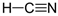1. Introduction
Comets are “Cosmic Shuttles” known to harbor organic molecules relevant to prebiotic chemistry, including hydrogen cyanide (HCN) [
1], ammonia (NH
3) [
2], formaldehyde (H
2CO) [
3], methanol (CH
3OH) [
4], formic acid (HCOOH) [
5], and phosphate-bearing minerals [
6], as confirmed by direct and indirect observational data from space missions such as Rosetta, e.g., ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis), ALMA (Atacama Large Millimeter/Submillimeter Array), and COSAC (gas analyzer Cometary Sampling and Composition) to comet 67P/Churyumov-Gerasimenko [
7]. The detection of these molecular precursors, also in dust [
8], is of crucial importance: they represent essential building blocks for the synthesis of complex organic molecules such as amino acids, sugars, nucleobases, nucleosides, and nucleotides, i.e., main components required for the emergence of life [
9]. Cometary nuclei likely are over most of their history at temperatures of about 50 K while their surfaces and the outer layers, where most chemistry is active, are subject to extreme thermal cycling ranging from cryogenic temperatures of ~50 K in the Oort cloud to over 300 K at ~1 AU and even above 1000 K for sungrazing comets [
10]. In addition, cometary surfaces are exposed to ultraviolet (UV) radiation, cosmic ray, and sporadic heating during perihelion passages [
11]. These distinctive conditions enable chemical reactions that might be energetically unfavorable or kinetically inaccessible under standard terrestrial conditions, making comets ideal natural laboratories for prebiotic chemistry [
12]. Within nuclei superficial regions, photochemical activation and radical-mediated pathways can facilitate reaction routes not typically available under ambient planetary conditions [
13].
Several synthetic pathways for nucleobases and sugars formation under such conditions have been proposed. These include the Strecker synthesis, radical polymerization of formamide (HCONH
2), and HCN-based oligomerization [
14,
15]. Compounds such as glycolaldehyde (HOCH
2-CHO) and formamide have been detected in cometary comae [
16] supporting the astrochemical plausibility of these pathways. The presence of minerals in cometary nuclei [
17], including silicates [
18], metal oxides, sulfides [
19], and phosphates, can provide catalytic sites that can influence chemical reactivity and selectivity. The role of comets in delivering prebiotic molecules to Earth, a key aspect of the pseudo-panspermia hypothesis, has gained increasing attention [
20]. Laboratory experiments simulating cometary impact conditions demonstrate the survival of complex organics encapsulated within icy or mineral matrices during atmospheric entry [
21]. A systematic understanding of the thermodynamic feasibility of these synthetic reactions under cometary conditions is still to be investigated.
In this study, a quantitative thermodynamic analysis is performed to evaluate whether reactions involved in the prebiotic synthesis of nucleobases, sugars, nucleosides, and nucleotides are thermodynamically viable on cometary surfaces. Calculations are based on standard-state Gibbs free energy changes, assuming solid-phase chemistry and excluding other intermediates unless explicitly supported by environmental justification. This approach aims to define the thermodynamic constraints governing organic reactions in cometary environments, some of which may be critical to prebiotic chemical evolution. It is important to note that the present work focuses exclusively on thermodynamic feasibility, neglecting kinetic barriers and catalytic effects that may operate on cometary surfaces. While this simplification allows for a systematic mapping of Gibbs free energy changes, it represents only part of the full prebiotic picture. The analysis directly connects to the broader question of life’s origins, since the cometary molecules evaluated here, bases, sugars, and simple nucleosides, are key precursors of ribonucleotides and thus of RNA itself.
