Bond Rearrangement Produces Oxygen from Carbon Dioxide
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
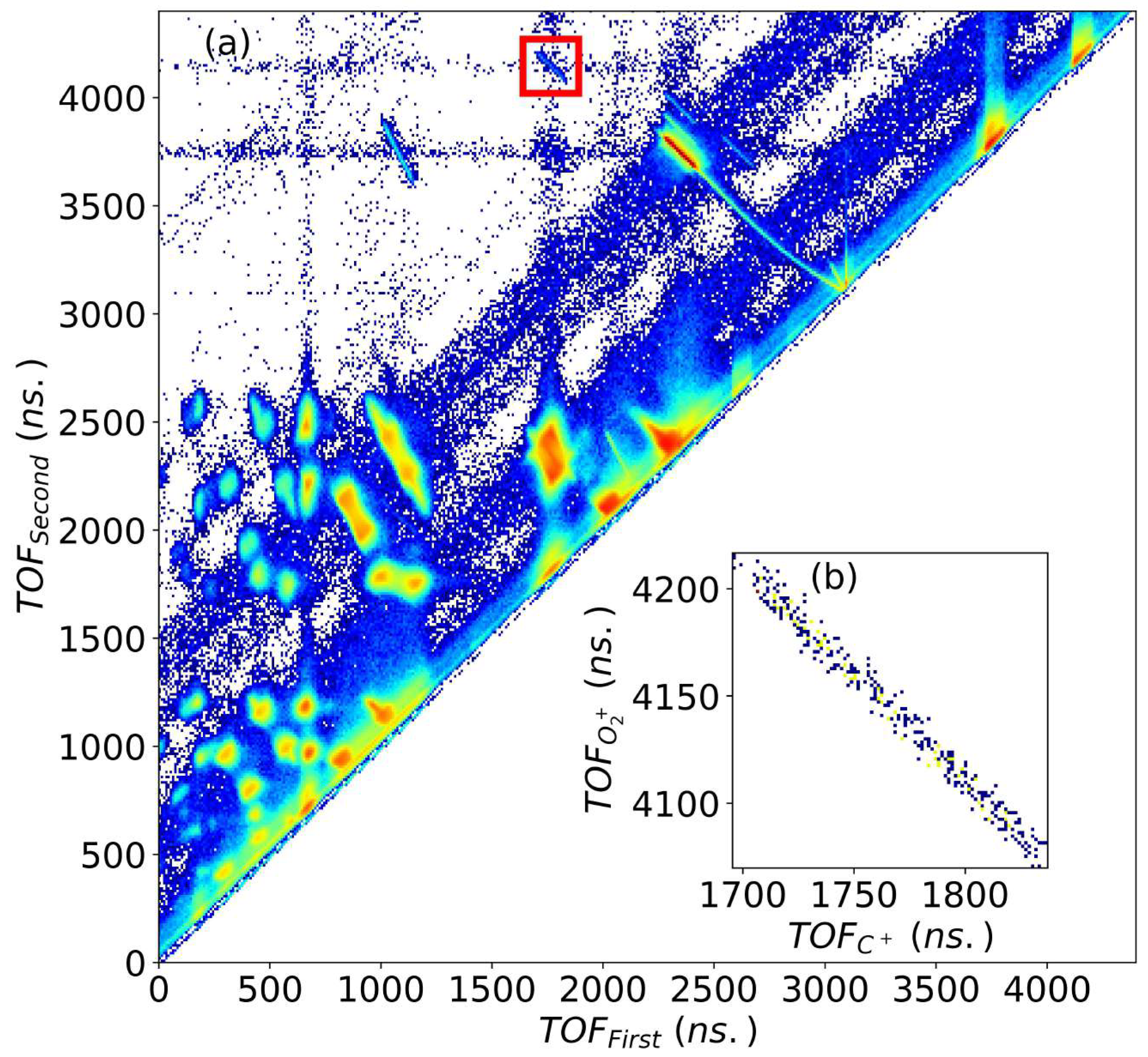
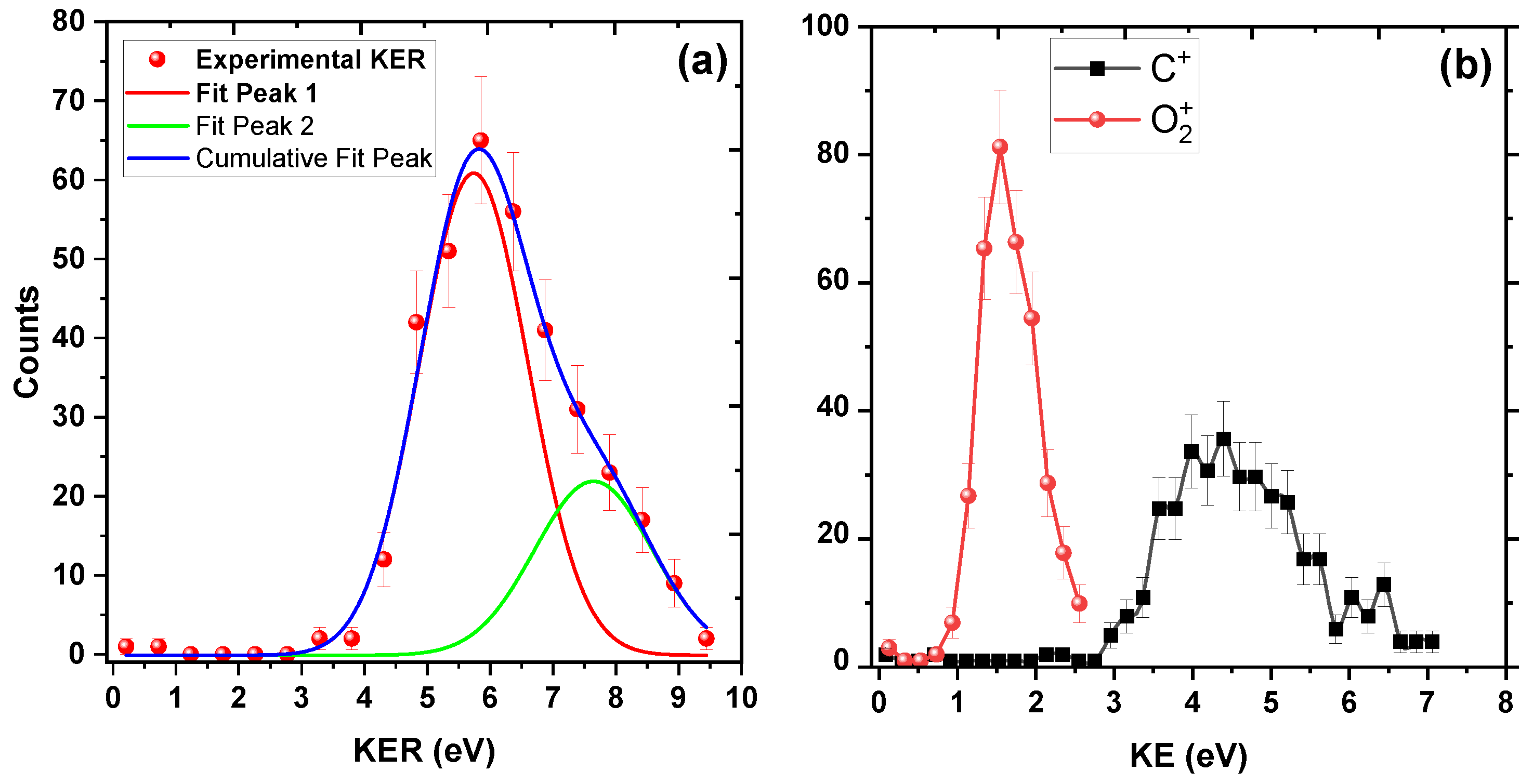
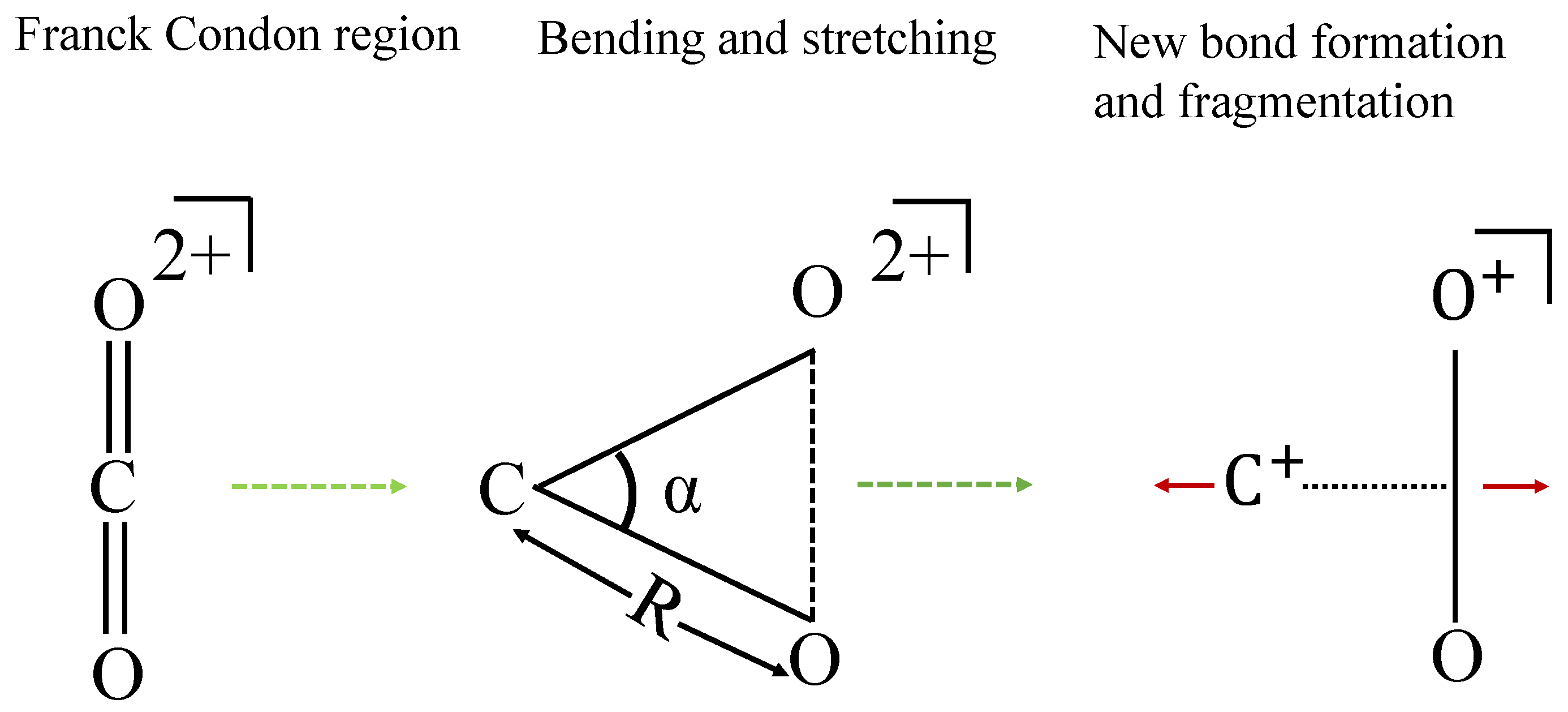
3.1. Dynamics
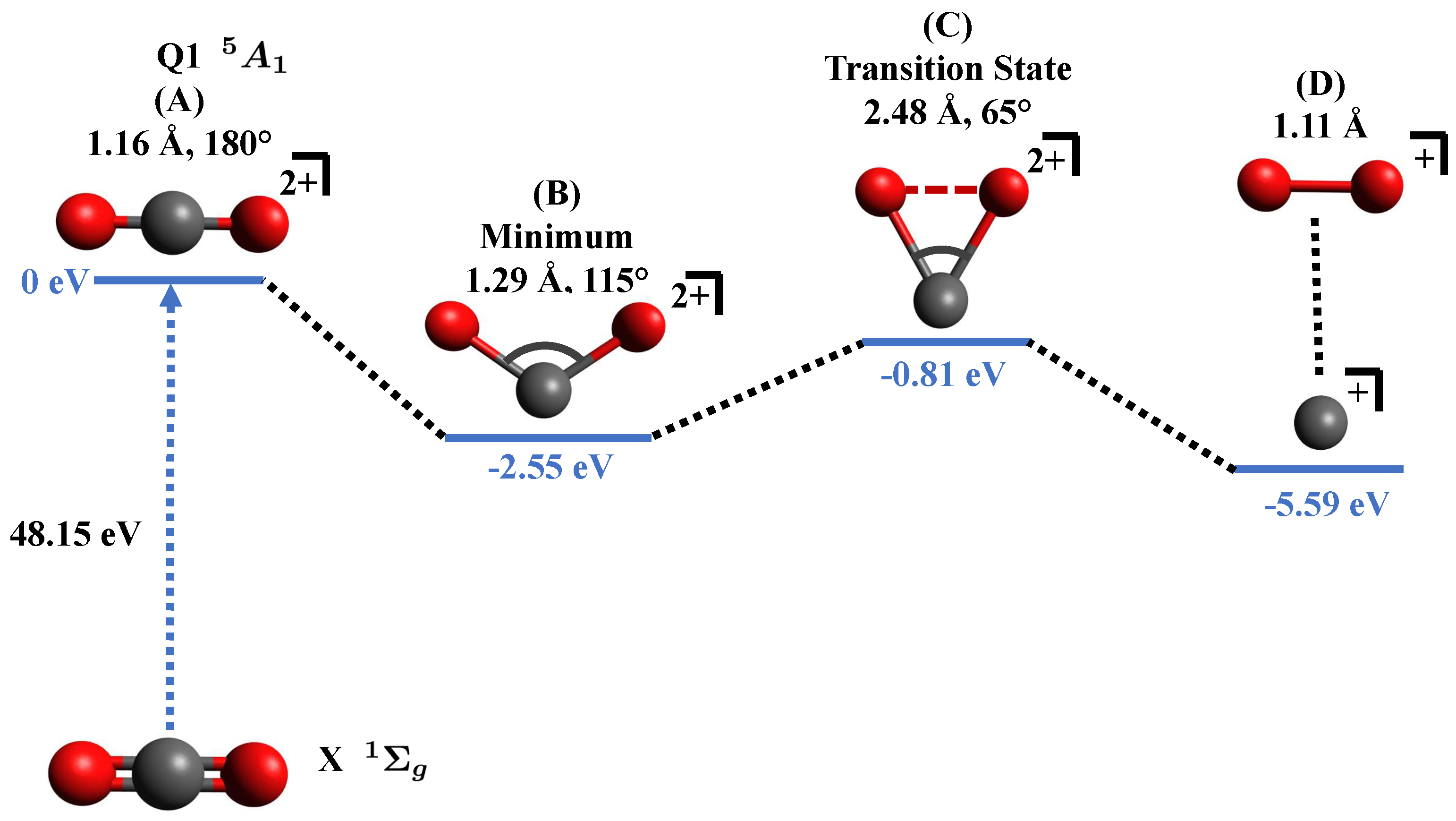
3.2. Theoretical Calculations
4. Branching Ratio
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hecht, M.H.; Hoffman, J.; Team, M. The Mars oxygen ISRU experiment (MOXIE) on the Mars 2020 Rover. In Proceedings of the 3rd International Workshop on Instrumentation for Planetary Mission, Pasadena, CA, USA, 24–27 October 2016; Volume 1980, p. 4130. [Google Scholar]
- Hu, H.; Larimian, S.; Erattupuzha, S.; Wen, J.; Baltuška, A.; Kitzler-Zeiler, M.; Xie, X. Laser-induced dissociative recombination of carbon dioxide. Phys. Rev. Res. 2019, 1, 033152. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Jiang, W.; Zhang, Y.; Jiang, Y.; Zhu, Z. Charge-encoded multi-photoion coincidence for three-body fragmentation of CO2 in the strong laser fields. J. Chem. Phys. 2022, 156, 134302. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Bapat, B. Electron-impact-like feature in triple fragmentation of under slow proton impact. Phys. Rev. A 2022, 105, 012801. [Google Scholar] [CrossRef]
