Fibroin Delays Chilling Injury of Postharvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage
Abstract
1. Introduction
2. Results
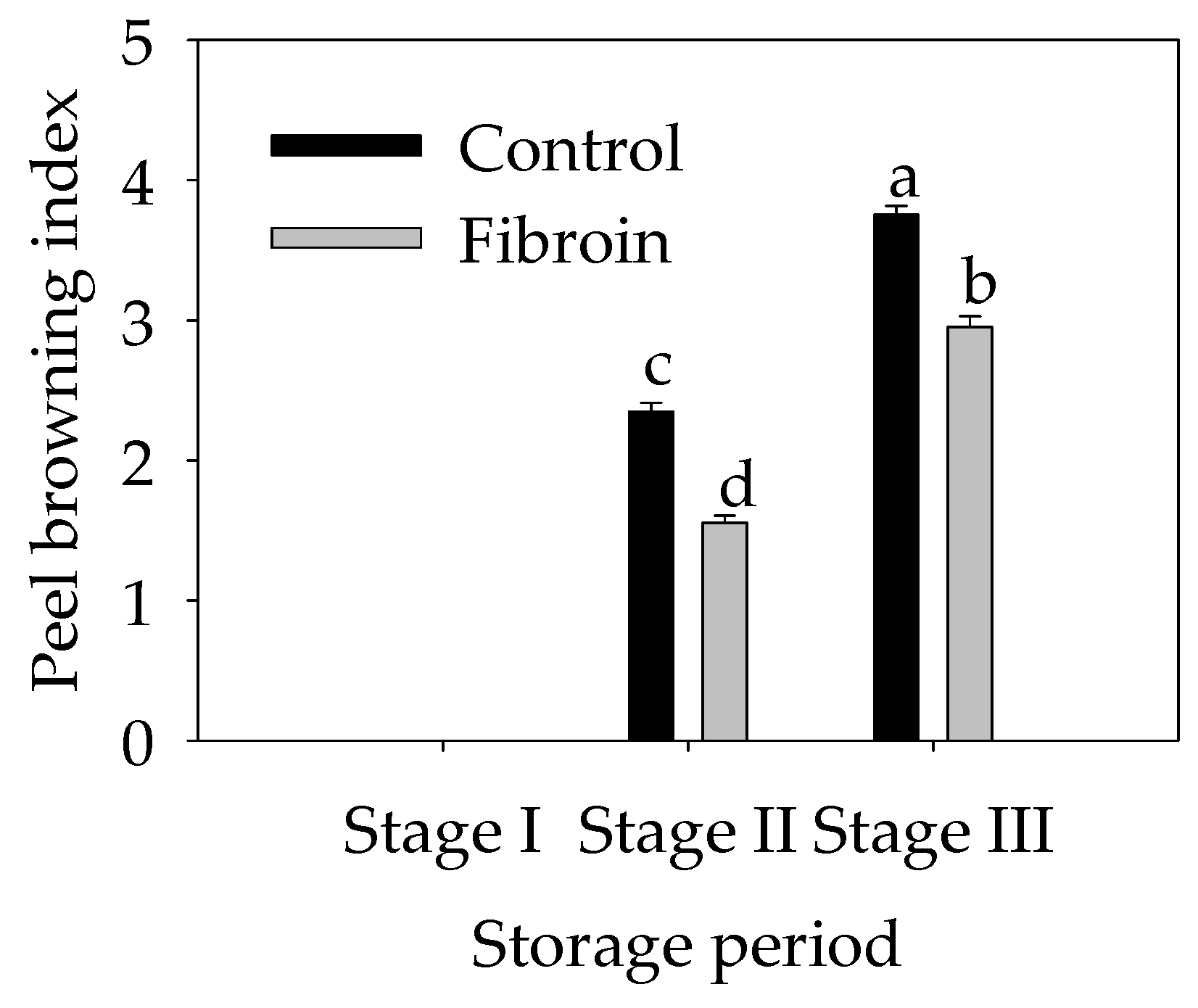
2.1. Changes in the Peel Browning Index of Harvested Banana Fruit during Cold Storage
2.2. Changes in Amino Acid Contents of Harvested Banana Fruit during Cold Storage
2.3. Changes in Primary Metabolites of Harvested Banana Fruit during Cold Storage
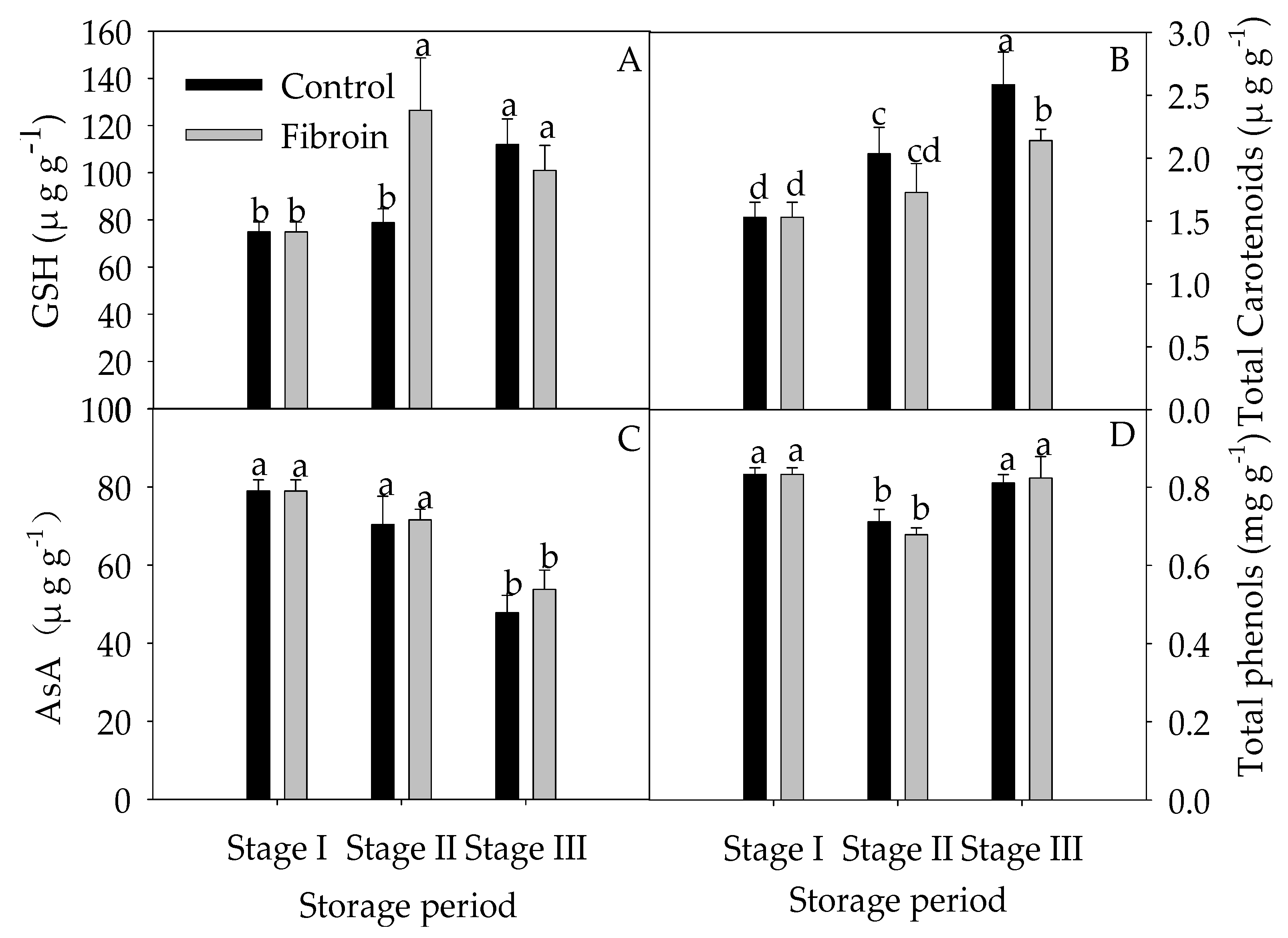
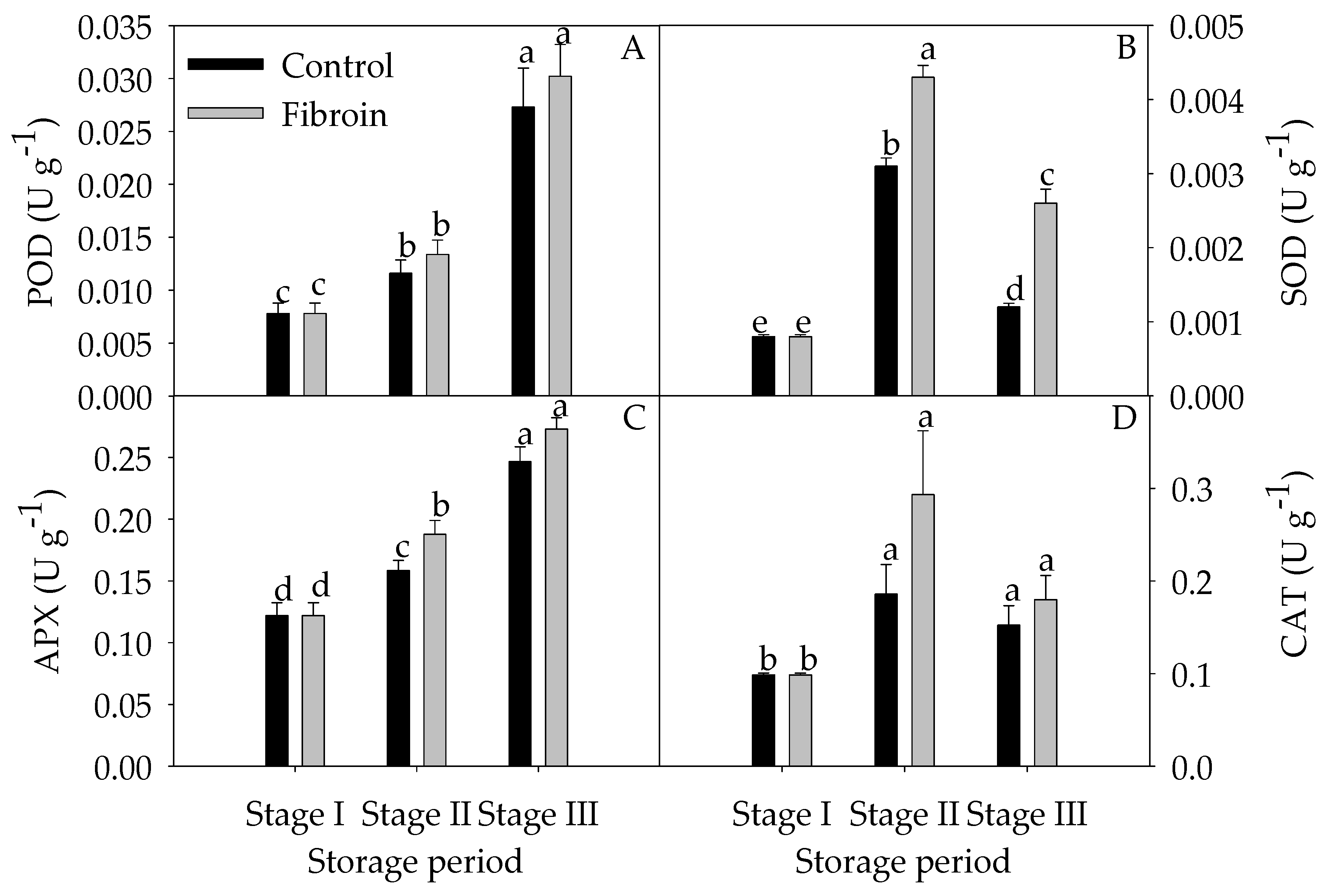
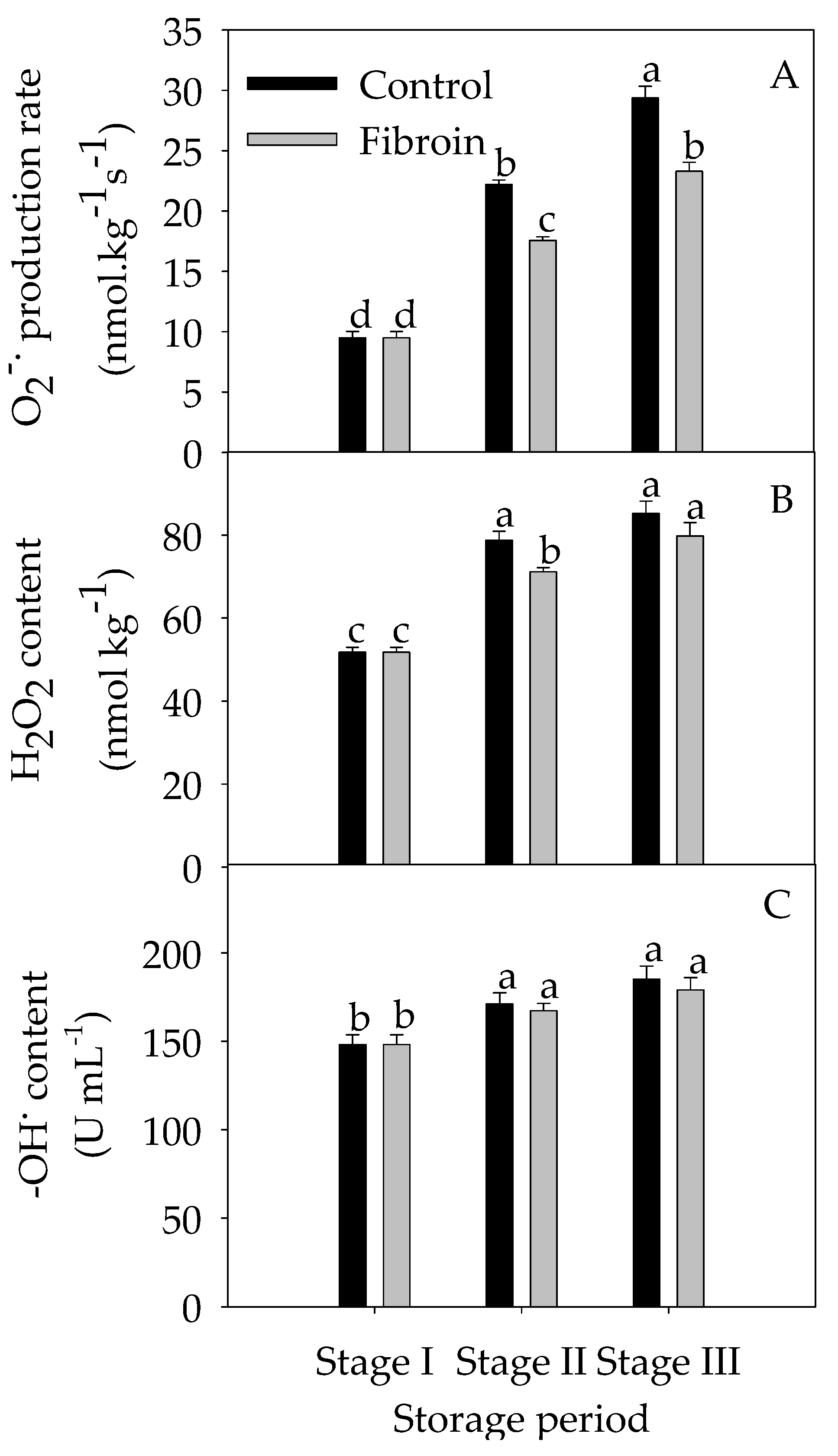
2.4. Changes in Antioxidants, Antioxidant Enzyme Activities, and ROS Levels in Peels of Harvested Banana Fruit during Cold Storage
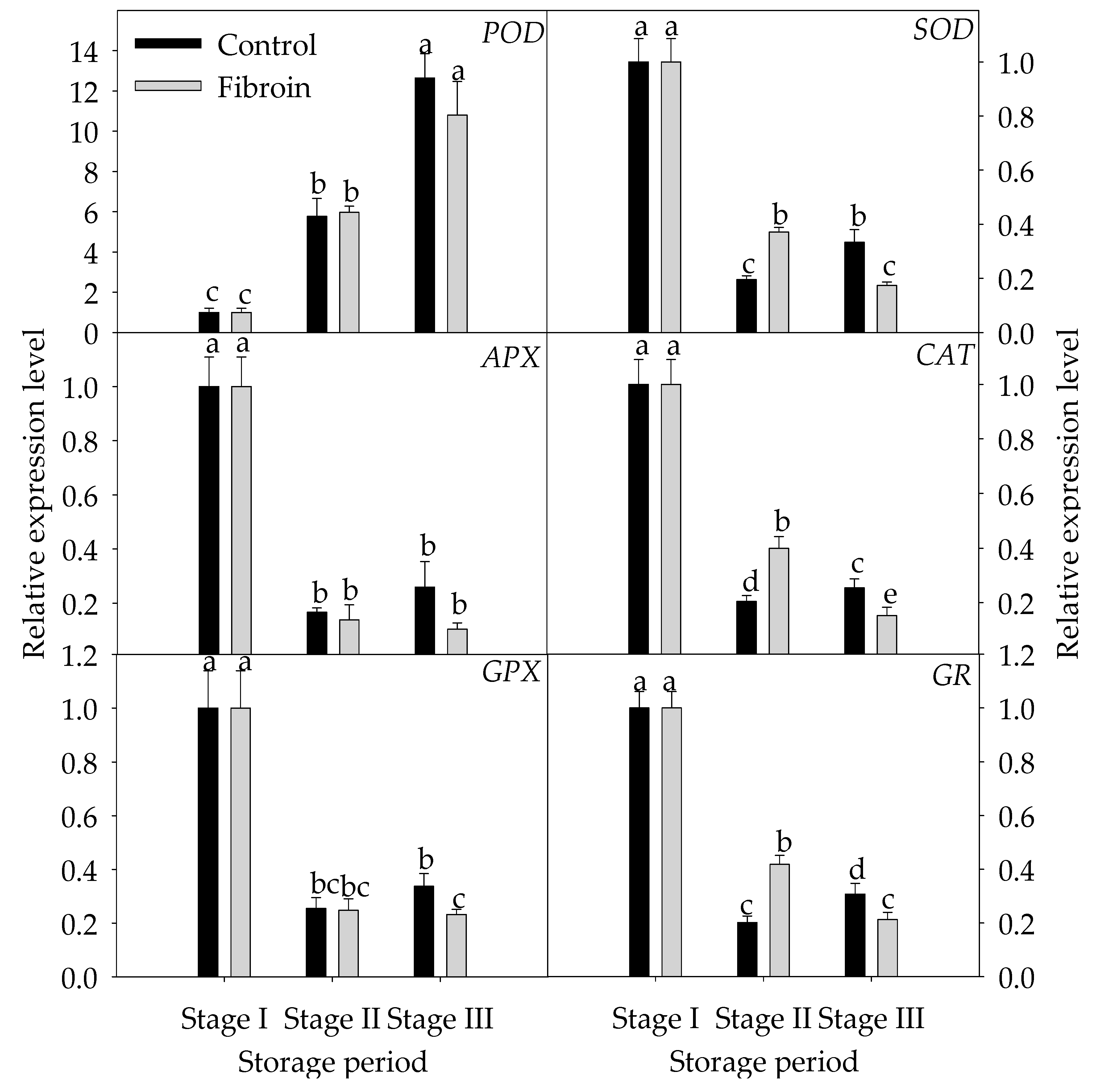
2.5. Changes in Expression Levels of Genes Related to Inhibition Mechanisms of Oxidative Damage and Peroxiredoxins in Peels of Harvested Banana Fruit during Cold Storage
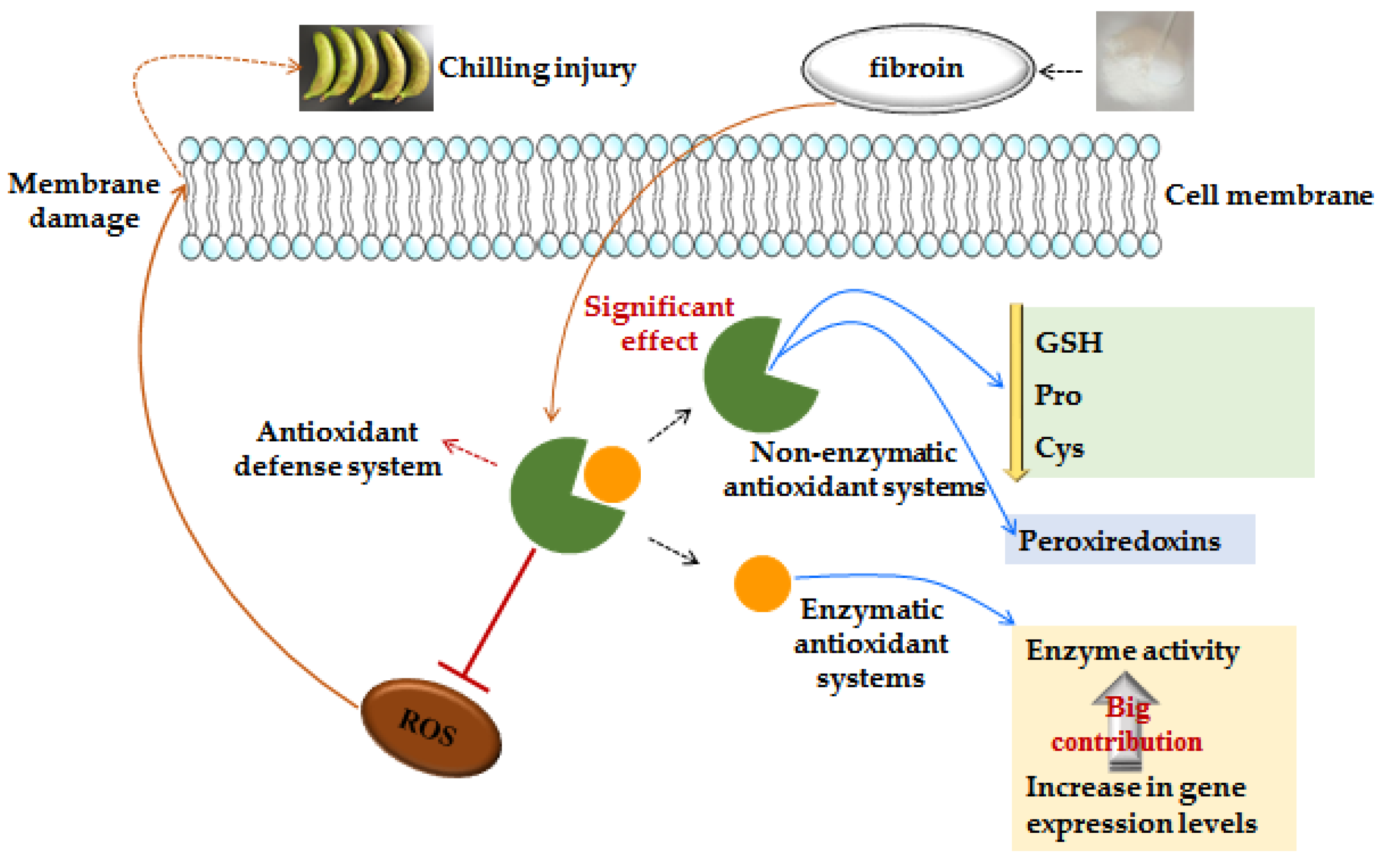
3. Discussion
3.1. Fibroin Treatment Reduced Chilling Injury of Banana Fruit during Cold Storage
3.2. Fibroin Treatment Alleviated Oxidative Damage on Banana Fruit during Cold Storage
3.3. Fibroin Treatment Improved Antioxidant Ability of Banana Fruit during Cold Storage
3.4. Fibroin Treatment Stimulated Repair Capability of Peroxiredoxins of Banana Fruit during Cold Storage
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Evaluation of Peel Browning Index
4.3. Extraction and Analyses of Amino Acid Content
4.4. Extraction and Analyses of Primary Metabolites
4.5. Measurement and Analyses of Glutathione (GSH), Ascorbic Acid (AsA), and Total Phenols
4.6. Determination of Total Carotenoids
4.7. Analyses of Enzyme Activities
