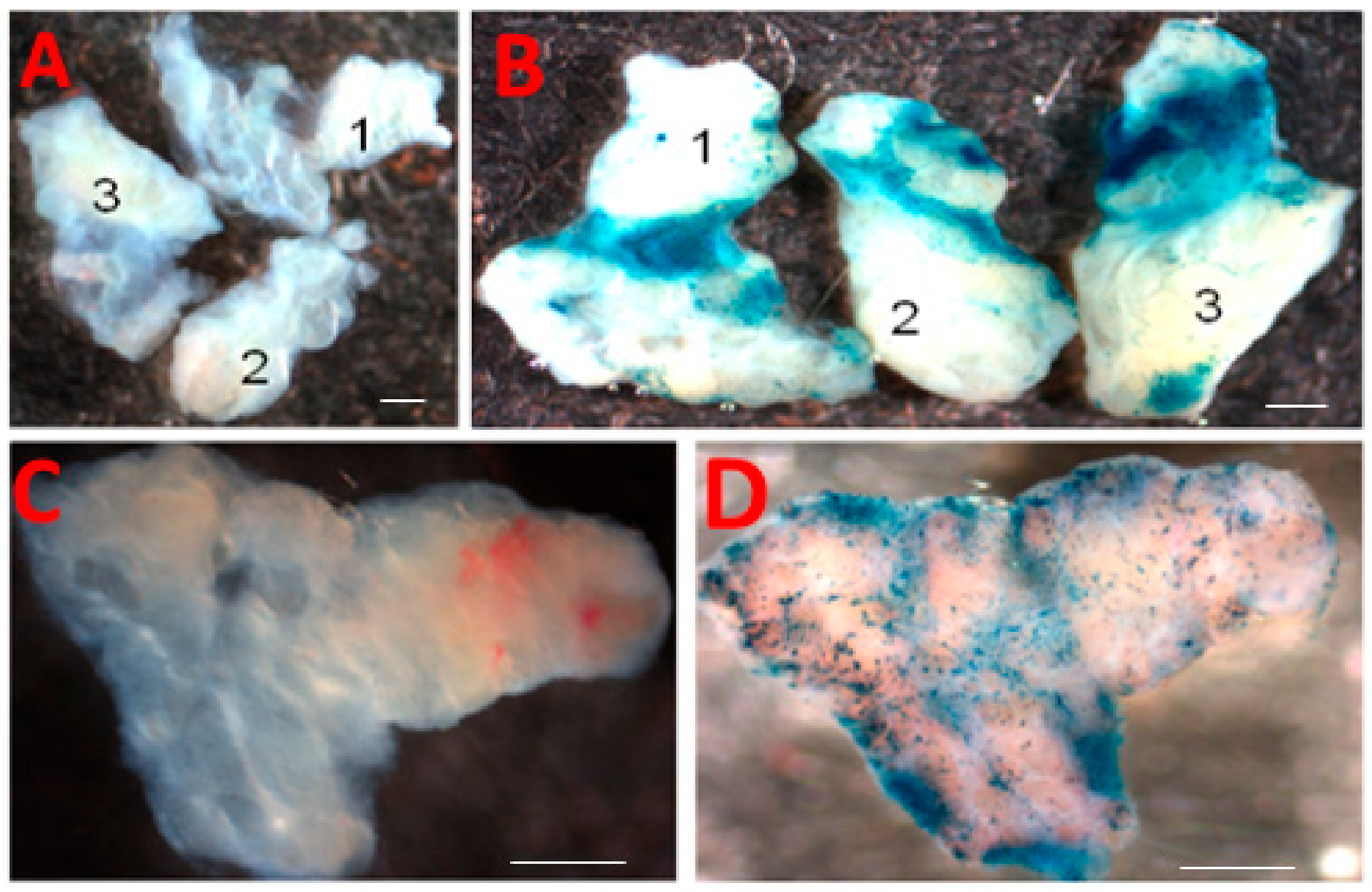
2.3. Adenovirus-Mediated Gene Delivery
2.4. LacZ Staining
2.5. Histology and Immunohistochemistry
3. Results and Discussion
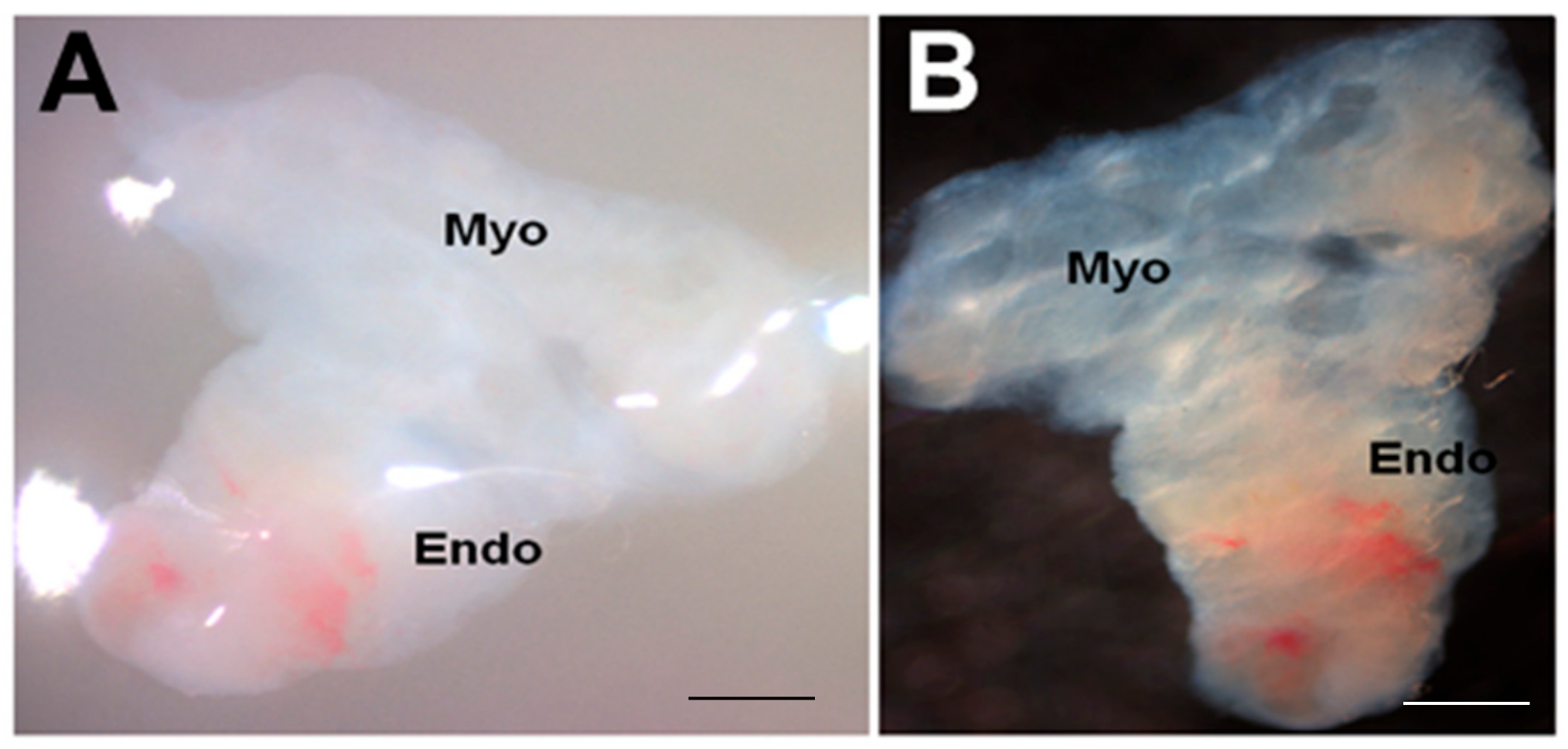
3.1. Establishment of Endometrial Tissue Air-Liquid Interface 3D Cultures
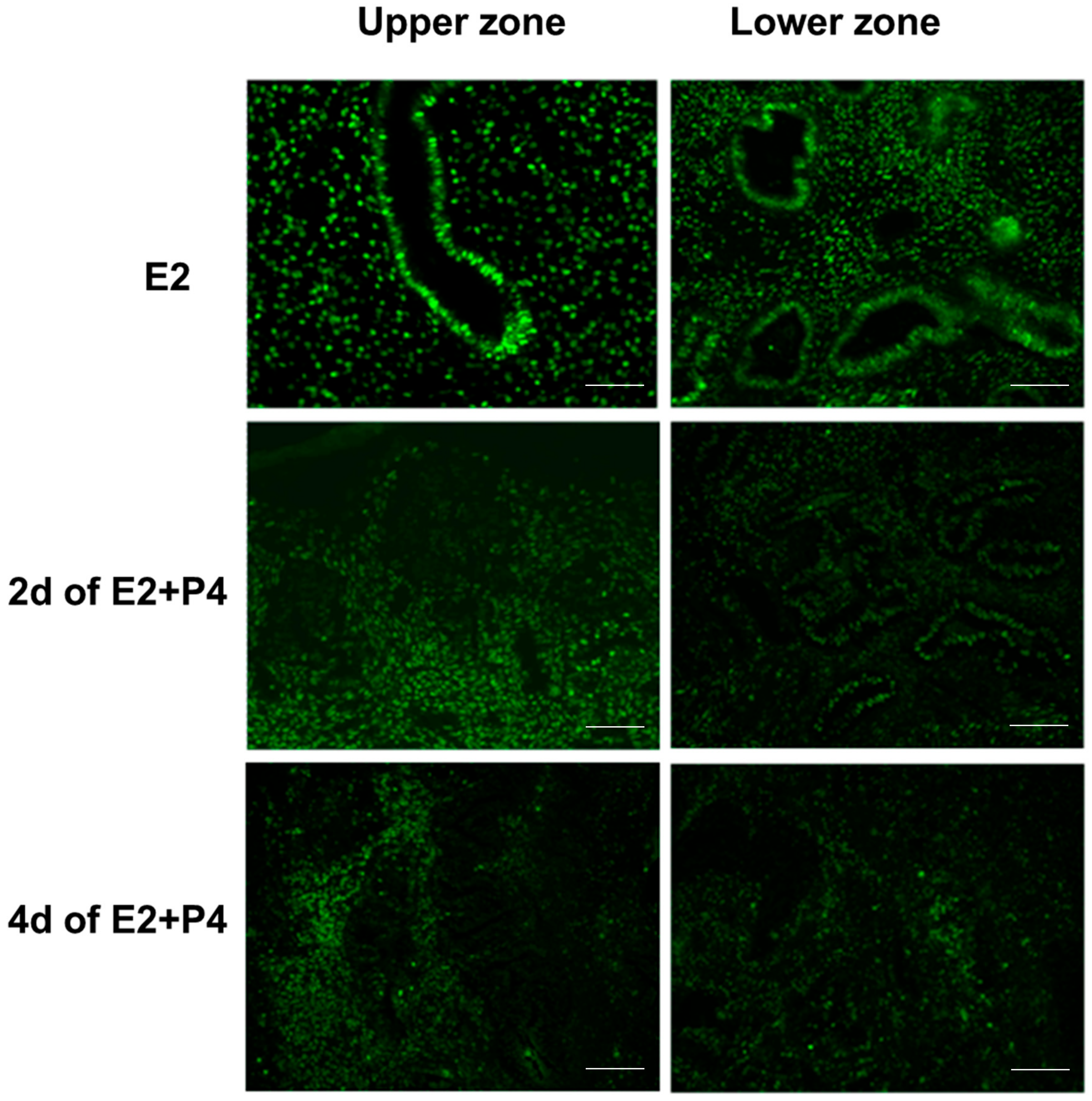
3.2. Evaluation of E2/P4 Responses in Endometrial Tissue Air-Liquid Interface 3D Cultures
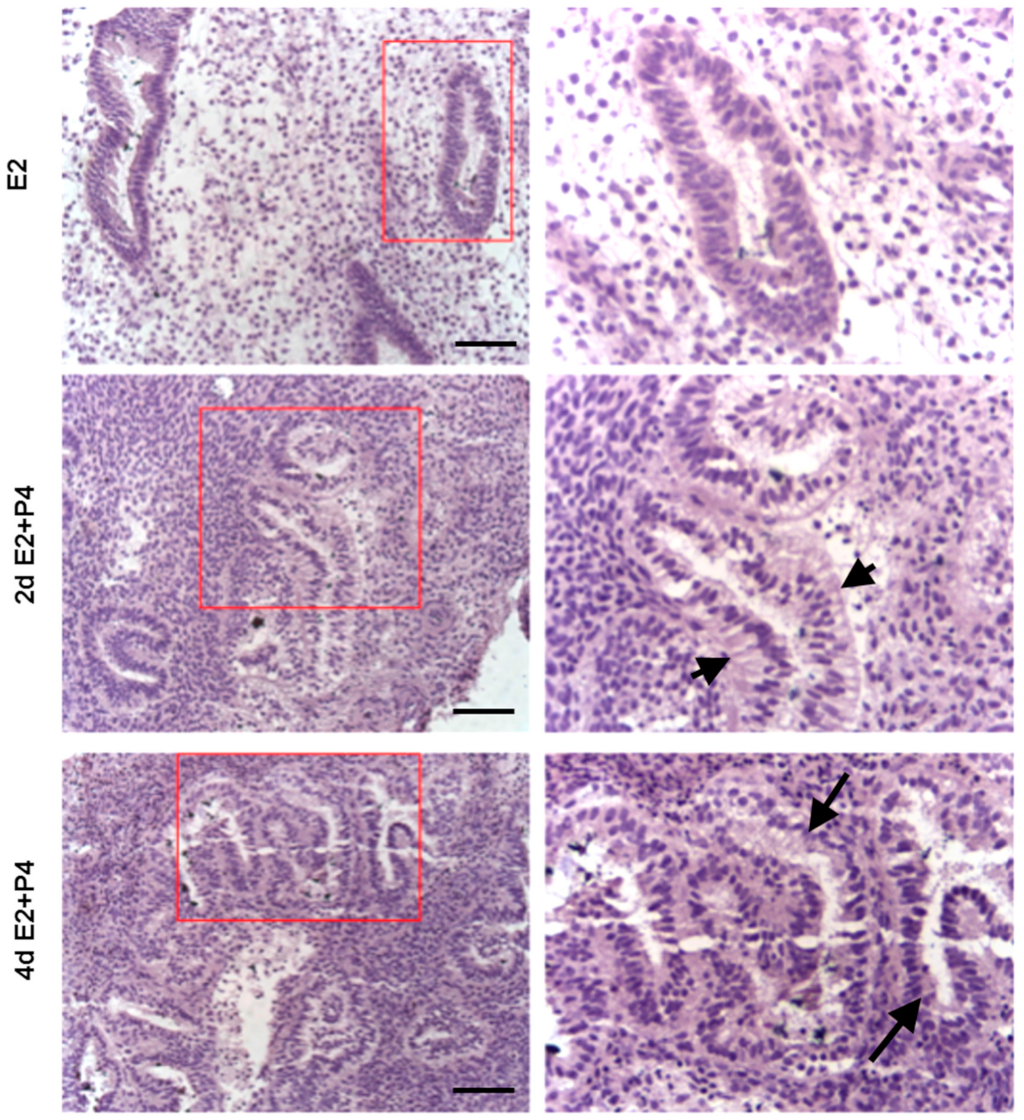
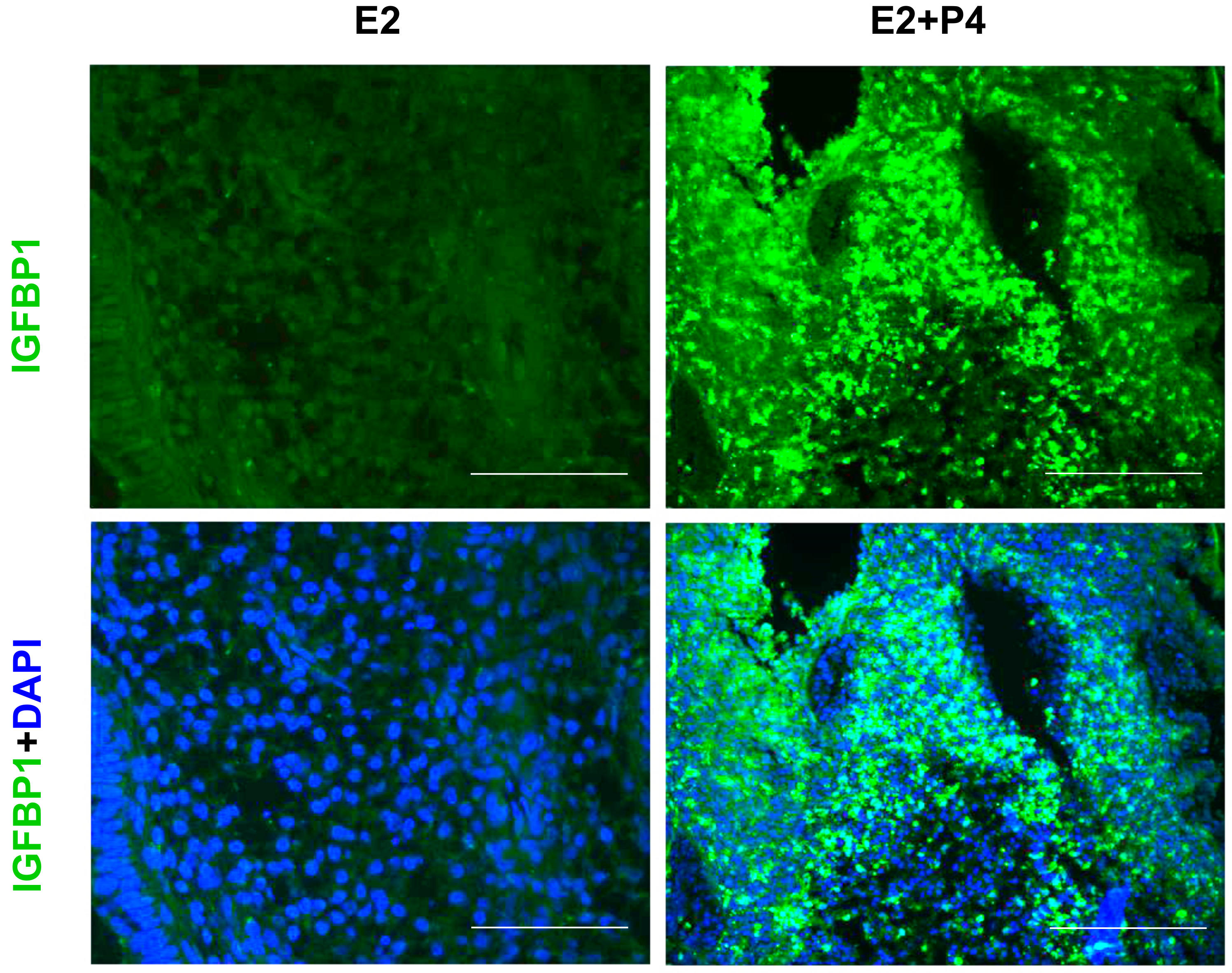
3.3. Induction of Glandular Remodeling and Decidualization in Endometrial Tissue Air-Liquid Interface 3D Cultures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocrinol. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.E.; Fraser, R.; Leslie, K.; Wallace, A.E.; James, J.L. Remodelling at the maternal-fetal interface: Relevance to human pregnancy disorders. Reproduction 2010, 140, 803–813. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 2010, 120, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Li, R.; DeMayo, F.J. Progesterone receptor regulation of uterine adaptation for pregnancy. Trends Endocrinol. Metab. 2018, 29, 481–491. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.S. A review of mechanisms of implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Solberg, H.; Rinkenberger, J.; Dano, K.; Werb, Z.; Lund, L.R. A functional overlap of plasminogen and MMPs regulates vascularization during placental development. Development 2003, 130, 4439–4450. [Google Scholar] [CrossRef][Green Version]
- Velicky, P.; Knofler, M.; Pollheimer, J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adhes. Migr. 2016, 10, 154–162. [Google Scholar] [CrossRef]
