Fragmentation of Multiply Charged C10H8 Isomers Produced in keV Range Proton Collision
Abstract
:1. Introduction
2. Computational Details
3. Experimental Details and Analysis
4. Results and Discussion
4.1. Normalisation Process
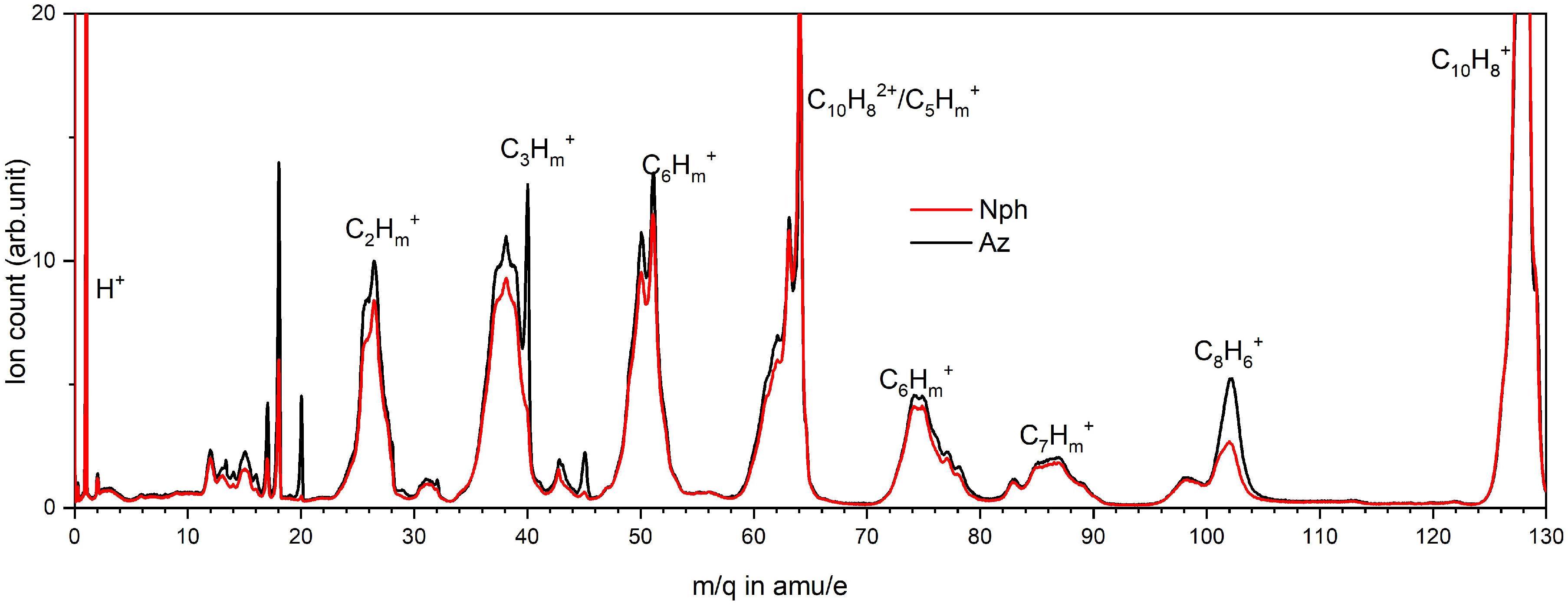
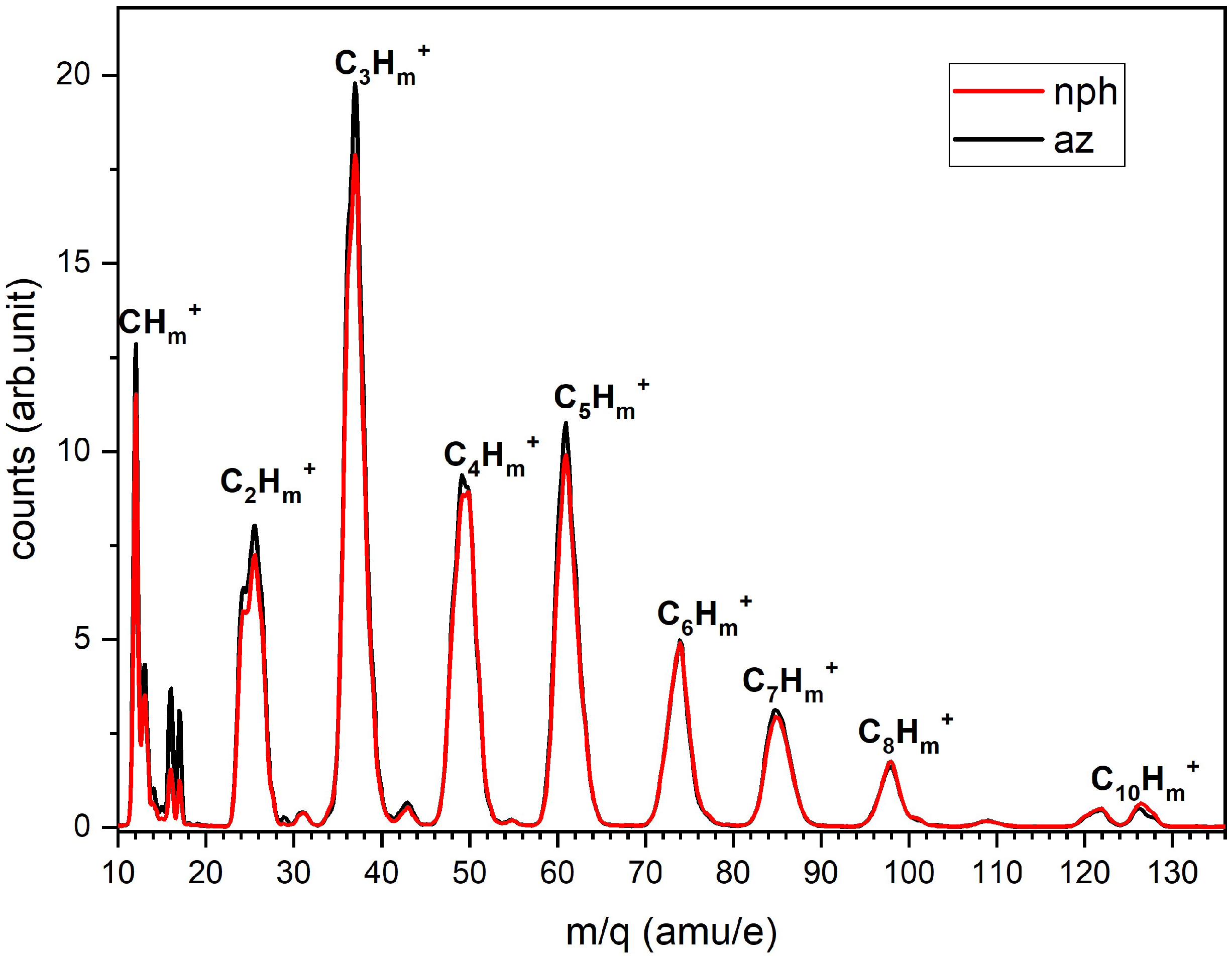
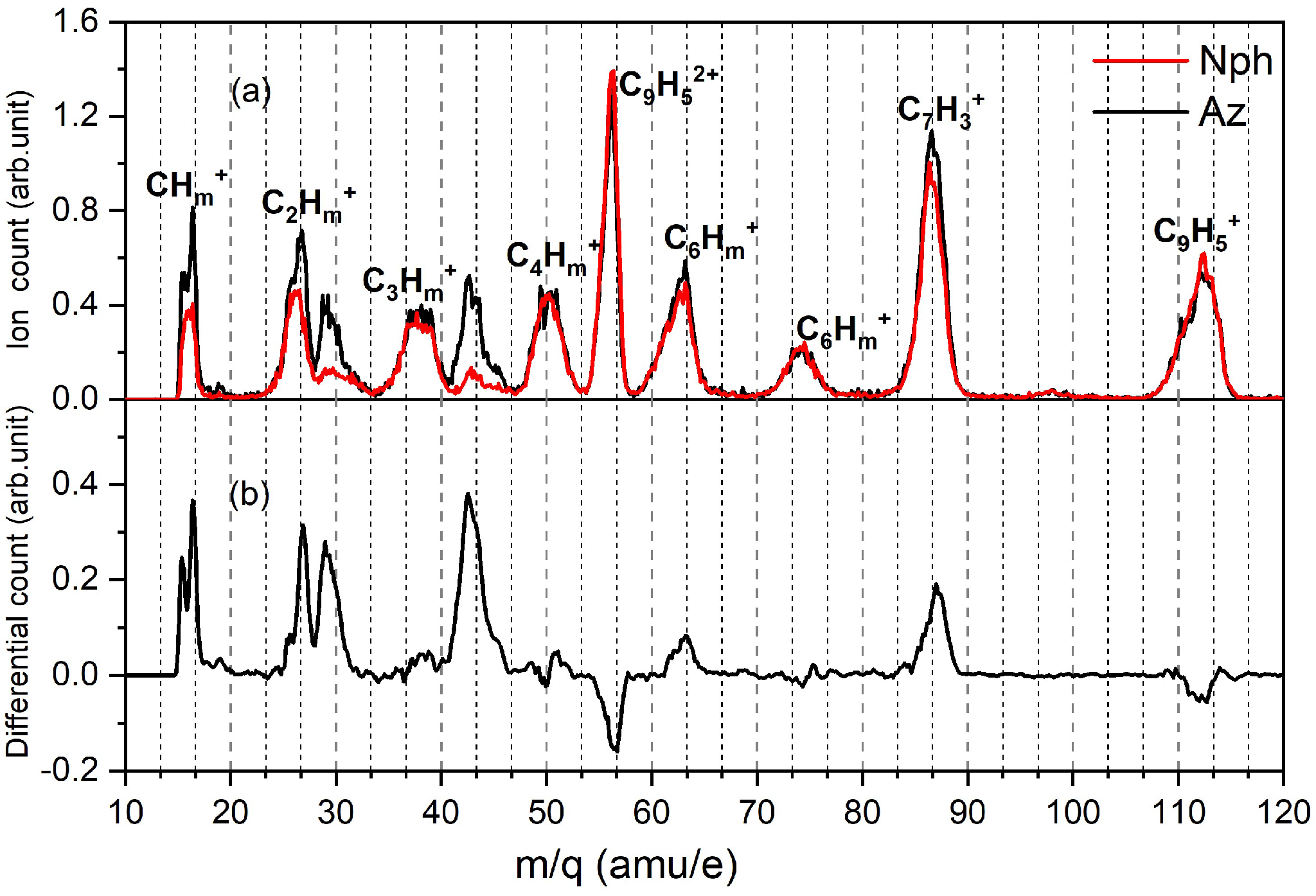
4.2. Single Hit Analysis
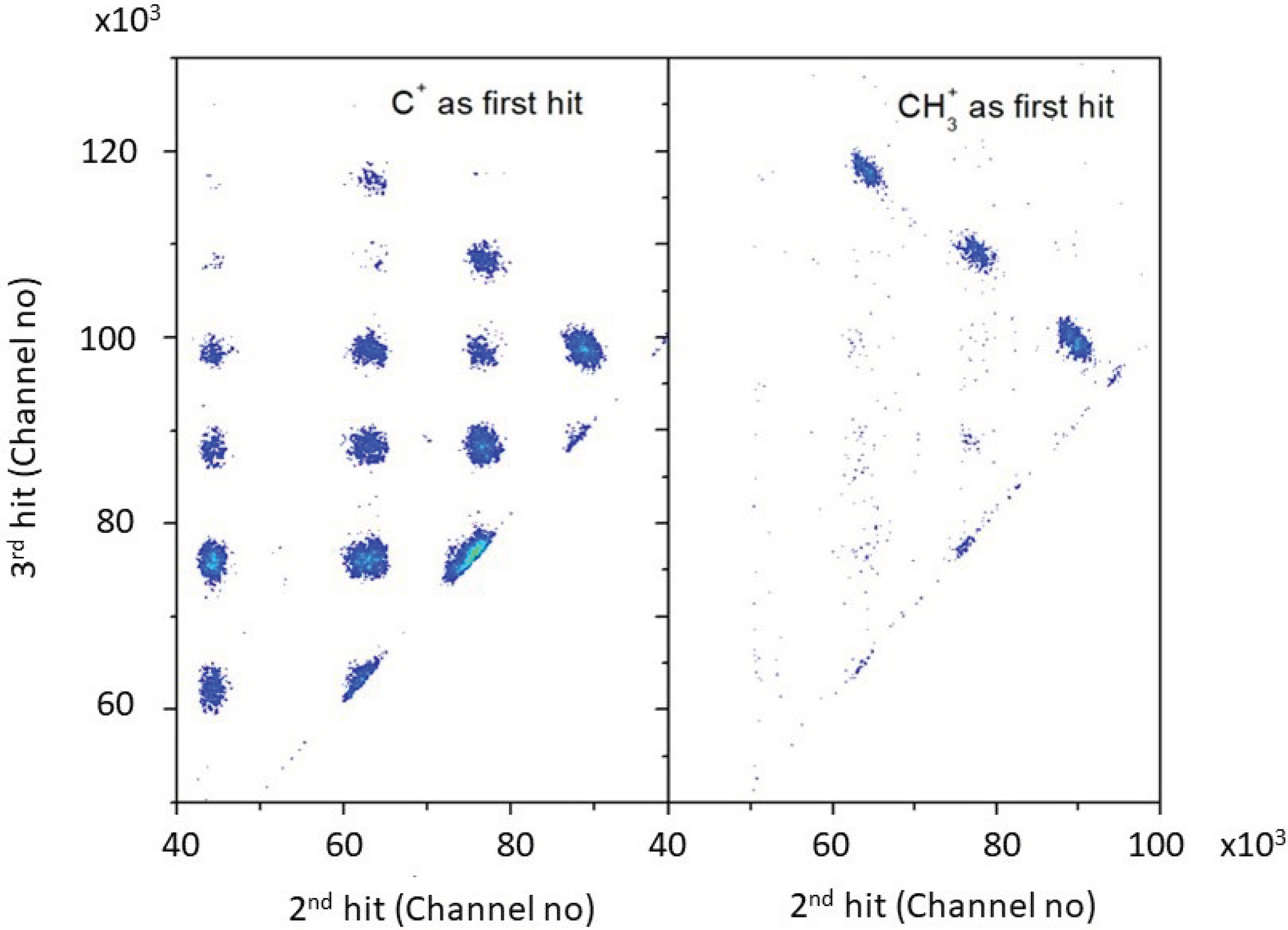
4.3. Coincidence Analysis of Double Hit Data
4.4. H+ Coincidence
4.5. C+, CH+, CH2+ Coincidence
4.6. CH3+ Coincidence
4.7. Multihit Analysis of CH3+ Channel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Brunt, R.J.; Wacks, M.E. Electron-Impact Studies of Aromatic Hydrocarbons. III. Azulene and Naphthalene. J. Chem. Phys. 1964, 41, 3195–3199. [Google Scholar] [CrossRef]
- Ruehl, E.; Price, S.D.; Leach, S. Single and double photoionization processes in naphthalene between 8 and 35 eV. J. Phys. Chem. 1989, 93, 6312–6321. [Google Scholar] [CrossRef]
- Jochims, H.; Rasekh, H.; Rühl, E.; Baumgärtel, H.; Leach, S. The photofragmentation of naphthalene and azulene monocations in the energy range 7–22 eV. Chem. Phys. 1992, 168, 159–184. [Google Scholar] [CrossRef]
- Jochims, H.; Baumgärtel, H.; Leach, S. Structure-dependent photostability of polycyclic aromatic hydrocarbon cations: Laboratory studies and astrophysical implications. Astrophys. J. 1999, 512, 500. [Google Scholar] [CrossRef]
- Cui, W.; Hadas, B.; Cao, B.; Lifshitz, C. Time-resolved photodissociation (TRPD) of the naphthalene and azulene cations in an ion trap/reflectron. J. Phys. Chem. A 2000, 104, 6339–6344. [Google Scholar] [CrossRef]