2. Thermodynamic Calculations
To evaluate the thermodynamic feasibility for seven key prebiotic reactions, involved in prebiotic synthesis of nucleobases, sugars, nucleosides, and nucleotides, to occur on cometary nuclei, I estimated the Gibbs free energy variation, ΔG, at temperature, T, spanning from 50 K to 1000 K, mimicking different cometary conditions. Standard enthalpies of formation (ΔH°
f) and molar entropies (S°) for the molecular species involved were collected from experimental databases, e.g., NIST (
www.nist.gov/publications, accessed on 20 April 2025), Cheméo (
www.chemeo.com, accessed on 20 April 2025), and from [
22] for adenosine and adenosine 5′-monophosphate. Where unavailable, values were estimated by analogy with structurally related molecules. All molecular states were considered in their condensed form, i.e., solid or liquid, except for CO which was treated as gas under standard conditions. This approximation is consistent with thermodynamic convention for condensed-phase systems, where activities of solids and liquids are typically set to unity.
The following thermodynamic equation was applied:
where ΔH and ΔS for each reaction were calculated from the sum of the products minus the sum of the reactants. In cases where only gas-phase values were available, e.g., cyanamide, aminoacetonitrile, the solid-state enthalpy of formation was calculated by subtracting the enthalpy of sublimation (ΔH_sub) from the gas-phase value:
Standard molar entropies were assumed to vary minimally. For AMP aqueous-phase values [
22] were used as an approximation for solid-state properties. All calculations and assumptions are summarized in
Table 1 and
Table 2. These calculations rely on standard-state assumptions, with activities of condensed species set to unity. While actual ΔG values depend on specific concentrations through ΔG = ΔG
0 + RT ln Q, such data are not available for cometary interiors. The approach focuses on identifying relative thermodynamic trends across a range of temperatures, rather than predicting reaction yields. Reactions with ΔG > 0 are considered thermodynamically unfeasible, reactions with ΔG < 0 are considered thermodynamically feasible, and reactions with ΔG ≈ 0 are considered thermodynamically neutral.
3. Results
3.1. Formation of Nitrogenous Bases via Strecker Synthesis
In the Strecker Synthesis, hydrogen cyanide (HCN), ammonia (NH
3), and formaldehyde (H
2CO) react to form aminoacetonitrile (NH
2CH
2CN), a relatively stable intermediate under cometary cryogenic conditions:
Under moderate UV irradiation, aminoacetonitrile can polymerize and subsequent cyclize to adenine (C
5H
5N
5):
I computed the ΔG for reaction (R2) at temperatures from 50 to 1000 K (
Table 2). My results confirm that this reaction is strongly exergonic across all surface-relevant temperatures, with ΔG decreasing from −402.5 kcal mol
−1 at 50 K to −450.0 kcal mol
−1 at 1000 K. At 200 K the calculated ΔG is −410.0 kcal mol
−1, driven almost entirely by the enthalpic gain associated with aromatic ring formation in adenine. The entropy term (TΔS) is minimal at low temperatures, reinforcing the enthalpy-dominant profile of the reaction. This makes the Strecker-based synthesis of adenine the most thermodynamically favorable pathway among all routes analyzed in this study. Even in deep-space cometary reservoirs (~50 K), the reaction remains spontaneous and exergonic.
It is important to emphasize that these findings are purely thermodynamic and do not address kinetic factors. Although UV photons could initiate polymerization and radical formation, kinetic barriers associated with cyclization are not considered here. An important limitation involves the potential transformation of aminoacetonitrile under transient aqueous conditions. Aminoacetonitrile can hydrolyze to glycine (NH
2CH
2COOH), a compound that has been detected in cometary samples [
23,
24,
25,
26]. While this competing reaction could reduce yields of adenine such kinetic considerations and environmental perturbations fall outside the scope of this purely thermodynamic evaluation.
3.2. Formation of Nitrogenous Bases via Radical Synthesis from Formamide
Formamide (HCONH
2), spectroscopically detected in cometary nuclei, can be an alternative precursor for nitrogenous base [
27] formation through radical-driven reactions induced by UV radiation and cosmic rays:
At T = 200 K, ΔG is −32.0 kcal mol
−1, indicating that the reaction is moderately exergonic. The exothermicity can derive from the formation of aromatic rings in adenine [
28]. As temperature increases, the entropy contribution becomes more favorable, and ΔG becomes increasingly negative, reaching −60.0 kcal mol
−1 for T = 1000 K (
Table 2). These results suggest that this pathway is thermodynamically viable over the entire surface temperature range of cometary bodies, although less favorable than the Strecker pathway (reaction 2). Under cometary UV exposure, formamide is prone to fragmentation into simpler species such as NH
3, CO
2, or HCN, potentially competing with nucleobase formation [
29].
