Preliminary Estimations of Insect Mediated Transfers of Mercury and Physiologically Important Fatty Acids from Water to Land
Abstract
1. Introduction
2. Material and Methods
2.1. Literature Search and Data Extraction
2.2. Calculation of Surface Area
2.3. Emergence of Insects
2.4. Estimates of Physiologically Important Fatty Acids in Aquatic Insects
2.5. Estimates of Mercury and Methylmercury Content in Aquatic Insects
2.6. Data Analyses
3. Results
3.1. Continental Exports of Physiologically Important Fatty Acids
3.2. Continental Exports of Mercury and Methylmercury
3.3. Global Exports of Physiologically Important Fatty Acids and Mercury
4. Discussion
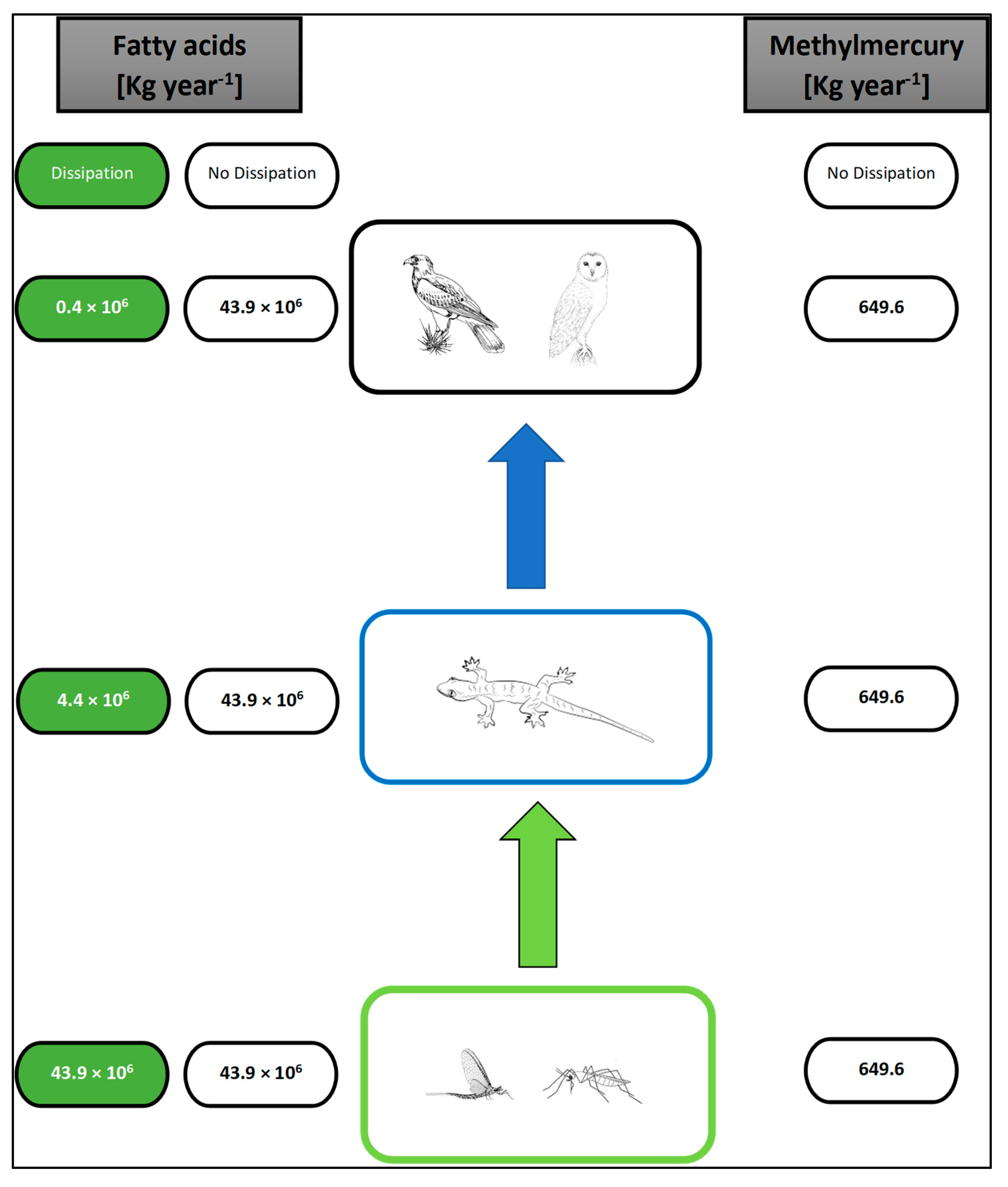
5. Implications
5.1. Wildlife
5.2. Climate Change
6. Additional Considerations and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, J.S.; Sato, T. Resource Subsidy Flows across Freshwater–Terrestrial Boundaries and Influence on Processes Linking Adjacent Ecosystems. Ecohydrology 2015, 8, 406–415. [Google Scholar] [CrossRef]
- Brett, M.T.; Bunn, S.E.; Chandra, S.; Galloway, A.W.E.; Guo, F.; Kainz, M.J.; Kankaala, P.; Lau, D.C.P.; Moulton, T.P.; Power, M.E.; et al. How Important Are Terrestrial Organic Carbon Inputs for Secondary Production in Freshwater Ecosystems? Freshw. Biol. 2017, 62, 833–853. [Google Scholar] [CrossRef]
- Lam, M.M.Y.; Martin-Creuzburg, D.; Rothhaupt, K.O.; Safi, K.; Yohannes, E.; Salvarina, I. Tracking Diet Preferences of Bats Using Stable Isotope and Fatty Acid Signatures of Faeces. PLoS ONE 2013, 8, e83452. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.V.; Fausch, K.D.; Saunders, W.C. Tangled Webs: Reciprocal Flows of Invertebrate Prey Link Streams and Riparian Zones. Freshw. Biol. 2005, 50, 201–220. [Google Scholar] [CrossRef]
- Larsen, S.; Muehlbauer, J.D.; Marti, E. Resource Subsidies between Stream and Terrestrial Ecosystems under Global Change. Glob. Change Biol. 2016, 22, 2489–2504. [Google Scholar] [CrossRef]
- Harris, H.E.; Baxter, C.V.; Davis, J.M. Wildfire and Debris Flows Affect Prey Subsidies with Implications for Riparian and Riverine Predators. Aquat. Sci. 2018, 80, 37. [Google Scholar] [CrossRef]
- Sabo, J.L.; Power, M.E. River–Watershed Exchange: Effects of Riverine Subsidies on Riparian Lizards and their Terrestrial Prey. Ecology 2002, 83, 1860–1869. [Google Scholar]
- Sabo, J.L.; Power, M.E. Numerical Response of Lizards to Aquatic Insects and Short-Term Consequences for Terrestrial Prey. Ecology 2002, 83, 3023–3036. [Google Scholar] [CrossRef]
- Hixson, S.M.; Arts, M.T. Climate Warming Is Predicted to Reduce Omega-3, Long-chain, Polyunsaturated Fatty Acid Production in Phytoplankton. Glob. Change Biol. 2016, 22, 2744–2755. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Lawrence, P.; Shipley, J.R.; Tollefson, T.N.; Winkler, D.W. Omega-3 Long-Chain Polyunsaturated Fatty Acids Support Aerial Insectivore Performance More than Food Quantity. Proc. Natl. Acad. Sci. 2016, 113, 10920–10925. [Google Scholar] [CrossRef]
- Twining, C.W.; Shipley, J.R.; Winkler, D.W. Aquatic Insects Rich in Omega-3 Fatty Acids Drive Breeding Success in a Widespread Bird. Ecol. Lett. 2018, 21, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.T.; Ackman, R.G.; Holub, B.J. “Essential Fatty Acids” in Aquatic Ecosystems: A Crucial Link between Diet and Human Health and Evolution. Can. J. Fish. Aquat. Sci. 2001, 58, 122–137. [Google Scholar] [CrossRef]
- Moyo, S.; Chari, L.D.; Villet, M.H.; Richoux, N.B. Decoupled Reciprocal Subsidies of Biomass and Fatty Acids in Fluxes of Invertebrates between a Temperate River and the Adjacent Land. Aquat. Sci. 2017, 79, 689–703. [Google Scholar] [CrossRef]
- Jackson, J.K.; Fisher, S.G. Secondary Production, Emergence, and Export of Aquatic Insects of a Sonoran Desert Stream. Ecology 1986, 67, 629–638. [Google Scholar] [CrossRef]
- Bell, J.G.; Ghioni, C.; Sargent, J.R. Fatty Acid Compositions of 10 Freshwater Invertebrates Which Are Natural Food Organisms of Atlantic Salmon Parr (Salmo salar): A Comparison with Commercial Diets. Aquaculture 1994, 128, 301–313. [Google Scholar] [CrossRef]