- West, B.; Joblin, C.; Blanchet, V.; Bodi, A.; Sztáray, B.; Mayer, P.M. On the dissociation of the naphthalene radical cation: New iPEPICO and tandem mass spectrometry results. J. Phys. Chem. A 2012, 116, 10999–11007. [Google Scholar] [CrossRef]
- Reitsma, G.; Zettergren, H.; Martin, S.; Brédy, R.; Chen, L.; Bernard, J.; Hoekstra, R.; Schlathölter, T. Activation energies for fragmentation channels of anthracene dications—Experiment and theory. J. Phys. B At. Mol. Opt. Phys. 2012, 45, 215201. [Google Scholar] [CrossRef]
- Postma, J.; Bari, S.; Hoekstra, R.; Tielens, A.; Schlathölter, T. Ionization and Fragmentation of Anthracene upon Interaction with keV Protons and α Particles. Astrophys. J. 2009, 708, 435. [Google Scholar] [CrossRef]
- West, B.; Castillo, S.R.; Sit, A.; Mohamad, S.; Lowe, B.; Joblin, C.; Bodi, A.; Mayer, P.M. Unimolecular reaction energies for polycyclic aromatic hydrocarbon ions. Phys. Chem. Chem. Phys. 2018, 20, 7195–7205. [Google Scholar] [CrossRef]
- Simon, A.; Champeaux, J.P.; Rapacioli, M.; Moretto Capelle, P.; Gadéa, F.X.; Sence, M. Dissociation of polycyclic aromatic hydrocarbons at high energy: MD/DFTB simulations versus collision experiments: Fragmentation paths, energy distribution and internal conversion: Test on the pyrene cation. Theor. Chem. Accounts 2018, 137, 1–11. [Google Scholar] [CrossRef]