4.8. Measurement of ROS
4.9. RNA Isolation and Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Joyce, D.C.; Jiang, W.; Lu, W. Effects of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.H.; Sun, J.Z.; Lin, Y.F.; Lin, Y.X.; Lin, M.S.; Hung, Y.C.; Ritenour, M.A.; Lin, H.T. The changes in metabolisms of membrane lipids and phenolics induced by Phomopsis longanae Chi infection in association with peel browning and disease occurrence of postharvest longan fruit. J. Agr. Food Chem. 2018, 66, 12794–12804. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Lin, Y.X.; Lin, H.T.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.H.; Wang, H. Hydrogen peroxide-induced peel browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxygen stress and superoxide dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Pinegin, B.; Vorobjeva, N.; Pashenkov, M.; Chernyak, B. The role of mitochondrial ROS in antibacterial immunity. J. Cell Physiol. 2018, 233, 3745–3754. [Google Scholar] [CrossRef]
- Hikaru, M.; Yoshinori, I. Effect of different postharvest temperatures on the accumulation of sugars, organic acids, and amino acids in the juice sacs of Satsuma mandarin (Citrus unshiu Marc.) fruit. J. Agr. Food Chem. 2012, 60, 9900–9909. [Google Scholar]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol. 2018, 59, 167–178. [Google Scholar] [CrossRef]
- Moradtalab, N. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Enviro. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, N.; Cerveau, D.; Couturier, J.; Reichheld, J.P.; Rey, P. Involvement of thiol-based mechanisms in plant development. Bba-Gen Subj. 2015, 1850, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. 1999, 50, 571–599. [Google Scholar]