- Ghioni, C.; Bell, J.G.; Sargent, J.R. Polyunsaturated Fatty Acids in Neutral Lipids and Phospholipids of Some Freshwater Insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 161–170. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; Wehr, J.D.; Perrone, A.A. Trophic Relations in a Stream Food Web: Importance of Fatty Acids for Macroinvertebrate Consumers. J. North. Am. Benthol. Soc. 2007, 26, 509–522. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Arts, M.T.; Sushchik, N.N. Preliminary Estimates of the Export of Omega-3 Highly Unsaturated Fatty Acids (EPA+DHA) from Aquatic to Terrestrial Ecosystems. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer New York: New York, NY, USA, 2009; pp. 179–210. [Google Scholar] [CrossRef]
- Burdon, F.J.; Harding, J.S. The Linkage between Riparian Predators and Aquatic Insects across a Stream-Resource Spectrum. Freshw. Biol. 2008, 53, 330–346. [Google Scholar] [CrossRef]
- Walters, D.M.; Fritz, K.M.; Otter, R.R. The Dark Side of Subsidies: Adult Stream Insects Export Organic Contaminants to Riparian Predators. Ecol. Appl. 2008, 18, 1835–1841. [Google Scholar] [CrossRef]
- Speir, S.L.; Chumchal, M.M.; Drenner, R.W.; Cocke, W.G.; Lewis, M.E.; Whitt, H.J. Methyl Mercury and Stable Isotopes of Nitrogen Reveal That a Terrestrial Spider Has a Diet of Emergent Aquatic Insects. Environ. Toxicol. Chem. 2014, 33, 2506–2509. [Google Scholar] [CrossRef]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Chumchal, M.M.; Drenner, R.W.; Greenhill, F.M.; Kennedy, J.H.; Courville, A.E.; Gober, C.A.A.; Lossau, L.O. Recovery of Aquatic Insect-Mediated Methylmercury Flux from Ponds Following Drying Disturbance. Environ. Toxicol. Chem. 2017, 36, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Chumchal, M.M.; Drenner, R.W.; Hall, M.N.; Polk, D.K.; Williams, E.B.; Ortega-Rodriguez, C.L.; Kennedy, J.H. Seasonality of Dipteran-Mediated Methylmercury Flux from Ponds. Environ. Toxicol. Chem. 2018, 37, 1846–1851. [Google Scholar] [CrossRef]
- Du, H.; Ma, M.; Igarashi, Y.; Wang, D. Biotic and Abiotic Degradation of Methylmercury in Aquatic Ecosystems: A Review. Bull. Environ. Contam. Toxicol. 2019. [Google Scholar] [CrossRef]
- Walters, D.M.; Rosi-Marshall, E.; Kennedy, T.A.; Cross, W.F.; Baxter, C.V. Mercury and Selenium Accumulation in the Colorado River Food Web, Grand Canyon, USA. Environ. Toxicol. Chem. 2015, 34, 2385–2394. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Gladysheva, E.E.; Sushchik, N.N. Preliminary Estimation of the Export of Omega-3 Polyunsaturated Fatty Acids from Aquatic to Terrestrial Ecosystems in Biomes via Emergent Insects. Ecol. Complex. 2019, 38, 140–145. [Google Scholar] [CrossRef]
- Wesner, J.S. Seasonal Variation in the Trophic Structure of a Spatial Prey Subsidy Linking Aquatic and Terrestrial Food Webs: Adult Aquatic Insects. Oikos 2010, 119, 170–178. [Google Scholar] [CrossRef]
- Lehner, B.; Döll, P. Development and Validation of a Global Database of Lakes, Reservoirs and Wetlands. J. Hydrol. 2004, 296, 1–22. [Google Scholar] [CrossRef]
- U.S. Defense Mapping Agency. Development of the Digital Chart of the World; Government Printing Office: Washington, DC, USA, 1992.
- Lehner, B.; Verdin, K.; Jarvis, A. New Global Hydrography Derived from Spaceborne Elevation Data. Eos Trans. Am. Geophys. Union 2008, 89, 93. [Google Scholar] [CrossRef]
- US Geological Survey. Long Term Archive; HYDRO1K [Internet]; U.S. Geological Survey: Reston, VA, USA, 2015.
- Allen, G.H.; Pavelsky, T.M. Global Extent of Rivers and Streams. Science 2018, 361, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Neteler, M.; Bowman, M.H.; Landa, M.; Metz, M. GRASS GIS: A Multi-Purpose Open Source GIS. Environ. Model. Softw. 2012, 31, 124–130. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project: Chicago, IL, USA, 2019; Available online: http://Qgis.Osgeo.Org (accessed on 12 January 2020).