- Duley, A.; Kelkar, A.H. Fragmentation Dynamics of CO2 q + (q = 2,3) in Collisions with 1 MeV Proton. Atoms 2023, 11, 75. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Mebel, A.M. Ab initio study of spin-forbidden unimolecular decomposition of carbon dioxide. Chem. Phys. 2000, 256, 169–176. [Google Scholar] [CrossRef]
- Grebenshchikov, S.Y. Photodissociation of carbon dioxide in singlet valence electronic states. I. Six multiply intersecting ab initio potential energy surfaces. J. Chem. Phys. 2013, 138, 224106. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, Y.C.; Yin, Q.Z.; Ng, C.Y.; Jackson, W.M. Evidence for direct molecular oxygen production in CO2 photodissociation. Science 2014, 346, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Gao, X.F.; Xuan, C.J.; Tian, S.X. Dissociative electron attachment to CO2 produces molecular oxygen. Nat. Chem. 2016, 8, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Larimian, S.; Erattupuzha, S.; Mai, S.; Marquetand, P.; González, L.; Baltuška, A.; Kitzler, M.; Xie, X. Molecular oxygen observed by direct photoproduction from carbon dioxide. Phys. Rev. A 2017, 95, 011404. [Google Scholar] [CrossRef]
- Long, J.; Furch, F.J.; Durá, J.; Tremsin, A.S.; Vallerga, J.; Schulz, C.P.; Rouzée, A.; Vrakking, M.J.J. Ion-ion coincidence imaging at high event rate using an in-vacuum pixel detector. J. Chem. Phys. 2017, 147, 