- Yamaguchi Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Brenckle, M.A.; Kaplan, D.L.; Omenetto, F.G. Silk fibroin as edible coating for perishable food preservation. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.J.; Li, T.T.; Yun, Z.; Duan, X.W.; Jiang, Y.M. Fibroin treatment inhibits chilling injury of banana fruit via energy regulation. Sci. Hortic. 2019, 248, 8–13. [Google Scholar] [CrossRef]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F.A. Prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef]
- Gabriel, R.; Stéphane, O.; Mireille, F.; Pierrette, F.L.; Berjeaud, J.M. Cysteine: A multifaceted amino acid involved in signaling, plant resistance and antifungal development. Plant Physiol. Bioch. 2018, 129, 77–89. [Google Scholar]
- Burdon, J.; Lallu, N.; Yearsley, C.; Osman, S.; Billing, D.; Boldingh, H. Postharvest conditioning of Satsuma mandarins for reduction of acidity and skin puffiness. Postharvest Biol. Tec. 2007, 43, 102–114. [Google Scholar] [CrossRef]
- Moellering, E.R.; Bagyalakshmi, M.; Christoph, B. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The free radical theory of aging. Antioxid. Redox Sign. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N.; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M.; et al. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl. Acad. Sci. USA 2016, 113, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, L.; Xu, J.; Xin, P.; Guo, H.; Wu, J.; Bai, L.; Wang, G.; Chu, J.; Zuo, J. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res. 2018, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2016, 22, 11–19. [Google Scholar] [CrossRef]