- Vadeboncoeur, Y.; McIntyre, P.B.; Vander Zanden, M.J. Borders of Biodiversity: Life at the Edge of the World’s Large Lakes. BioScience 2011, 61, 526–537. [Google Scholar] [CrossRef]
- Gray, L.J. Emergence Production and Export of Aquatic Insects from a Tallgrass Prairie Stream. Southwest. Nat. 1989, 34, 313–318. [Google Scholar] [CrossRef]
- Stagliano, D.M.; Benke, A.C.; Anderson, D.H. Emergence of Aquatic Insects from 2 Habitats in a Small Wetland of the Southeastern USA: Temporal Patterns of Numbers and Biomass. J. North. Am. Benthol. Soc. 1998, 17, 37–53. [Google Scholar] [CrossRef]
- Francis, T.B.; Schindler, D.E.; Moore, J.W. Aquatic Insects Play a Minor Role in Dispersing Salmon-Derived Nutrients into Riparian Forests in Southwestern Alaska. Can. J. Fish. Aquat. Sci. 2006, 63, 2543–2552. [Google Scholar] [CrossRef]
- Goedkoop, W.; Sonesten, L.; Ahlgren, G.; Boberg, M. Fatty Acids in Profundal Benthic Invertebrates and Their Major Food Resources in Lake Erken, Sweden: Seasonal Variation and Trophic Indications. Can. J. Fish. Aquat. Sci. 2000, 57, 2267–2279. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Yurchenko, Y.A.; Gladyshev, M.I.; Belevich, O.E.; Kalachova, G.S.; Kolmakova, A.A. Comparison of Fatty Acid Contents and Composition in Major Lipid Classes of Larvae and Adults of Mosquitoes (Diptera: Culicidae) from a Steppe Region. Insect Sci. 2012, 20, 585–600. [Google Scholar] [CrossRef]
- Raitif, J.; Plantegenest, M.; Agator, O.; Piscart, C.; Roussel, J.-M. Seasonal and Spatial Variations of Stream Insect Emergence in an Intensive Agricultural Landscape. Sci. Total Environ. 2018, 644, 594–601. [Google Scholar] [CrossRef]
- Salvarina, I.; Gravier, D.; Rothhaupt, K.-O. Seasonal Insect Emergence from Three Different Temperate Lakes. Limnologica 2017, 62, 47–56. [Google Scholar] [CrossRef]
- Silina, A.E. Emergence of Amphibiotic Insects from a Floodplain Lake in the Usman Forest in the Central Russian Forest Steppe. Contemp. Probl. Ecol. 2016, 9, 421–436. [Google Scholar] [CrossRef]
- Dreyer, J.; Townsend, P.A.; Iii, J.C.H.; Hoekman, D.; Vander Zanden, M.J.; Gratton, C. Quantifying Aquatic Insect Deposition from Lake to Land. Ecology 2015, 96, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Borisova, E.V.; Makhutova, O.N.; Gladyshev, M.I.; Sushchik, N.N. Fluxes of Biomass and Essential Polyunsaturated Fatty Acids from Water to Land via Chironomid Emergence from a Mountain Lake. Contemp. Probl. Ecol. 2016, 9, 446–457. [Google Scholar] [CrossRef]
- Djomina, I.V.; Yermokhin, M.V.; Polukonova, N.V. Substance and Energy Flows Formed by the Emergence of Amphibiotic Insects across the Water–Air Boundary on the Floodplain Lakes of the Volga River. Contemp. Probl. Ecol. 2016, 9, 407–420. [Google Scholar] [CrossRef]
- Tremblay, A.; Cloutier, L.; Lucotte, M. Total Mercury and Methylmercury Fluxes via Emerging Insects in Recently Flooded Hydroelectric Reservoirs and a Natural Lake. Sci. Total Environ. 1998, 219, 209–221. [Google Scholar] [CrossRef]
- Sherk, T.; Rau, G. Emergence of Chironomidae from Findley Lake and Two Ponds in the Cascade Mountains, U.S.A. Netherland J. Aquat. Ecol. 1992, 26, 321–330. [Google Scholar] [CrossRef]