3.3. Orò-Type Polymerization
HCN, i.e., one of the most abundant organic molecules detected in astrophysical environments and potentially involved in prebiotic chemistry, is known to undergo spontaneous polymerization under cryogenic conditions, forming a macromolecular material referred to as HCN polymer or polyamino-cyanomethylene [
30]. This solid-state polymer is stable due to a combination of two factors: (i) extensive hydrogen bonding, conjugated structure, and (ii) entrapment within water ice matrices, which reduce molecular mobility and degradation in cold environments. The breakdown of this polymer can produce adenine and potentially other purine derivatives, according to the following overall reactions:
If we consider the polymer as 6HCN:
These reactions have attracted significant interest as a possible reservoir mechanism, as they could store biologically relevant molecules, releasing them only upon conditions that allow depolymerization.
At 200 K the ΔG is approximately −98 kcal mol
−1 and the reaction becomes even more favorable at higher temperatures, reaching values near −130 kcal mol
−1 at 1000 K. This derives both from the aromatic stabilization of adenine and from the increased entropy associated with the release of volatile by-products such as NH
3, and CO
2 during breakdown. Although HCN polymers may kinetically require activation energy or aqueous conditions for nucleobase release [
31], such kinetic considerations and related activation mechanisms are not considered here. Thus, cometary environments are thermodynamically ideal for the preservation and accumulation of HCN polymers, but the potential transition to prebiotic purines upon planetary delivery is possibly ruled by kinetic factors in addition to thermodynamics.
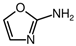
3.4. Cyanamide-Based Reactions
Cyanamide (NH
2CN), another plausible cometary molecule, reacts with glycolaldehyde (HOCH
2CHO) to form intermediates leading to nucleobases:
The structural stability of 2-aminooxazole, attributed to its ring closure and intramolecular hydrogen bonding, is significant under cryogenic conditions, where reduced molecular motion enhances the persistence of such intermediates. This makes it a promising candidate for long-lived cometary reservoirs of nucleotide precursors. Thermodynamic calculations show that this reaction is endergonic under cometary-relevant temperatures. At 200 K the ΔG is approximately +16 kcal mol
−1, indicating that the reaction is not spontaneous under typical surface conditions of cometary bodies. Even at the lowest evaluated temperature (50 K) the ΔG remains positive, and only at elevated temperatures approaching 1000 K does the reaction become thermodynamically favorable (
Table 2). The formation of 2-aminooxazole is thermodynamically unfeasible across most of the full cometary temperature range considered here.
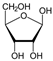
3.5. Formation of Pentose Sugars and Nucleosides/Nucleotides
Two primary pathways have been proposed for pentose sugar formation under prebiotic conditions. The first is the classical Formose reaction, where formaldehyde (H
2CO) undergoes autocatalytic polymerization to ribose:
An alternative pathway involves Glycolaldehyde Polymerization, utilizing glycolaldehyde (HOCH
2CHO):
Thermodynamic analysis confirms that the formation of ribose from formaldehyde is exergonic, with ΔG, calculated at 200 K, is approximately −76 kcal mol−1 and even more favorable at higher temperatures.
Compared to the classical Formose reaction pathway, the alternative ribose synthesis route shown in Equation (10) is slightly more exergonic. At 298 K, the ΔG for this alternative pathway is approximately −49.2 kJ/mol, while the Formose pathway yields a net ΔG of approximately −45.1 kJ/mol. Although the difference is modest, it suggests that the alternative route may be thermodynamically more favorable under standard conditions. Formaldehyde and glycolaldehyde are chemically versatile to undergo alternative reactions, including the Cannizzaro reaction, where two H
2CO molecules yield formic acid (HCOOH) and methanol (CH
3OH). From a thermodynamic standpoint, the Cannizzaro reaction is strongly exergonic (ΔG ≈ −55.4 kJ/mol at 298 K) and thus represents a competitive pathway for formaldehyde consumption. However, it does not lead to sugar formation and may act as a thermodynamic sink, diverting carbon away from more complex prebiotic molecules. Moreover, the Formose reaction lacks selectivity and tends to produce complex mixtures of sugars and polyhydroxy oligomers, with little control over chain length or stereochemistry [
32]. While low temperatures may slow degradation, thermodynamic feasibility alone does not guarantee ribose stability or accumulation.