- Vinitha, M.V.; Najeeb, P.K.; Kala, A.; Bhatt, P.; Safvan, C.P.; Vig, S.; Kadhane, U.R. Plasmon excitation and subsequent isomerization dynamics in naphthalene and azulene under fast proton interaction. J. Chem. Phys. 2018, 149, 194303. [Google Scholar] [CrossRef]
- Bagdia, C.; Biswas, S.; Mandal, A.; Bhattacharjee, S.; Tribedi, L.C. Ionization and fragmentation of fluorene upon 250 keV proton impact. Eur. Phys. J. D 2021, 75, 1–7. [Google Scholar]
- Leach, S. The formation and destruction of doubly-charged polycyclic aromatic hydrocarbon cations in the interstellar medium. J. Electron Spectrosc. Relat. Phenom. 1986, 41, 427–438. [Google Scholar] [CrossRef]
- Witt, A.N.; Gordon, K.D.; Vijh, U.P.; Sell, P.H.; Smith, T.L.; Xie, R.H. The excitation of extended red emission: New constraints on its carrier from hubble space telescope observations of NGC 7023. Astrophys. J. 2006, 636, 303. [Google Scholar] [CrossRef]
- Malloci, G.; Joblin, C.; Mulas, G. Theoretical evaluation of PAH dication properties. Astron. Astrophys. 2007, 462, 627–635. [Google Scholar] [CrossRef]
- Monfredini, T.; Quitián-Lara, H.M.; Fantuzzi, F.; Wolff, W.; Mendoza, E.; Lago, A.F.; Sales, D.A.; Pastoriza, M.G.; Boechat-Roberty, H.M. Destruction and multiple ionization of PAHs by X-rays in circumnuclear regions of AGNs. Mon. Not. R. Astron. Soc. 2019, 488, 451–469. [Google Scholar]
- Leach, S.; Eland, J.; Price, S. Formation and dissociation of dications of naphthalene, azulene and related heterocyclic compounds. J. Phys. Chem. 1989, 93, 7575–7583. [Google Scholar] [CrossRef]
- Leach, S.; Eland, J.; Price, S. Formation and dissociation of dications of naphthalene-d8. J. Phys. Chem. 1989, 93, 7583–7593. [Google Scholar] [CrossRef]