- Freitag, H. Composition and Longitudinal Patterns of Aquatic Insect Emergence in Small Rivers of Palawan Island, the Philippines. Int. Rev. Hydrobiol. 2004, 89, 375–391. [Google Scholar] [CrossRef]
- Nakano, S.; Murakami, M. Reciprocal Subsidies: Dynamic Interdependence between Terrestrial and Aquatic Food Webs. Proc. Natl. Acad. Sci. 2001, 98, 166–170. [Google Scholar] [CrossRef]
- Poepperl, R. Benthic Secondary Production and Biomass of Insects Emerging from a Northern German Temperate Stream. Freshw. Biol. 2001, 44, 199–211. [Google Scholar] [CrossRef]
- Whiles, M.R.; Goldowitz, B.S. Hydrologic Influences on Insect Emergence Production from Central Platte River Wetlands. Ecol. Appl. 2001, 11, 1829–1842. [Google Scholar] [CrossRef]
- Rundio, D.E.; Lindley, S.T. Reciprocal Fluxes of Stream and Riparian Invertebrates in a Coastal California Basin with Mediterranean Climate. Ecol. Res. 2012, 27, 539–550. [Google Scholar] [CrossRef]
- Popova, O.N.; Haritonov, A.Y.; Sushchik, N.N.; Makhutova, O.N.; Kalachova, G.S.; Kolmakova, A.A.; Gladyshev, M.I. Export of Aquatic Productivity, Including Highly Unsaturated Fatty Acids, to Terrestrial Ecosystems via Odonata. Sci. Total Environ. 2017, 581–582, 40–48. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Kowarik, C.; Straile, D. Cross-Ecosystem Fluxes: Export of Polyunsaturated Fatty Acids from Aquatic to Terrestrial Ecosystems via Emerging Insects. Sci. Total Environ. 2017, 577, 174–182. [Google Scholar] [CrossRef]
- Makhutova, O.N.; Borisova, E.V.; Shulepina, S.P.; Kolmakova, A.A.; Sushchik, N.N. Fatty Acid Composition and Content in Chironomid Species at Various Life Stages Dominating in a Saline Siberian Lake. Contemp. Probl. Ecol. 2017, 10, 230–239. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Gladyshev, M.I.; Moskvichova, A.V.; Makhutova, O.N.; Kalachova, G.S. Comparison of Fatty Acid Composition in Major Lipid Classes of the Dominant Benthic Invertebrates of the Yenisei River. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 111–122. [Google Scholar] [CrossRef]
- Tremblay, A.; Lucotte, M.; Rheault, I. Methylmercury in a Benthic Food Web of Two Hydroelectric Reservoirs and a Natural Lake of Northern Québec (Canada). Water. Air. Soil Pollut. 1996, 91, 255–269. [Google Scholar] [CrossRef]
- Gorski, P.R.; Cleckner, L.B.; Hurley, J.P.; Sierszen, M.E.; Armstrong, D.E. Factors Affecting Enhanced Mercury Bioaccumulation in Inland Lakes of Isle Royale National Park, USA. Sci. Total Environ. 2003, 304, 327–348. [Google Scholar] [CrossRef]
- Cremona, F.; Planas, D.; Lucotte, M. Assessing the Importance of Macroinvertebrate Trophic Dead Ends in the Lower Transfer of Methylmercury in Littoral Food Webs. Can. J. Fish. Aquat. Sci. 2008, 65, 2043–2052. [Google Scholar] [CrossRef]
- Tweedy, B.N. Effects of Fish on Emergent Insects and Their Transport of Methyl Mercury from Ponds [Electronic Resource]. UMI Thesis, Texas Christian University, Fort Worth, TX, USA, 2012. [Google Scholar]
- Rizzo, A.; Arcagni, M.; Arribére, M.A.; Bubach, D.; Guevara, S.R. Mercury in the Biotic Compartments of Northwest Patagonia Lakes, Argentina. Chemosphere 2011, 84, 70–79. [Google Scholar] [CrossRef]