- Pollheimer, J.; Knofler, M. Signalling pathways regulating the invasive differentiation of human trophoblasts: A review. Placenta 2005, 26 (Suppl. SA), S21–S30. [Google Scholar] [CrossRef]
- Schatz, F.; Guzeloglu-Kayisli, O.; Arlier, S.; Kayisli, U.A.; Lockwood, C.J. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum. Reprod. Update 2016, 22, 497–515. [Google Scholar] [CrossRef]
- Makieva, S.; Giacomini, E.; Ottolina, J.; Sanchez, A.M.; Papaleo, E.; Vigano, P. Inside the endometrial cell signaling subway: Mind the gap(s). Int. J. Mol. Sci. 2018, 19, 2477. [Google Scholar] [CrossRef]
- Yu, J.; Berga, S.L.; Johnston-MacAnanny, E.B.; Sidell, N.; Bagchi, I.C.; Bagchi, M.K.; Taylor, R.N. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology 2016, 157, 2432–2446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrinol. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.J. Mechanisms of normal and abnormal endometrial bleeding. Menopause 2011, 18, 408–411. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, H.J.; Lin, C.; Long, L.; Yang, X.; Zhang, H.; Taylor, H.; Min, W. CD34(+)KLF4(+) stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 2019, 27, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 6848–6857. [Google Scholar] [CrossRef] [PubMed]
- Bellofiore, N.; Rana, S.; Dickinson, H.; Temple-Smith, P.; Evans, J. Characterization of human-like menstruation in the spiny mouse: Comparative studies with the human and induced mouse model. Hum. Reprod. 2018, 33, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Bläuer, M.; Heinonen, P.K.; Martikainen, P.M.; Tomás, E.; Ylikomi, T. A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum. Reprod. 