- Kissoudis, C.; Wiel, C.V.D.; Visser, R.G.F.; Linden, G.V.D. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 1–20. [Google Scholar] [CrossRef]
- Rosalie, R.; Léchaudel, M.; Dhuique-Mayer, C.; Dufossé, L.; Joas, J. Antioxidant and enzymatic responses to oxidative stress induced by cold temperature storage and ripening in mango (Mangifera Indica L. cv. ‘Cogshall’) in Relation to carotenoid content. J. Plant Physiol. 2018, 224–225, 75–85. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van, B.F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2017, 239, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Benítez-González, A.M.; Stinco, C.M.; Meléndez-Martínez, A.J. Antioxidants (carotenoids and phenolics) profile of cherry tomatoes as influenced by deficit irrigation, ripening and cluster. Food Chem. 2017, 240, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y. Effect of temperature preconditioning on catalase, peroxidase, and superoxide dismutase in chilled zucchini squash. Postharvest Biol. Tech. 1995, 5, 67–76. [Google Scholar] [CrossRef]
- Nicolas, N.; Valérie, C.; José, G.; Eric, G.; Masakazu, H.; Pascal, R.; Knaff, D.B.; Emmanuelle, I.; Jean-Pierre, J.; Nicolas, R. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar]
- Passaia, G.; Fonini, L.S.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; Mariath, J.E.D.A.; Margis, R.; Margis-Pinheiro, M. The mitochondrial glutathione peroxidase GPX3 is essential for H2O2 homeostasis and root and shoot development in rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jang, M.J.; Noh, H.Y.; Lee, H.J.; Sukeweenadhi, J.; Kim, J.H.; Kim, S.Y.; Kwon, W.S.; Yang, D.C. Molecular