- Arcagni, M.; Juncos, R.; Rizzo, A.; Pavlin, M.; Fajon, V.; Arribére, M.A.; Horvat, M.; Ribeiro Guevara, S. Species and Habitat-Specific Bioaccumulation of Total Mercury and Methylmercury in the Food Web of a Deep Oligotrophic Lake. Sci. Total Environ. 2018, 612, 1311–1319. [Google Scholar] [CrossRef]
- Dominique, Y.; Maury-Brachet, R.; Muresan, B.; Vigouroux, R.; Richard, S.; Cossa, D.; Mariotti, A.; Boudou, A. Biofilm and Mercury Availability as Key Factors for Mercury Accumulation in Fish (Curimata cyprinoides) from a Disturbed Amazonian Freshwater System. Environ. Toxicol. Chem. 2009, 26, 45–52. [Google Scholar] [CrossRef]
- Harding, K.M.; Gowland, J.A.; Dillon, P.J. Mercury Concentration in Black Flies Simulium Spp. (Diptera, Simuliidae) from Soft-Water Streams in Ontario, Canada. Environ. Pollut. 2006, 143, 529–535. [Google Scholar] [CrossRef]
- Nagorski, S.A.; Engstrom, D.R.; Hudson, J.P.; Krabbenhoft, D.P.; Hood, E.; DeWild, J.F.; Aiken, G.R. Spatial Distribution of Mercury in Southeastern Alaskan Streams Influenced by Glaciers, Wetlands, and Salmon. Environ. Pollut. 2014, 184, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.J.; Carter, J.L.; Fend, S.V.; Luoma, S.N.; Alpers, C.N.; Taylor, H.E. Metal Exposure in a Benthic Macroinvertebrate, Hydropsyche californica, Related to Mine Drainage in the Sacramento River. Can. J. Fish. Aquat. Sci. 2000, 57, 380–390. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Boaz, L.E.; Hossler, K. Fluvial Geomorphology and Aquatic-to-Terrestrial Hg Export Are Weakly Coupled in Small Urban Streams of Columbus, Ohio. Water Resour. Res. 2016, 52, 2822–2839. [Google Scholar] [CrossRef]
- Dukerschein, J.T.; Wiener, J.G.; Rada, R.G.; Steingraeber, M.T. Cadmium and Mercury in Emergent Mayflies (Hexagenia Bilineata) from the Upper Mississippi River. Arch. Environ. Contam. Toxicol. 1992, 23, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1990. [Google Scholar]
- Polis, G.A.; Anderson, W.B.; Holt, R.D. Toward an Integration of Landscape and Food Web Ecology: The Dynamics of Spatially Subsidized Food Webs. Annu. Rev. Ecol. Syst. 1997, 28, 289–316. [Google Scholar] [CrossRef]
- Kautza, A.; Sullivan, S.M.P. Shifts in Reciprocal River-Riparian Arthropod Fluxes along an Urban-Rural Landscape Gradient. Freshw. Biol. 2015, 60, 2156–2168. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Han, Y.-J.; Chen, C.Y.; Evers, D.C.; Lambert, K.F.; Holsen, T.M.; Kamman, N.C.; Munson, R.K. Mercury Contamination in Forest and Freshwater Ecosystems in the Northeastern United States. BioScience 2007, 57, 17–28. [Google Scholar] [CrossRef]
- Martinez del Rio, C.; McWilliams, S.R. How Essential Fats Affect Bird Performance and Link Aquatic Ecosystems and Terrestrial Consumers. Proc. Natl. Acad. Sci. USA 2016, 113, 11988–11990. [Google Scholar] [CrossRef]