- Sittler, E.C.; Hartle, R.; Bertucci, C.; Coates, A.; Cravens, T.; Dandouras, I.; Shemansky, D. Energy deposition processes in Titan’s upper atmosphere and its induced magnetosphere. In Titan from Cassini-Huygens; Springer: New York, NY, USA, 2010; pp. 393–453. [Google Scholar]
- Smith, H.; Mitchell, D.; Johnson, R.; Paranicas, C. Investigation of energetic proton penetration in Titan’s atmosphere using the Cassini INCA instrument. Planet. Space Sci. 2009, 57, 1538–1546. [Google Scholar] [CrossRef]
- Sillanpää, I.; Johnson, R.E. The role of ion-neutral collisions in Titan’s magnetospheric interaction. Planet. Space Sci. 2015, 108, 73–86. [Google Scholar] [CrossRef]
- Cravens, T.; Robertson, I.; Ledvina, S.; Mitchell, D.; Krimigis, S.; Waite, J., Jr. Energetic ion precipitation at Titan. Geophys. Res. Lett. 2008, 35, L03103. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09: (Revision D.01). Inc. Wallingford CT 2009, 121, 150–166. [Google Scholar]
- Krems, M.; Zirbel, J.; Thomason, M.; DuBois, R.D. Channel electron multiplier and channelplate efficiencies for detecting positive ions. Rev. Sci. Instrum. 2005, 76, 093305. [Google Scholar]
- Santos, J.C.; Fantuzzi, F.; Quitián-Lara, H.M.; Martins-Franco, Y.; Menéndez-Delmestre, K.; Boechat-Roberty, H.M.; Oliveira, R.R. Multiply charged naphthalene and its C10H8 isomers: Bonding, spectroscopy, and implications in AGN environments. Mon. Not. R. Astron. Soc. 2022, 512, 4669–4682. [Google Scholar]