2005, 20, 864–871. [Google Scholar] [CrossRef]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Deane, J.A.; Cousins, F.L.; Gargett, C.E. Endometrial organoids: in vitro models for endometrial research and personalized medicine. Biol. Reprod. 2017, 97, 781–783. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Dhakal, P.; Behura, S.K.; Schust, D.J.; Spencer, T.E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 23132–23142. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Wiwatpanit, T.; Lu, Z.; Davaadelger, B.; Kim, J.J. generation of multicellular human primary endometrial organoids. J. Vis. Exp. 2019, 152, e60384. [Google Scholar] [CrossRef] [PubMed]
- Wiwatpanit, T.; Murphy, A.R.; Lu, Z.; Urbanek, M.; Burdette, J.E.; Woodruff, T.K.; Kim, J.J. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2019, dgz100. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knöfler, M.; et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef] [PubMed]
- Padykula, H.A.; Coles, L.G.; Okulicz, W.C.; Rapaport, S.I.; Mc Cracken, J.A.; King, N.W.; Longcope, C.; Kaiserman-Abramof, I.R. The basalis of the primate endometrium: A bifunctional germinal compartment. Biol. Reprod. 1989, 40, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.R.; Critchley, H.O.; Slayden, O.D.; Menrad, A.; Chwalisz, K.; Baird, D.T.; Brenner, R.M. Progesterone withdrawal up-regulates vascular endothelial growth factor receptor type 2 in the superficial zone stroma of the human and macaque endometrium: Potential relevance to menstruation. J. Clin. Endocrinol. Metab. 2000, 85, 3442–3452. [Google Scholar]
- Germeyer, A.; Hamilton, A.E.; Laughlin, L.S.; Lasley, B.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. Cellular expression and hormonal regulation of neuropilin-1 and -2 messenger ribonucleic Acid in the human and rhesus macaque endometrium. J. Clin. Endocrinol. Metab. 2005, 90, 1783–1790. [Google Scholar] [CrossRef][Green Version]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef]