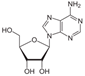
The subsequent synthesis of nucleosides proceeds through condensation reactions, e.g.,
Thermodynamic calculations show that reaction (R9) is mildly endergonic across cometary-relevant temperatures. At 200 K, ΔG is approximately +3.0 kcal mol−1, decreasing slightly at higher temperatures, e.g., +2.0 kcal mol−1 at 300 K, and becoming marginally exergonic only above ~600–800 K (−1.0 to −3.0 kcal mol−1). These values indicate that the reaction is thermodynamically neutral under standard cometary conditions. While actual directionality may depend on concentration gradients (ΔG = ΔG0 + RT ln Q), such data are not available for cometary interiors. Therefore, only standard-state behavior is evaluated here.
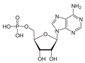
Subsequent phosphorylation, assisted by UV-activated phosphate minerals, can lead to nucleotide formation:
Reaction (R10), in contrast, is strongly endergonic under all cometary-like conditions. At 200 K the reaction has a ΔG of approximately +66.0 kcal mol−1 and remains energetically unfavorable even at 1000 K (ΔG ≈ +50.0 kcal mol−1). These values indicate that AMP formation is thermodynamically unfeasible across the full cometary temperature range.
4. Discussion
This study provides a thermodynamic evaluation of key reactions leading to nitrogenous bases, sugars, nucleosides, and nucleotides across a wide temperature range (50–1000 K) (
Figure 1).
The results reveal systematic trends that differentiate which types of molecular synthesis are thermodynamically feasible under cometary temperatures and which are thermodynamically prohibited, regardless of environmental complexity The surface temperature of comets spans orders of magnitude depending on their orbital history and family. Long-period comets originating from the Oort Cloud spend most of their lifetime at cryogenic temperatures (~50 K), interrupted by brief warming during solar approaches [
33,
34]. Jupiter Family Comets (JFCs) typically oscillate between 4–6 AU and 1 AU, experiencing intermediate thermal cycling (100–250 K) [
33]. In contrast, sungrazing comets, such as those in the Kreutz group, can reach surface temperatures exceeding 800–1000 K during perihelion [
12] (
Figure 2). Assessing thermodynamic feasibility across this spectrum provides a map of chemical potential within cometary systems.
The formation of aromatic nucleobases from simple precursors is consistently thermodynamically favorable across all temperature regimes. The Strecker-type polymerization of aminoacetonitrile to adenine is strongly exergonic (ΔG from −402 kcal mol−1 at 50 K to −450 kcal mol−1 at 1000 K), indicating potential synthesis under even the Oort Cloud conditions. Hydrolysis of HCN polymers to adenine is also highly favorable (ΔG ≈ −92 to −130 kcal mol−1), with growing exergonicity at higher temperatures due to growing entropy contributions. This supports the potential for nucleobase accumulation under both cold and warm cometary conditions, driven by different dominant processes. The formamide-derived pathway to adenine is moderately exergonic, transitioning from ΔG ≈ −27 kcal mol−1 at 50 K to ≈−60 kcal mol−1 at 1000 K. While viable at all temperatures, its favorability is lower compared to HCN-based pathways. Some pathways shift from endergonic to exergonic ΔG values as temperature rises, suggesting conditional thermodynamic feasibility. For instance, 2-aminooxazole synthesis from cyanamide and glycolaldehyde is endergonic below ~600–700 K (ΔG ≈ +23 kcal mol−1 at 50 K, +16 kcal mol−1 at 200 K) but becomes exergonic above ~800 K. This implies that such pyrimidine precursors cannot form under cold cometary conditions and require elevated temperatures for thermodynamic favorability.