- Lee, J.W.; Stockett, M.H.; Ashworth, E.K.; Navarro Navarrete, J.E.; Gougoula, E.; Garg, D.; Ji, M.; Zhu, B.; Indrajith, S.; Zettergren, H.; et al. Cooling dynamics of energized naphthalene and azulene radical cations. J. Chem. Phys. 2023, 158, 174305. [Google Scholar] [PubMed]
- Ławicki, A.; Holm, A.I.; Rousseau, P.; Capron, M.; Maisonny, R.; Maclot, S.; Seitz, F.; Johansson, H.A.; Rosén, S.; Schmidt, H.T.; et al. Multiple ionization and fragmentation of isolated pyrene and coronene molecules in collision with ions. Phys. Rev. A 2011, 83, 022704. [Google Scholar]
- Champeaux, J.P.; Moretto-Capelle, P.; Cafarelli, P.; Deville, C.; Sence, M.; Casta, R. Is the dissociation of coronene in stellar winds a source of molecular hydrogen? application to the HD 44179 nebula. Mon. Not. R. Astron. Soc. 2014, 441, 1479–1487. [Google Scholar] [CrossRef]
- Holm, A.I.; Johansson, H.A.; Cederquist, H.; Zettergren, H. Dissociation and multiple ionization energies for five polycyclic aromatic hydrocarbon molecules. J. Chem. Phys. 2011, 134, 044301. [Google Scholar]
- Martínez, J.I.; Alonso, J.A. Hydrogen quenches the size effects in carbon clusters. Phys. Chem. Chem. Phys. 2019, 21, 10402–10410. [Google Scholar]
- Wang, H.; Frenklach, M. Calculations of rate coefficients for the chemically activated reactions of acetylene with vinylic and aromatic radicals. J. Phys. Chem. 1994, 98, 11465–11489. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Lu, W.; Xu, B.; Ahmed, M.; Morozov, A.N.; Mebel, A.M.; Howlader, A.H.; Wnuk, S.F. Molecular mass growth through ring expansion in polycyclic aromatic hydrocarbons via radical–radical reactions. Nat. Commun. 2019, 10, 3689. [Google Scholar]