013919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jochim, B.; Feizollah, P.; Rajput, J.; Ziaee, F.; P., K.R.; Kaderiya, B.; Borne, K.; Malakar, Y.; Berry, B.; et al. Strong-field-induced bond rearrangement in triatomic molecules. Phys. Rev. A 2019, 99, 053412. [Google Scholar] [CrossRef]
- Öhrwall, G.; Sant’Anna, M.M.; Stolte, W.C.; Dominguez-Lopez, I.; Dang, L.T.N.; Schlachter, A.S.; Lindle, D.W. Anion and cation formation following core-level photoexcitation of CO2. J. Phys. B At. Mol. Opt. Phys. 2002, 35, 4543. [Google Scholar] [CrossRef]
- Laksman, J.; Månsson, E.P.; Grunewald, C.; Sankari, A.; Gisselbrecht, M.; Céolin, D.; Sorensen, S.L. Role of the Renner-Teller effect after core hole excitation in the dissociation dynamics of carbon dioxide dication. J. Chem. Phys. 2012, 136, 104303. [Google Scholar] [CrossRef] [PubMed]
- Eland, J.H.D.; Zagorodskikh, S.; Squibb, R.J.; Mucke, M.; Sorensen, S.L.; Feifel, R. Carbon dioxide ion dissociations after inner shell excitation and ionization: The origin of site-specific effects. J. Chem. Phys. 2014, 140, 184305. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shushkov, P.; Miller III, T.F.; Giapis, K.P. Direct dioxygen evolution in collisions of carbon dioxide with surfaces. Nat. Commun. 2019, 10, 2294. [Google Scholar] [CrossRef]