The Formose reaction is exergonic across all temperatures considered, i.e., ΔG from −71.5 kcal mol−1 at 50 K to −100 kcal mol−1 at 1000 K. Side reactions, e.g., Cannizzaro disproportionation, and the low selectivity of sugar formation reduce the chance of ribose accumulation. Ribose is also chemically fragile, prone to racemization and decomposition under warming. Although cold trapping may preserve it at low temperatures, its persistence at biologically relevant fidelity is questionable.
The reaction of adenine and ribose to form adenosine lies thermodynamically near neutral with ΔG ranging from +4.5 kcal mol−1 at 50 K to ≈−5 kcal mol−1 at 1000 K. This suggests marginal thermodynamic favorability only at high temperatures, and infeasibility in cold regions. No spontaneous condensation is expected under cryogenic conditions. The phosphorylation of adenosine to AMP is thermodynamically prohibited at all temperatures (ΔG from +69 kcal mol−1 at 50 K to +50 kcal mol−1 at 1000 K). No known cometary thermal environment can make this step favorable under standard-state thermodynamics.
This analysis confirms that cometary environments cannot spontaneously support full nucleotide synthesis. However, early steps such as nitrogenous base and simple sugar formation are thermodynamically feasible, especially under cold conditions that stabilize intermediates and suppress side reactions. Upon planetary delivery, cometary materials encounter new environmental regimes: (i) higher temperatures (273–373 K), (ii) atmospheric pressure (~1 bar), (iii) metal-rich surfaces, which may enable kinetically slow or thermodynamically hindered reactions. While this study does not explore these mechanisms, the thermodynamic groundwork established here could provide a baseline to assess the plausibility of such transformations.
A further limitation of this work is the assumption of isolated, bare molecules. In reality, cometary surfaces host complex mixtures and abundant solid phases, e.g., silicates, metal oxides, phosphates, ices, which can alter both thermodynamic states and kinetic accessibility. Adsorption of organics onto ice matrices or mineral surfaces can change Gibbs free energy by stabilizing intermediates, lowering enthalpic costs, or altering entropy contributions. Laboratory simulations have shown that catalytic minerals such as montmorillonite clays or phosphate-rich phases can promote condensation reactions otherwise unfavorable in the bulk phase. Similarly, hydrogen-bonded networks in water–ice mixtures can provide stabilization that is not captured by standard-state values. As a concrete example, the adenine-ribose condensation step (reaction R9) has a calculated ΔG of +3.0 kcal mol
−1 at 200 K under standard-state assumptions (
Table 2), i.e., slightly endergonic. Adsorption enthalpies reported for adenine on montmorillonite or silica surfaces are in the range of –10 to –20 kcal mol
−1 [
27]. If a conservative stabilization of –15 kcal mol
−1 is considered, the effective free energy change becomes ΔG_eff ≈ –12 kcal mol
−1, rendering the condensation exergonic. This should not be interpreted as catalysis in the kinetic sense, which lowers activation barriers without altering thermodynamic balances, but rather as a shift in the thermodynamic reference state: the reaction no longer proceeds from isolated adenine and ribose but from adsorbed species with different enthalpic baselines. Such stabilization illustrates how surface-bound molecules can qualitatively change the thermodynamic feasibility of key steps, even if kinetic accessibility remains an independent limitation.
These results also carry implications for the RNA world hypothesis. The cometary synthesis of adenine, ribose, and other simple sugars represents not only general organic molecules but a supply of precise precursors required for ribonucleotide assembly. Although nucleotide formation itself is thermodynamically prohibited in cometary settings, delivery of such precursors to the early Earth could have provided essential inputs for ribonucleotide synthesis under terrestrial conditions. In particular, comet-derived purines, ribose, and HCN polymers may have contributed to the abiotic formation of ribonucleotides, thereby feeding into the emergence of RNA as the first informational and catalytic biopolymer. Beyond its role as an informational polymer, RNA has also been proposed as a metabolic scaffold in early evolution, where ribozymes could have catalyzed primitive reactions linking energy transduction to molecular complexity [
35,
36]. This perspective reinforces the significance of cometary delivery of ribonucleotide precursors, as it could have supported both genetic and metabolic innovation at life’s origin.