characterization of two glutathione peroxidase genes of Panax ginseng and their expression analysis against environmental stresses. Gene 2014, 535, 33–41. [Google Scholar] [CrossRef]
- Wang, X.; Fang, G.; Yang, J.; Li, Y. A thioredoxin-dependent glutathione peroxidase (OsGPX5) is required for rice normal development and salt stress tolerance. Plant Mol. Biol. Rep. 2017, 35, 1–10. [Google Scholar] [CrossRef]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Vieira, D.S.C. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jao, S.C.; Nanduri, S.; Starke, D.W.; Mieyal, J.J.; Qin, J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry 1998, 37, 17145–17156. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Q.; Zhou, Y.; Yun, Z.; Duan, X.; Jiang, Y. l -Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest Biol. Tec. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Liu, P.; Liu, S.; Luo, T.; Jin, S.; Xu, Q.; Xu, J.; Cheng, Y.; Deng, X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. Bmc Plant Biol. 2013, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Zhong, Y.; Zheng, J.; Ali, M.; Liu, G.D.; Zheng, X.L. L-ascorbic acid metabolism in an ascorbate-rich kiwifruit (Actinidia. Eriantha, Benth.) cv. ‘white’ during postharvest. Plant Physiol. Biochem. 2018, 124, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Q.; Jiang, Y.; Duan, X.; Qu, H. Enhanced chilling tolerance of banana fruit treated with malic acid prior to low-temperature storage. Postharvest Biol. Technol. 2016, 111, 209–213. [Google Scholar]