- Lindeman, R.L. The Trophic-Dynamic Aspect of Ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in Aquatic Ecosystems and Their Transfer to the Land. Prostaglandins Other Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kainz, M.J.; Hager, H.H.; Rasconi, S.; Kahilainen, K.K.; Amundsen, P.-A.; Hayden, B. Polyunsaturated Fatty Acids in Freshwater Fishes Increase with Total Lipids Irrespective of Feeding Sources and Trophic Position. UiT Munin 2017. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Anishchenko, O.V.; Makhutova, O.N.; Kolmakov, V.I.; Kalachova, G.S.; Kolmakova, A.A.; Dubovskaya, O.P. Efficiency of Transfer of Essential Polyunsaturated Fatty Acids versus Organic Carbon from Producers to Consumers in a Eutrophic Reservoir. Oecologia 2011, 165, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Hixson, S.M.; Sharma, B.; Kainz, M.J.; Wacker, A.; Arts, M.T. Production, Distribution, and Abundance of Long-Chain Omega-3 Polyunsaturated Fatty Acids: A Fundamental Dichotomy between Freshwater and Terrestrial Ecosystems. Environ. Rev. 2015, 23, 414–424. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Evers, D. The Effects of Methylmercury on Wildlife: A Comprehensive Review and Approach for Interpretation. Encycl. Anthr. 2018, 5, 181–194. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Finlay, J.C.; Nater, E.A. Mercury Bioaccumulation in a Stream Network. Environ. Sci. Technol. 2009, 43, 7016–7022. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Blum, J.D.; Kwon, S.Y.; Finlay, J.C.; Balogh, S.J.; Nollet, Y.H. Sources and Transfers of Methylmercury in Adjacent River and Forest Food Webs. Environ. Sci. Technol. 2012, 46, 10957–10964. [Google Scholar] [CrossRef]
- Fuschino, J.R.; Guschina, I.A.; Dobson, G.; Yan, N.D.; Harwood, J.L.; Arts, M.T. Rising Water Temperatures Alter Lipid Dynamics and Reduce N-3 Essential Fatty Acid Concentrations in Scenedesmus obliquus (Chlorophyta)1. J. Phycol. 2011, 47, 763–774. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Dubovskaya, O.P.; Buseva, Z.F.; Makhutova, O.N.; Fefilova, E.B.; Feniova, I.Y.; Semenchenko, V.P.; Kolmakova, A.A.; Kalachova, G.S. Fatty Acid Composition of Cladocera and Copepoda from Lakes of Contrasting Temperature. Freshw. Biol. 2015, 60, 373–386. [Google Scholar] [CrossRef]
- Malison, R.L.; Benjamin, J.R.; Baxter, C.V. Measuring Adult Insect Emergence from Streams: The Influence of Trap Placement and a Comparison with Benthic Sampling. J. North. Am. Benthol. Soc. 2010, 29, 647–656. [Google Scholar] [CrossRef]
- Edwards, E.D.; Huryn, A.D. Effect of Riparian Land Use on Contributions of Terrestrial Invertebrates to Streams. Hydrobiologia 1996, 337, 151–159. [Google Scholar] [CrossRef]
- Wipfli, M.S. Terrestrial Invertebrates as Salmonid Prey and Nitrogen Sources in Streams: Contrasting Old-Growth and Young-Growth Riparian Forests in Southeastern Alaska, U.S.A. Can. J. Fish. Aquat. Sci. 1997, 54, 1259–1269. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W. Introduction to the Aquatic Insects of North America, 3rd ed.; Kendall/Hunt: Dubuque, IA, USA, 1996. [Google Scholar]
- Shia, R.-L.; Seigneur, C.; Pai, P.; Ko, M.; Sze, N.D. Global Simulation of Atmospheric Mercury Concentrations and Deposition Fluxes. J. Geophys. Res. Atmos. 1999, 104, 23747–23760. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury Pollution in Asia: A Review of the Contaminated Sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar] [CrossRef]