5. Conclusions
I performed a quantitative thermodynamic analysis of seven key reactions involved in prebiotic synthesis of nucleobases, sugars, nucleosides, and nucleotides. For each pathway, I computed the Gibbs free energy change (ΔG) over a wide temperature range (50–1000 K), chosen to reflect the diverse thermal regimes observed across different cometary families: from cryogenic preservation in the Oort Cloud to intense solar heating in sungrazers and perihelion-active Jupiter Family Comets. By systematically mapping ΔG across this spectrum, I capture both cold-trapping potential and temperature-driven reactivity, offering a unified framework for understanding cometary chemistry across evolutionary timescales. The results reveal a strong temperature dependence across pathways and clear thermodynamic hierarchy. Several nucleobase-forming reactions are highly exergonic even at cryogenic temperatures, e.g., −400 kcal mol−1 at 200 K, while nucleotide assembly remains thermodynamically prohibited up to 1000 K. These trends help to delineate which molecular classes can thermodynamically form and accumulate on comets, and which require non-cometary environments. By integrating cometary temperatures regimes, this study defines the thermodynamic boundaries of cometary prebiotic and organic chemistry and supports the evaluation of exogenous molecular delivery during early chemical evolution. Aromatic nucleobase synthesis, e.g., adenine from aminoacetonitrile, formamide, or HCN polymers, is thermodynamically favorable at all evaluated temperatures. These reactions are spontaneous even at 50 K, supporting the idea that cometary environments can enable the synthesis and long-term preservation of nitrogenous bases without requiring solar heating or kinetic forcing. Sugar synthesis via the Formose reaction is also thermodynamically favorable, though selectivity limitations and competition with exergonic side reactions such as Cannizzaro disproportionation may reduce net yields of ribose. Ribose stability remains temperature-sensitive, with degradation above ~250 K unless buffered by mineral matrices or ice encapsulation. Other reactions, such as 2-aminooxazole formation from cyanamide and glycolaldehyde, show ΔG > 0 at low temperatures and become exergonic only above 700 K, reflecting a temperature-induced shift in thermodynamic. These reactions are prohibited in cryogenic environments but feasible at higher temperatures, such as during perihelion passages or planetary impact heating.
Nucleoside formation is thermodynamic neutral at low T (from 50 to 400 K), and becomes exergonic at high temperatures, especially >700 K. This suggests limited feasibility under cold conditions and possible progression only under higher-temperature regimes. In contrast, phosphorylation to form nucleotides, e.g., AMP, thermodynamically unfeasible across the entire cometary temperature range, with ΔG consistently above +50 kcal mol−1.
Overall, cometary environments are thermodynamically favorable for the synthesis and preservation of nitrogenous bases and simple sugars, especially under low-temperature conditions. Full nucleotide assembly exceeds the thermodynamic limits of cometary settings, supporting a two-phase model of chemical evolution: (i) comets as low-temperature synthesis and storage environments for key intermediates and (ii) planetary surfaces as settings for thermodynamically demanding processes leading to functional biopolymers. In particular, the results reinforce the idea that cometary delivery may have supplied purines, ribose, and HCN-derived polymers that later converged toward RNA synthesis under terrestrial conditions. This provides a thermodynamic underpinning for the RNA world hypothesis, in which comet-delivered precursors fed into the first genetic and catalytic biopolymer. At the same time, our framework should be regarded as a baseline, since it neglects kinetic barriers, catalytic influences, and adsorption effects, all of which may further modulate feasibility in realistic cometary environments. Thus, while kinetic barriers and environmental complexities remain to be addressed, the present thermodynamic framework delineates which cometary reactions are feasible in principle, thereby identifying the most plausible pathways through which exogenous delivery could have contributed to the emergence of RNA-centered biochemistry on the early Earth.