- Amemori, S.; Ootani, A.; Aoki, S.; Fujise, T.; Shimoda, R.; Kakimoto, T.; Shiraishi, R.; Sakata, Y.; Tsunada, S.; Iwakiri, R.; et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G923–G929. [Google Scholar] [CrossRef]
- Fan, X.; Rai, A.; Kambham, N.; Sung, J.F.; Singh, N.; Petitt, M.; Dhal, S.; Agrawal, R.; Sutton, R.E.; Druzin, M.L.; et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Investig. 2014, 124, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.R.; Slayden, O.D.; Mah, K.; Chwalisz, K.; Brenner, R.M. Antiprogestin-releasing intrauterine devices: A novel approach to endometrial contraception. Contraception 2007, 75 (Suppl. S6), S104–S111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slayden, O.D.; Chwalisz, K.; Brenner, R.M. Reversible suppression of menstruation with progesterone antagonists in rhesus macaques. Hum. Reprod. 2001, 16, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Haluska, G.J.; Wells, T.R.; Hirst, J.J.; Brenner, R.M.; Sadowsky, D.W.; Novy, M.J. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: Evidence for functional progesterone withdrawal at parturition. J. Soc. Gynecol. Investig. 2002, 9, 125–136. [Google Scholar] [CrossRef]
- Fan, X.; Krieg, S.; Hwang, J.Y.; Dhal, S.; Kuo, C.J.; Lasley, B.L.; Brenner, R.M.; Nayak, N.R. Dynamic regulation of Wnt7a expression in the primate endometrium: Implications for postmenstrual regeneration and secretory transformation. Endocrinology 2012, 153, 1063–1069. [Google Scholar] [CrossRef]