- Ruenroengklin, N.; Yang, B.; Lin, H.; Chen, F.; Jiang, Y. Degradation of anthocyanin from litchi fruit peel by H2O2 and hydroxyl radical. Food Chem. 2009, 116, 995–998. [Google Scholar] [CrossRef]
- Kuang, J.F.; Chen, J.Y.; Luo, M.; Wu, K.Q.; Sun, W.; Jiang, Y.M.; Lu, W.J. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 2012, 63, 441–454. [Google Scholar] [CrossRef]

| Amino Acid Name | Treatment | Amino Acid Content (mg kg−1) | ||
|---|---|---|---|---|
| Stage I | Stage II | Stage III | ||
| Asp | Control | 95.7 ± 5.0 a | 105.2 ± 4.2 b | 105 ± 2.7 b |
| Fibroin | 95.7 ± 5.0 a | 116.8 ± 6.3 b | 125.5 ± 3.1 c | |
| Thr | Control | 10.1 ± 0.2 a | 8.1 ± 0.7 bc | 7.2 ± 0.8 c |
| Fibroin | 10.1 ± 0.2 a | 8.9 ± 0.3 b | 9.5 ± 1.0 ab | |
| Ser | Control | 37.2 ± 0.5 a | 35.4 ± 2.1 a | 31.3 ± 1.5 b |
| Fibroin | 37.2 ± 0.5 a | 36 ± 1.8 a | 34 ± 2.6 ab | |
| Asn | Control | 145.6 ± 2.5 a | 135.2 ± 5.6 b | 106.3 ± 3.9 e |
| Fibroin | 145.6 ± 2.5 a | 159.4 ± 6.8 c | 189.5 ± 2.4 d | |
| Glu | Control | 51.3 ± 4.3 a | 19.3 ± 1.2 b | 19.6 ± 2.5 b |
| Fibroin | 51.3 ± 4.3 a | 25.4 ± 2.3 c | 20.9 ± 1.1 d | |
| Gly | Control | 18.4 ± 1.6 a | 15.3 ± 1.8 a | 3.4 ± 0.6 b |
| Fibroin | 18.4 ± 1.6 a | 3.6 ± 0.5 b | 3.7 ± 0.7 b | |
| Ala | Control | 22.5 ± 2.0 a | 37.5 ± 1.8 b | 3.2 ± 0.1 c |
| Fibroin | 22.5 ± 2.0 a | 37.9 ± 2.6 b | 40.9 ± 4.6 b | |
| Val | Control | 3.1 ± 0.2 a | 3.2 ± 0.1 a | 1.3 ± 0.1 b |
| Fibroin | 3.1 ± 0.2 a | 3.6 ± 0.3 a | 3.8 ± 0.2 a | |
| Cys | Control | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| Fibroin | 0.0 ± 0.0 a | 2.1 ± 0.1 b | 4.3 ± 0.3 c | |
| Met | Control | 2.3 ± 0.1 a | 1.2 ± 0.2 b | 3.4 ± 0.4 c |
| Fibroin | 2.3 ± 0.1 a | 1.3 ± 0.1 b | 3.7 ± 0.1 c | |