- Smita, G.; Barreiro-Lage, D.; Walsh, N.; Bart, O.; Sorensen, S.L.; Díaz-Tendero, S.; Mathieu, G. The origin of enhanced O2 production from photoionized CO2 clusters. Commun. Chem. 2022, 5, 16. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, S.; Wang, E.; Xu, J.; Gao, Y.; Zhu, X.; Guo, D.; Ma, B.; Zhao, D.; Zhang, S.; et al. Fragmentation Dynamics of a Carbon Dioxide Dication Produced by Ion Impact. J. Phys. Chem. Lett. 2022, 13, 7594–7599. [Google Scholar] [CrossRef] [PubMed]
- Dörner, R.; Mergel, V.; Jagutzki, O.; Spielberger, L.; Ullrich, J.; Moshammer, R.; Schmidt-Böcking, H. Cold Target Recoil Ion Momentum Spectroscopy: A ‘momentum microscope’ to view atomic collision dynamics. Phys. Rep. 2000, 330, 95–192. [Google Scholar] [CrossRef]
- Ullrich, J.; Moshammer, R.; Dorn, A.; Dörner, R.; Schmidt, L.P.H.; Schmidt-Böcking, H. Recoil-ion and electron momentum spectroscopy: Reaction-microscopes. Rep. Prog. Phys. 2003, 66, 1463. [Google Scholar] [CrossRef]
- Agnihotri, A.N.; Kelkar, A.H.; Kasthurirangan, S.; Thulasiram, K.V.; Desai, C.A.; Fernandez, W.A.; Tribedi, L.C. An ECR ion source-based low-energy ion accelerator: Development and performance. Phys. Scr. 2011, 2011, 014038. [Google Scholar] [CrossRef]
- Khan, A.; Tribedi, L.C.; Misra, D. A recoil ion momentum spectrometer for molecular and atomic fragmentation studies. Rev. Sci. Instrum. 2015, 86, 043105. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, M.A.K.A.; Nrisimhamurty, M.; Kumar, K.; Mukherjee, J.; Tribedi, L.C.; Khan, A.; Misra, D. Development of a cold target recoil ion momentum spectrometer and a projectile charge state analyzer setup to study electron transfer processes in highly charged ion–atom/molecule collisions. Rev. Sci. Instrum. 2022, 93, 113313. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.A.; Cheng, L.; Harding, M.E.; Lipparini, F.; Stopkowicz, S.; Jagau, T.C.; Szalay, P.G.; Gauss, J.; Stanton, J.F. Coupled-cluster techniques for computational chemistry: The CFOUR program package. J. Chem. Phys. 2020, 152, 214108. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.F.; Bartlett, R.J. The equation of motion coupled-cluster method. A systematic biorthogonal approach to molecular excitation energies, transition probabilities, and excited state properties. J. Chem. Phys. 1993, 98, 7029–7039. [Google Scholar] [CrossRef]
- Goings, J.J.; Caricato, M.; Frisch, M.J.; Li, X. Assessment of low-scaling approximations to the equation of motion coupled-cluster singles and doubles equations. J. Chem. Phys. 2014, 141, 164116. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Jo/rgensen, P. Coupled cluster response functions. J. Chem. Phys. 1990, 93, 3333–3344. [Google Scholar] [CrossRef]