- Zuber, T.J. Endometrial biopsy. Am. Fam. Physician 2001, 63, 1131–1135. [Google Scholar]
- Kuntz, C. Endometrial biopsy. Can. Fam. Physician 2007, 53, 43–44. [Google Scholar]
- Zaino, R.J.; Kauderer, J.; Trimble, C.L.; Silverberg, S.G.; Curtin, J.P.; Lim, P.C.; Gallup, D.G. Reproducibility of the diagnosis of atypical endometrial hyperplasia: A Gynecologic Oncology Group study. Cancer 2006, 106, 804–811. [Google Scholar] [CrossRef]
- Allison, K.H.; Reed, S.D.; Voigt, L.F.; Jordan, C.D.; Newton, K.M.; Garcia, R.L. Diagnosing endometrial hyperplasia: Why is it so difficult to agree? Am. J. Surg. Pathol. 2008, 32, 691–698. [Google Scholar] [CrossRef]
- Fadare, O.; Roma, A.A.; Mhawech-Fauceglia, P.; Parkash, V.; Rabban, J.T. The diagnosis of mucinous lesions in endometrial samplings by gynaecological pathologists: An analysis of diagnostic reproducibility. Pathology 2018, 50, 276–285. [Google Scholar] [CrossRef]
- Usubutun, A.; Mutter, G.L.; Saglam, A.; Dolgun, A.; Ozkan, E.A.; Ince, T.; Akyol, A.; Bulbul, H.D.; Calay, Z.; Eren, F.; et al. Reproducibility of endometrial intraepithelial neoplasia diagnosis is good, but influenced by the diagnostic style of pathologists. Mod. Pathol. 2012, 25, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, A.; Burney, R.O.; F O, D.; D’Hooghe, T.; Giudice, L. Update on biomarkers for the detection of endometriosis. Biomed. Res. Int. 2015, 2015, 130854. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.L.; Lu, Y.; Whiteley, K.J.; Holmyard, D.; Hemberger, M.; Pfarrer, C.; Cross, J.C. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002, 250, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Ain, R.; Canham, L.N.; Soares, M.J. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: Novel endocrine phenotype and regulation. Dev. Biol. 2003, 260, 176–190. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Dunn, C.L.; Kelly, R.W.; Critchley, H.O. Decidualization of the human endometrial stromal cell: An enigmatic transformation. Reprod. Biomed. Online 2003, 7, 151–161. [Google Scholar] [CrossRef]
- Clark, D.A. The use and misuse of animal analog models of human pregnancy disorders. J. Reprod. Immunol. 2014, 103, 1–8. [Google Scholar] [CrossRef]
- Kurita, T.; Medina, R.; Schabel, A.B.; Young, P.; Gama, P.; Parekh, T.V.; Brody, J.; Cunha, G.R.; Osteen, K.G.; Bruner-Tran, K.L.; et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 2005, 73, 313–322. [Google Scholar] [CrossRef]
- Van den Brand, A.D.; Rubinstein, E.; de Jong, P.C.; van den Berg, M.; van Duursen, M.B.M. Primary endometrial 3D co-cultures: A comparison between human and rat endometrium. J. Steroid Biochem. Mol. Biol. 2019, 194, 105458. [Google Scholar] [CrossRef]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of macrophages in pregnancy and related complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef]
- Brar, A.K.; Frank, G.R.; Kessler, C.A.; Cedars, M.I.; Handwerger, S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 1997, 6, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gaide Chevronnay, H.P.; Galant, C.; Lemoine, P.; Courtoy, P.J.; Marbaix, E.; Henriet, P. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 2009, 150, 5094–5105. [Google Scholar] [CrossRef] [PubMed]