- Kingston, R.; Guilhaus, M.; Brenton, A.; Beynon, J. Multiple ionization, charge separation and charge stripping reactions involving polycyclic aromatic compounds. Org. Mass Spectrom. 1985, 20, 406–412. [Google Scholar] [CrossRef]
- Reitsma, G.; Zettergren, H.; Boschman, L.; Bodewits, E.; Hoekstra, R.; Schlathölter, T. Ion–polycyclic aromatic hydrocarbon collisions: Kinetic energy releases for specific fragmentation channels. J. Phys. At. Mol. Opt. Phys. 2013, 46, 245201. [Google Scholar]
- Dyakov, Y.A.; Ni, C.K.; Lin, S.; Lee, Y.; Mebel, A. Ab initio and RRKM study of photodissociation of azulene cation. Phys. Chem. Chem. Phys. 2006, 8, 1404–1415. [Google Scholar] [CrossRef]
- Solano, E.A.; Mayer, P.M. A complete map of the ion chemistry of the naphthalene radical cation? DFT and RRKM modeling of a complex potential energy surface. J. Chem. Phys. 2015, 143, 104305. [Google Scholar] [PubMed]
- Huang, J.; Oka, T. Constraining the size of the carrier of the λ5797. 1 diffuse interstellar band. Mol. Phys. 2015, 113, 2159–2168. [Google Scholar]
- Steglich, M.; Maity, S.; Maier, J.P. Visible absorptions of potential diffuse ISM hydrocarbons: C9H9 and C9H5 radicals. Astrophys. J. 2016, 830, 145. [Google Scholar]
- Maity, S.; Steglich, M.; Maier, J.P. Gas Phase Detection of Benzocyclopropenyl. J. Phys. Chem. 2015, 119, 10849–10853. [Google Scholar]






| Structure | E of Dication | E of Trication | |