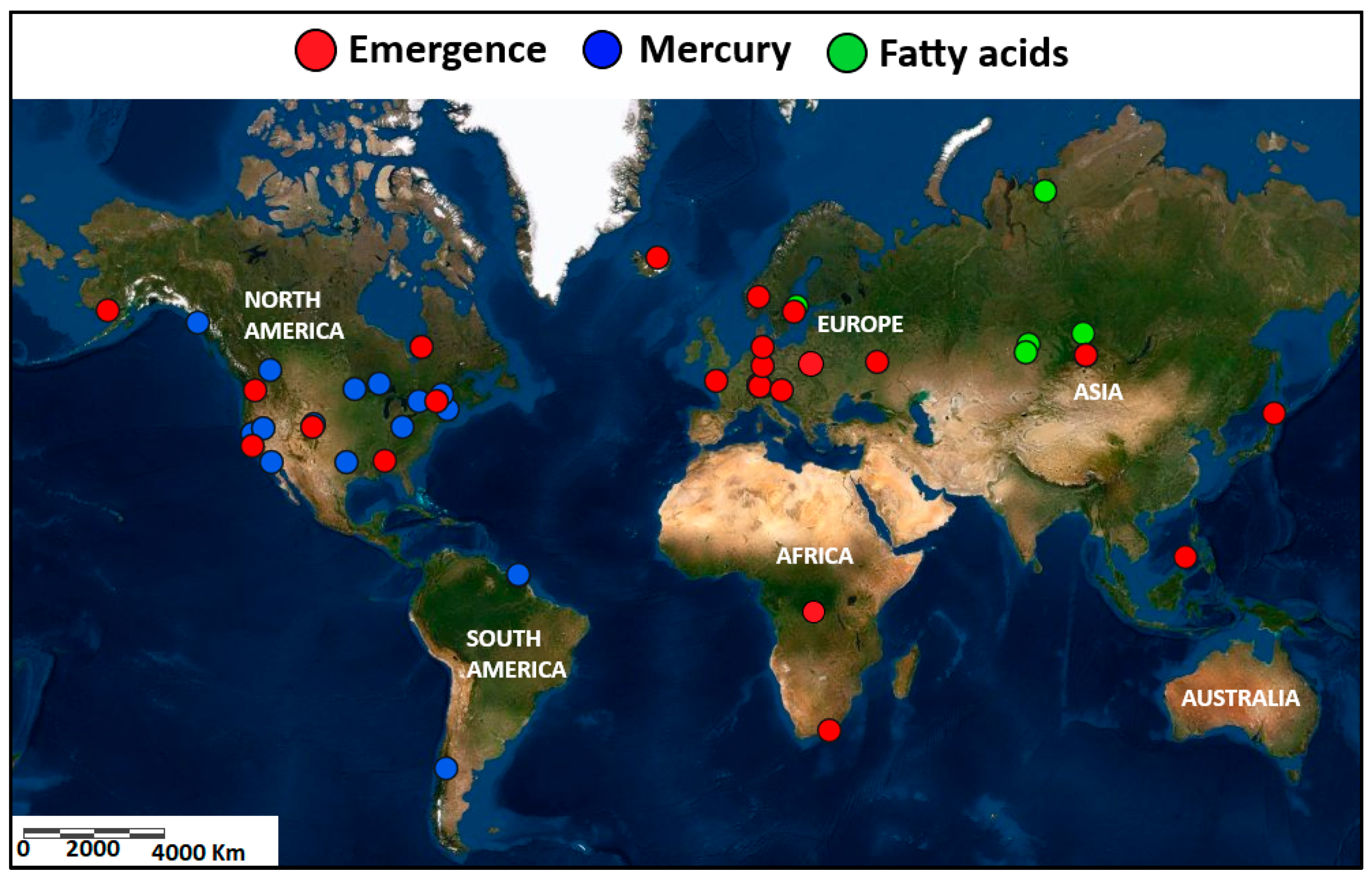
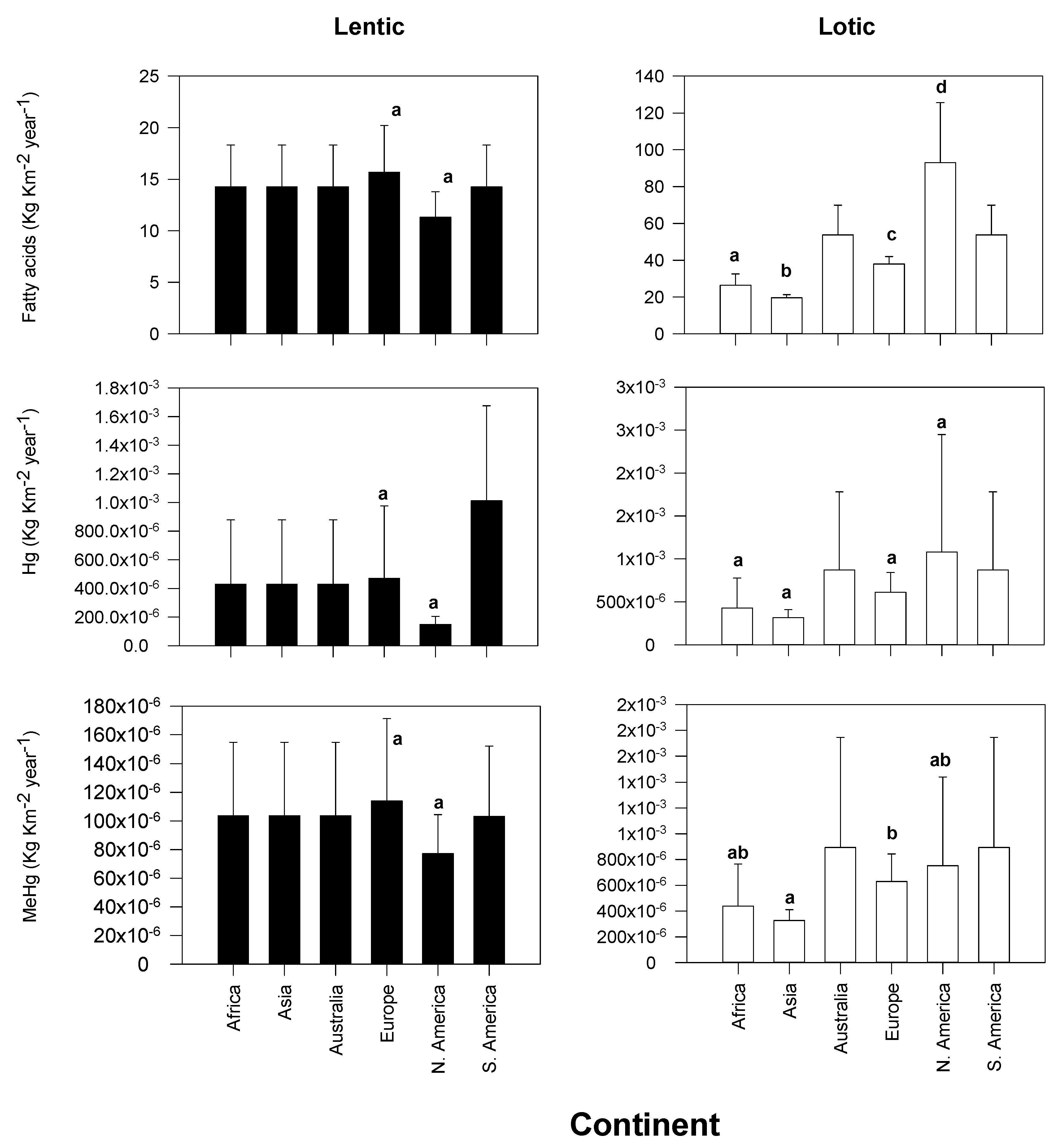
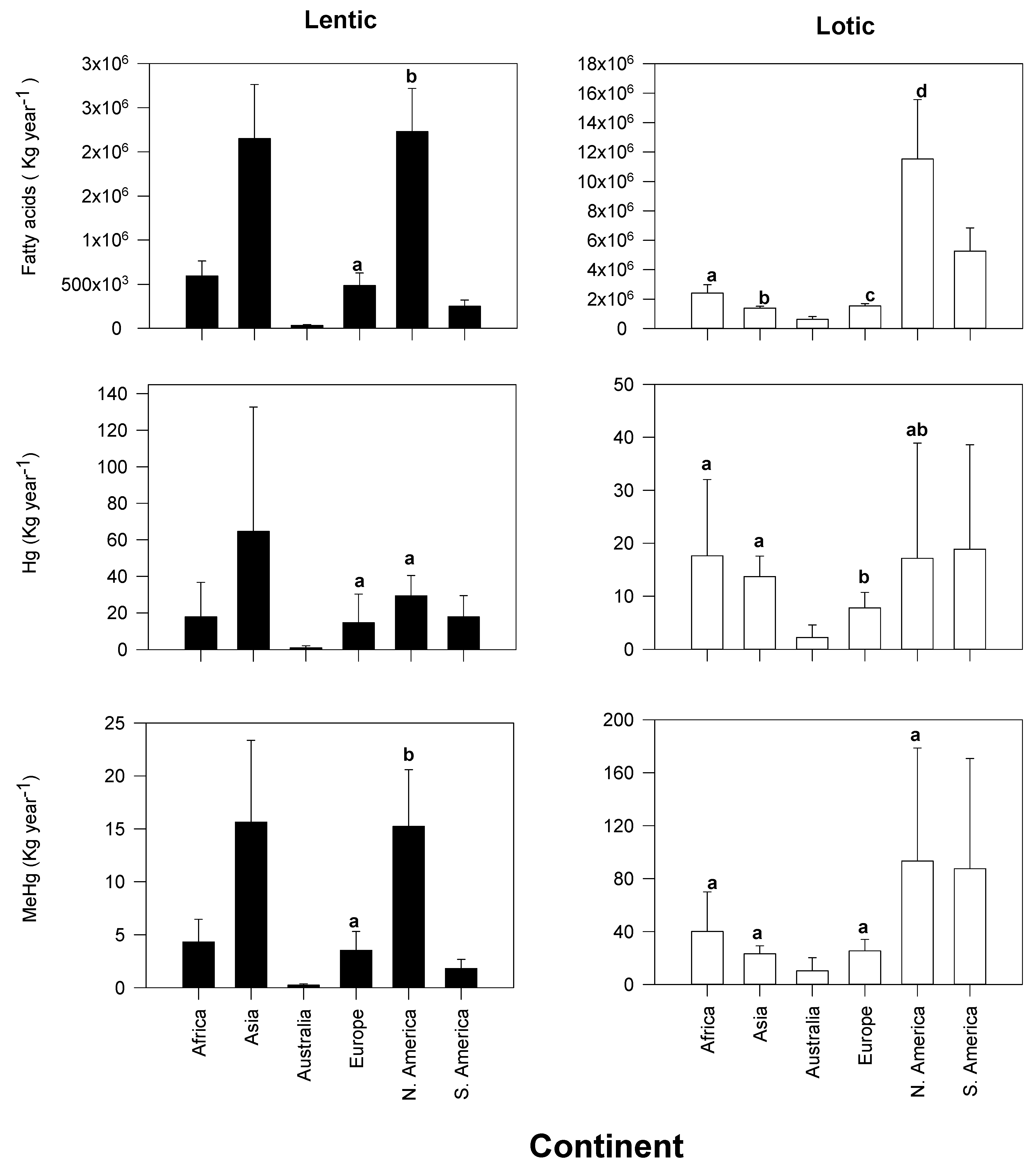
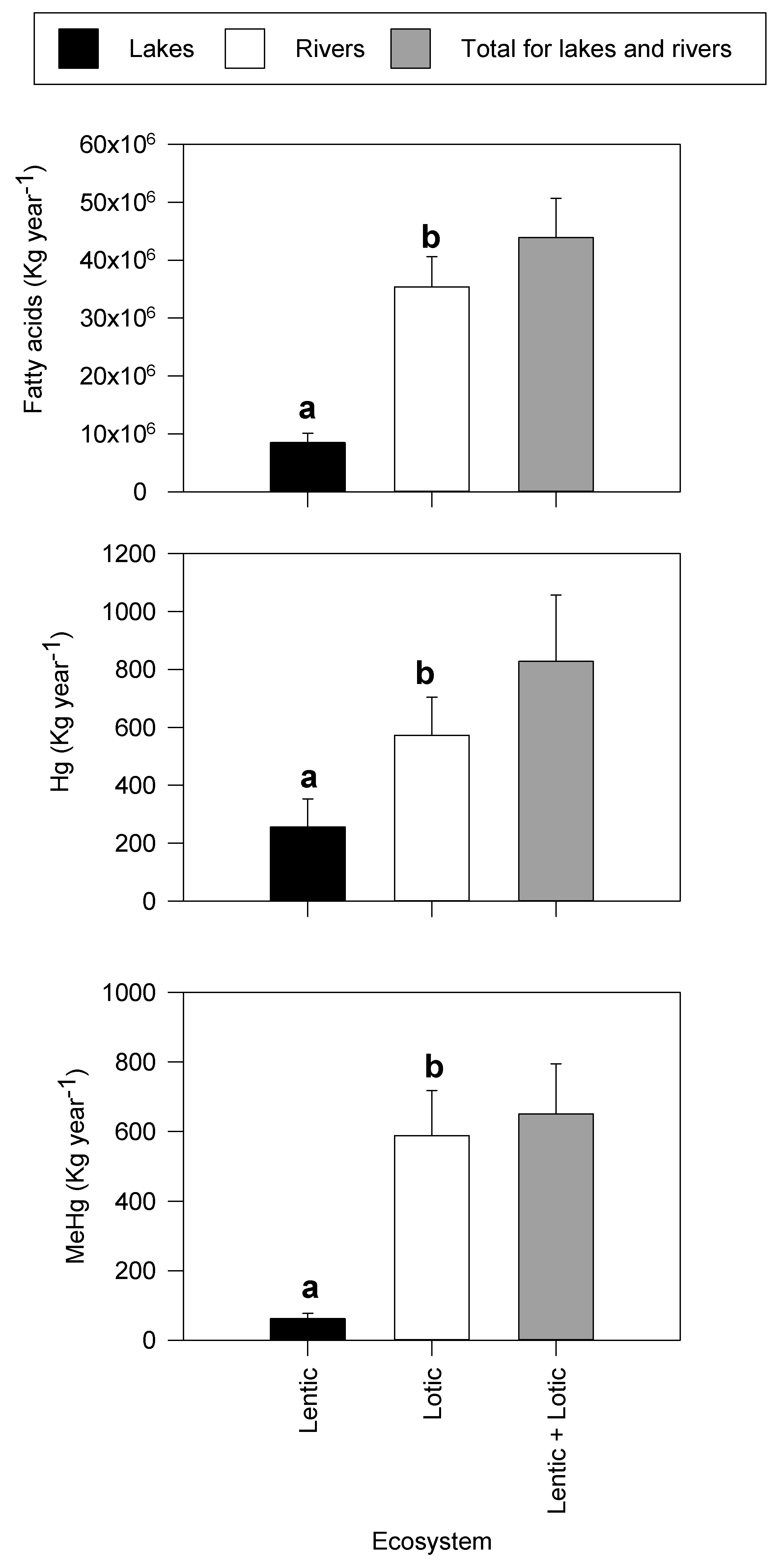
| Continent | Taxa | Emergence | Reference |
|---|---|---|---|
| Europe | |||
| Chironomidae, Ephemeroptera, Trichoptera | 4.0 | [42] | |
| Community | 1.8 | [43] | |
| Community | 1.4 | [43] | |
| Community | 1.1 | [43] | |
| Community | 2.4 | [44] | |
| Chironomidae | 1.9 | [45] | |
| Chironomidae | 0.2 | [46] | |
| Community | 0.2 a | [47] | |
| Average ± SD | 1.6 ± 1.2 | ||
| Coefficient of variation (%) | 70.9 | ||
| North America | |||
| Chironomidae | 1.5 b | [38] | |
| Community | 1.1 c | [48] | |
| Chironomidae | 0.2 d | [49] | |
| Chironomidae | 1.9 | [14] | |
| Average ± SD | 1.2 ± 0.6 | ||
| Coefficient of variation (%) | 53.8 | ||
| Average ± SD | 1.5 ± 1.0 | ||
| Coefficient of variation (%) | 70 |
| Continent | Taxa | Emergence | Reference |
|---|---|---|---|
| Africa | |||
| Trichoptera | 0.5 e | [14] | |
| Community | 4.0 e | [14] | |
| Average ± SD | 2.2 ± 1.7 | ||
| Coefficient of variation (%) | 78.6 | ||
| Asia | |||
| Community | 2.1 f | [50] | |
| Community | 1.2 g | [51] | |
| Average | 1.7 ± 0.5 | ||
| Coefficient of variation (%) | 27.3 | ||
| Europe | |||
| Diptera, Trichoptera, Ephemeroptera | 1.7 | [52] | |
| Ephemeroptera, Plecoptera, Trichoptera | 3.6 h | [14] | |
| Ephemeroptera, Plecoptera, Trichoptera | 5.0 h | [14] | |
| Community | 5.4 h | [14] | |
| Community | 2.6 h | [14] | |
| Community | 2.6 h | [14] | |
| Community | 3.7 h | [14] | |
| Community | 3.7 h | [14] | |
| Community | 2.0 h | [14] | |
| Community | 2.6 h | [14] | |
| Community | 3.2 h | [14] | |
| Chironomidae | 1.9 h | [14] | |
| Average | 3.2 ± 1.1 | ||
| Coefficient of variation (%) | 35.7 | ||
| North America | |||
| Diptera, Chironomidae | 1.2 i | [53] | |
| Trichoptera, Ephemeroptera, Plecoptera, Diptera | 6.6 j | [54] | |
| Ephemeroptera, Plecoptera, Trichoptera | 0.3 | [39] | |
| Chironomidae, Ephemeroptera, Trichopetra | 23.1 h | [14] | |
| Community | 5.3 | [14] | |
| Community | 7.1 | [14] | |
| Average ± SD | 7.8 ± 9.2 | ||
| Coefficient of variation (%) | 117.4 | ||
| Average ± SD | 4.5 ± 4.5 | ||
| Coefficient of variation (%) | 100.4 |
| Continent | Taxa | EPA +DHA | Reference |
|---|---|---|---|
| Lentic | |||
| Odonata | 8.27 k | [55] | |
| Chironomidae | 11.9 | [46] | |
| Community | 17.8 l | [56] | |
| Chironomidae | 4.0 | [40] | |
| Chironomidae | 7.0 | [40] | |
| Ephemeroptera | 11.3 | [27] | |
| Chironomidae | 10.1 | [57] | |
| Culicidae | 6.77 | [41] | |
| Average ± SD | 9.6 ± 3.9 | ||
| Coefficient of variation (%) | 41 | ||
| Lotic | |||
| Trichopteram | 11.6 | [58] | |
| Ephemeropteram | 12.8 | [58] | |
| Chironomidaem | 7.7 | [58] | |
| Chironomidae | 18.1 | [18] | |
| Trichoptera | 9.4 | [27] | |
| Average ± SD | 11.9 ± 3.6 | ||
| Coefficient of variation (%) | 30 |
| Continent | Taxa | Total Mercury | Methylmercury | Reference |
|---|---|---|---|---|
| Lentic | ||||
| North America | ||||
| Trichoptera, Diptera | n 4.2 × 10−4 | n 1.6 × 10−4 | [48] | |
| Coleoptera | 1.8 × 10−4 | 1.1 × 10−4 | [59] | |
| Ephemeroptera | 1.3 × 10−4 | 1.4 × 10−5 | [59] | |