- Gaide Chevronnay, H.P.; Selvais, C.; Emonard, H.; Galant, C.; Marbaix, E.; Henriet, P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim. Biophys. Acta 2012, 1824, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bałkowiec, M.; Maksym, R.B.; Włodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol. Med. Rep. 2018, 18, 3123–3136. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Fazleabas, A.T.; Lessey, B.A.; Guo, H.; Toole, B.P.; Nowak, R.A. Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J. Clin. Endocrinol. Metab. 2006, 91, 2358–2365. [Google Scholar] [CrossRef][Green Version]
- Braundmeier, A.G.; Nowak, R.A. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. Am. J. Reprod. Immunol. 2006, 56, 201–214. [Google Scholar] [CrossRef]
- Brenner, R.M.; Slayden, O.D. Molecular and functional aspects of menstruation in the macaque. Rev. Endocrinol. Metab. Disord. 2012, 13, 309–318. [Google Scholar] [CrossRef]
- Fasciani, A.; Bocci, G.; Xu, J.; Bielecki, R.; Greenblatt, E.; Leyland, N.; Casper, R. Three-dimensional in vitro culture of endometrial explants mimics the early stages of endometriosis. Fertil. Steril. 2003, 80, 1137–1143. [Google Scholar] [CrossRef]
- Esfandiari, N.; Ai, J.; Nazemian, Z.; Javed, M.H.; Gotlieb, L.; Casper, R.F. Expression of glycodelin and cyclooxygenase-2 in human endometrial tissue following three-dimensional culture. Am. J. Reprod. Immunol. 2007, 57, 49–54. [Google Scholar] [CrossRef]
- Khazaei, M.; Montaseri, A.; Casper, R.F. Letrozole stimulates the growth of human endometrial explants culturedd in three-dimensional fibrin matrix. Fertil. Steril. 2009, 91 (Suppl. S5), 2172–2176. [Google Scholar] [CrossRef]
- Greaves, E.; Critchley, H.O.D.; Horne, A.W.; Saunders, P.T.K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet. Gynecol. Scand. 2017, 96, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Tseng, H.; Gage, J.A.; Mani, A.; Desai, P.; Leonard, F.; Liao, A.; Longo, M.; Refuerzo, J.S.; Godin, B. Magnetically bioprinted human myometrial 3D cell rings as a model for uterine contractility. Int. J. Mol. Sci. 2017, 18, 683. [Google Scholar] [CrossRef] [PubMed]