|---|---|---|---|
| Nph | 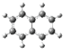 | 0 | 0 |
| Az |  | 0.41 | 0.29 |
| A |  | 3.07 | 2.20 |
| B | 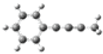 | 1.96 | 1.14 |
| Structure | E of Monocation | E of Dication | |
|---|---|---|---|
| A | 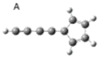 | 0.9 | 0 |
| B | 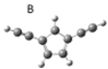 | 1.11 | 0.45 |
| C |  | 1.33 | 0.52 |
| D | 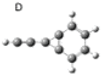 | 0 | 0.49 |
| E |  | 0.27 | 0.02 |
| F | 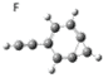 | 0.22 | 1.17 |
| G |  | 1.54 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinitha, M.V.; Bhatt, P.; Safvan, C.P.; Vig, S.; Kadhane, U.R. Fragmentation of Multiply Charged C10H8 Isomers Produced in keV Range Proton Collision. Atoms 2023, 11, 138. https://doi.org/10.3390/atoms11110138
Vinitha MV, Bhatt P, Safvan CP, Vig S, Kadhane UR. Fragmentation of Multiply Charged C10H8 Isomers Produced in keV Range Proton Collision. Atoms. 2023; 11(11):138. https://doi.org/10.3390/atoms11110138
Chicago/Turabian StyleVinitha, Meloottayil V., Pragya Bhatt, Cholakka P. Safvan, Sarita Vig, and Umesh R. Kadhane. 2023. "Fragmentation of Multiply Charged C10H8 Isomers Produced in keV Range Proton Collision" Atoms 11, no. 11: 138. https://doi.org/10.3390/atoms11110138
APA StyleVinitha, M. V., Bhatt, P., Safvan, C. P., Vig, S., & Kadhane, U. R. (2023). Fragmentation of Multiply Charged C10H8 Isomers Produced in keV Range Proton Collision. Atoms, 11(11), 138. https://doi.org/10.3390/atoms11110138