| Hemiptera | 2.6 × 10−4 | 1.2 × 10−4 | [59] | |
| Odonata | 1.4 × 10−4 | 1.0 × 10−4 | [59] | |
| Trichoptera | 1.3 × 10−4 | 4.9 × 10−5 | [59] | |
| Trichoptera | 4.9 × 10−4 | 2.5 × 10−5 | [60] | |
| Odonata | 1.1 × 10−4 | 5.7 × 10−5 | [60] | |
| Ephemeroptera | 1.1 × 10−4 | 2.1 × 10−5 | [60] | |
| Coleoptera | 1.5 × 10−4 | 2.0 × 10−5 | [60] | |
| Trichoptera | 3.8 × 10−5 | 1.6 × 10−5 | [60] | |
| Odonata | 7.1 × 10−5 | 4.8 × 10−5 | [60] | |
| Ephemeroptera | 7.5 × 10−5 | 1.9 × 10−5 | [60] | |
| Odonata | 9.7 × 10−5 | 1.1 × 10−4 | [61] | |
| Ephemeroptera | 1.1 × 10−4 | 7.9 × 10−5 | [61] | |
| Trichoptera | 5.0 × 10−5 | 3.7 × 10−5 | [61] | |
| Diptera | 6.9 × 10−5 | 3.6 × 10−5 | [61] | |
| Odonata | - | 1.3 × 10−4 | [62] | |
| Diptera | - | 7.9 × 10−5 | [62] | |
| Trichoptera | - | 8.9 × 10−5 | [62] | |
| Average ± SD | 1.3 × 10−4 ± 8.9 × 10−5 | 6.6 × 10−5 ± 4.3 × 10−5 | ||
| Coefficient of variation (%) | 70 | 65 | ||
| South America | ||||
| Diptera | o 1.3 × 10−3 | - | [63] | |
| Ephemeroptera | 5.7 × 10−4 | - | [63] | |
| Odonata | 1.7 × 10−4 | - | [63] | |
| Plecoptera | 2.0 × 10−3 | - | [63] | |
| Trichoptera | 3.1 × 10−4 | - | [63] | |
| Community | 2.0 × 10−4 | 3.4 × 10−5 | [64] | |
| Community | 2.8 × 10−4 | 1.9 × 10−4 | [65] | |
| Average ± SD | 6.9 × 10−4 ± 6.4 × 10−4 | 7.0 × 10−5 ± 4.9 × 10−5 | ||
| Coefficient of variation (%) | 93 | 68 | ||
| Average ± SD | 2.9 × 10−4 ± 4.4 × 10−4 | 7.0 × 10−5 ± 4.9 × 10−5 | ||
| Coefficient of variation (%) | 150 | 70 |
| Continent | Taxa | Total Mercury | Methylmercury | Reference |
|---|---|---|---|---|
| Lotic | ||||
| North America | ||||
| Diptera | p 4.5 × 10−4 | p 2.0 ×10−4 | [66] | |
| Ephemeroptera | q 3.4 × 10−5 | q 1.8 × 10−5 | [67] | |
| Trichoptera | 5.1 × 10−5 | * | [68] | |
| Community | 2.7 × 10−4 | * | [69] | |
| Ephemeroptera | 8.1 × 10−5 | * | [70] | |
| Plecoptera | 6.1 × 10−5 | 7.3 × 10−5 | ||
| Diptera | 2.0 × 10−5 | * | [22] | |
| Average ± SD | 1.4 × 10−4 ± 1.5 × 10−4 | 9.6 × 10−5 ± 7.5 × 10−5 | ||
| Coefficient of variation (%) | 108 | 78 | ||
| South America | ||||
| Community | 5.7 × 10−4 | 5.0 × 10−4 | [65] | |
| Average ± SD | 1.9 × 10−4 ± 2.0 × 10−4 | 2.0 × 10−4 ± 1.9 × 10−4 | ||
| Coefficient of variation (%) | 104 | 95 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, S. Preliminary Estimations of Insect Mediated Transfers of Mercury and Physiologically Important Fatty Acids from Water to Land. Biomolecules 2020, 10, 129. https://doi.org/10.3390/biom10010129
Moyo S. Preliminary Estimations of Insect Mediated Transfers of Mercury and Physiologically Important Fatty Acids from Water to Land. Biomolecules. 2020; 10(1):129. https://doi.org/10.3390/biom10010129
Chicago/Turabian StyleMoyo, Sydney. 2020. "Preliminary Estimations of Insect Mediated Transfers of Mercury and Physiologically Important Fatty Acids from Water to Land" Biomolecules 10, no. 1: 129. https://doi.org/10.3390/biom10010129
APA StyleMoyo, S. (2020). Preliminary Estimations of Insect Mediated Transfers of Mercury and Physiologically Important Fatty Acids from Water to Land. Biomolecules, 10(1), 129. https://doi.org/10.3390/biom10010129