- Stavreus-Evers, A.; Hovatta, O.; Eriksson, H.; Landgren, B.M. Development and characterization of an endometrial tissue culture system. Reprod. Biomed. Online 2003, 7, 243–249. [Google Scholar] [CrossRef]
- Schafer, W.R.; Fischer, L.; Roth, K.; Jullig, A.K.; Stuckenschneider, J.E.; Schwartz, P.; Weimer, M.; Orlowska-Volk, M.; Hanjalic-Beck, A.; Kranz, I.; et al. Critical evalulation of human endometrial explants as an ex vivo model system: A molecular approach. Mol. Hum. Reprod. 2011, 17, 255–265. [Google Scholar] [CrossRef]
- Dudley, D.J.; Hatasaka, H.H.; Branch, D.W.; Hammond, E.; Mitchell, M.D. A human endometrial explant system: Validation and potential applications. Am. J. Obstet. Gynecol. 1992, 167, 1774–1780. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muruganandan, S.; Fan, X.; Dhal, S.; Nayak, N.R. Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration. Biomolecules 2020, 10, 136. https://doi.org/10.3390/biom10010136
Muruganandan S, Fan X, Dhal S, Nayak NR. Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration. Biomolecules. 2020; 10(1):136. https://doi.org/10.3390/biom10010136
Chicago/Turabian StyleMuruganandan, Shanmugam, Xiujun Fan, Sabita Dhal, and Nihar R. Nayak. 2020. "Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration" Biomolecules 10, no. 1: 136. https://doi.org/10.3390/biom10010136
APA StyleMuruganandan, S., Fan, X., Dhal, S., & Nayak, N. R. (2020). Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration. Biomolecules, 10(1), 136. https://doi.org/10.3390/biom10010136