| P-Ser | Control | 10.6 ± 0.3 a | 10.2 ± 1.5 a | 10.1 ± 0.6 a |
| Fibroin | 10.6 ± 0.3 a | 10.3 ± 2.4 a | 11.5 ± 0.3 b | |
| Ile | Control | 2.8 ± 0.1 a | 3.5 ± 0.2 b | 4.3 ± 0.1 c |
| Fibroin | 2.8 ± 0.1 a | 3.7 ± 0.3 b | 2.6 ± 0.2 a | |
| Tyr | Control | 2.0 ± 0.1 a | 3.1 ± 0.3 bc | 2.8 ± 0.5 bc |
| Fibroin | 2.0 ± 0.1 a | 2.7 ± 0.2 b | 3.4 ± 0.3 c | |
| Phe | Control | 2.8 ± 0.1 a | 2.2 ± 0.3 b | 1.3 ± 0.1 c |
| Fibroin | 2.8 ± 0.1 a | 1.5 ± 0.1 c | 2.9 ± 0.2 a | |
| β-Ala | Control | 3.6 ± 0.3 a | 3.2 ± 0.2 a | 3.1 ± 0.3 a |
| Fibroin | 3.6 ± 0.3 a | 3.2 ± 0.3 a | 5.3 ± 0.6b | |
| P-Eta | Control | 3.3 ± 0.2 a | 2.1 ± 0.2b | 3.0 ± 0.1 a |
| Fibroin | 3.3 ± 0.2 a | 3.9 ± 0.3 c | 3.2 ± 0.3 a | |
| GABA | Control | 7.2 ± 0.5 a | 5.6 ± 0.4 b | 6.8 ± 0.4 ab |
| Fibroin | 7.2 ± 0.5 a | 6.4 ± 0.7 ab | 9.7 ± 0.2 c | |
| His | Control | 67 ± 5.3 a | 58 ± 4.1 b | 50 ± 3.7 c |
| Fibroin | 67 ± 5.3 a | 60 ± 3.6 ab | 67 ± 2.9 a | |
| Orn | Control | 3.9 ± 0.5 a | 2.2 ± 0.2 b | 2.0 ± 0.3 b |
| Fibroin | 3.9 ± 0.5 a | 2.2 ± 0.1 b | 2.1 ± 0.1 b | |
| Lys | Control | 4.6 ± 0.2 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| Fibroin | 4.6 ± 0.4 a | 1.0 ± 0.1 c | 2.7 ± 0.3 d | |
| Arg | Control | 6.5 ± 0.6 a | 5.4 ± 0.3 b | 3.3 ± 0.7 d |
| Fibroin | 6.5 ± 0.6 a | 13.2 ± 0.6 c | 6.4 ± 0.2 a | |
| Pro | Control | 0.0 ± 0.0 a | 2.3 ± 0.1 b | 2.5 ± 0.1 b |
| Fibroin | 0.0 ± 0.0 a | 4.7 ± 0.3 c | 7.3 ± 0.3 d | |
| Name | Primary Metabolite Content (µg Ribitol g−1 Fresh Weight (FW)) | |||||
|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | ||||
| Control | Fibroin | Control | Fibroin | Control | Fibroin | |
| Amino Acids | ||||||