- Caricato, M. Exploring Potential Energy Surfaces of Electronic Excited States in Solution with the EOM-CCSD-PCM Method. J. Chem. Theory Comput. 2012, 8, 5081–5091. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Pople, J.A.; Nesbet, R.K. Self-Consistent Orbitals for Radicals. J. Chem. Phys. 1954, 22, 571–572. [Google Scholar] [CrossRef]

| Branching Ratio | |||
|---|---|---|---|
| S. No. | Beam | : | |
| 1 | 240 keV | 6 | 0.15 ± 0.01 : 99.85 ± 0.01 |
| 2 | 62 keV | 12 | 0.17 ± 0.01 : 99.83 ± 0.01 |
| 3 | 240 keV | 12 | 0.17 ± 0.01 : 99.83 ± 0.01 |
| 4 | 240 keV | 16 | 0.18 ± 0.01 : 99.83 ± 0.01 |
| 5 | 160 keV | 20 | 0.17 ± 0.02 : 99.83 ± 0.02 |
| 6 | 200 keV | 40 | 0.15 ± 0.01 : 99.85 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Mukherjee, J.; Singh, H.; Misra, D. Bond Rearrangement Produces Oxygen from Carbon Dioxide. Atoms 2024, 12, 25. https://doi.org/10.3390/atoms12040025
Kumar K, Mukherjee J, Singh H, Misra D. Bond Rearrangement Produces Oxygen from Carbon Dioxide. Atoms. 2024; 12(4):25. https://doi.org/10.3390/atoms12040025
Chicago/Turabian StyleKumar, Kamal, Jibak Mukherjee, Harpreet Singh, and Deepankar Misra. 2024. "Bond Rearrangement Produces Oxygen from Carbon Dioxide" Atoms 12, no. 4: 25. https://doi.org/10.3390/atoms12040025
APA StyleKumar, K., Mukherjee, J., Singh, H., & Misra, D. (2024). Bond Rearrangement Produces Oxygen from Carbon Dioxide. Atoms, 12(4), 25. https://doi.org/10.3390/atoms12040025