| l-Norvaline | 3.3 ± 0.7 a | 3.3 ± 0.7 a | 1.9 ± 0.1 bc | 1.6 ± 0.2 c | 1.5 ± 0.07 d | 2.1 ± 0.1 b |
| l-Serine | 11.3 ± 2.4 a | 11.3 ± 2.4 a | 7.2 ± 0.3 b | 6.8 ± 0.7 b | 5.9 ± 0.7 b | 10.8 ± 1.3 c |
| l-Threonine | 4.8 ± 0.3 a | 4.8 ± 0.3 a | 2.3 ± 0.1 b | 1.9 ± 0.3 b | 1.8 ± 0.2 b | 2.1 ± 0.5 b |
| Glycine | 3.3 ± 0.5 c | 3.3 ± 0.5 c | 11.3 ± 0.1 a | 9.5 ± 0.4 b | 9.4 ± 0.2 b | 10.8 ± 1.3 a |
| l-Proline | 62.5 ± 9.4 a | 62.5 ± 9.4 a | 12.1 ± 0.2 b | 9.7 ± 0.5 b | 9.7 ± 0.3 b | 10.0 ± 0.5 b |
| l-Aspartic acid | 58.5 ± 17.8 a | 58.5 ± 17.8 a | 32.2 ± 1.0 c | 26.4 ± 1.2 d | 22.3 ± 1.0 e | 34.6 ± 2.2 b |
| l-Asparagine | 1.8 ± 0.6 e | 1.8 ± 0.6 e | 7.3 ± 0.1 b | 5.9 ± 0.1 c | 3.6 ± 0.2 d | 9.3 ± 0.2 a |
| l-Valine | 106.0 ± 17.8 a | 106.0 ± 17.8 a | 3.8 ± 0.2 d | 5.0 ± 0.1 c | 3.4 ± 0.2 d | 6.4 ± 2.2 b |
| Sugars | ||||||
| Glucopyranose | 17.3 ± 4.7 a | 17.3 ± 4.7 a | 11.4 ± 0.7 a | 5.8 ± 1.7 c | 8.2 ± 0.3 c | 14.8 ± 1.0 b |
| α-d-Glucopyranoside | 1510.0 ± 334.1 a | 1510.0 ± 334.1 a | 786.2 ± 14.8 b | 660.5 ± 66.2 c | 801.8 ± 25.9 b | 817.9 ± 30.3 b |
| 2-Keto-d-gluconic acid | 11.7 ± 0.9 a | 11.7 ± 0.9 a | 4.8 ± 0.5 c | 2.6 ± 0.8 d | 3.4 ± 0.2 d | 6.1 ± 0.4 b |
| dtABLE-Glucuronic acid | 0.9 ± 0.6 d | 0.9 ± 0.6 d | 4.2 ± 0.2 a | 2.2 ± 0.6 c | 1.8 ± 0.2 c | 3.5 ± 0.3 b |
| Organic acids | ||||||
| Propanoic acid | 77.1 ± 5.3 a | 77.1 ± 5.3 a | 7.1 ± 0.7 b | 4.7 ± 0.8 b | 6.5 ± 0.6 b | 4.9 ± 2.2 b |
| Citric acid | 125.7 ± 12.0 a | 125.7 ± 12.0 a | 99.3 ± 1.1 b | 86.7 ± 12.0 b | 71.8 ± 0.8 c | 125.2 ± 3.7 d |
| Malic acid | 108.4 ± 26.2 a | 108.4 ± 26.2 a | 73.9 ± 2.3 c | 62.1 ± 7.6 c | 73.6 ± 1.8 c | 95.3 ± 3.1 b |
| 6-Hydroxy-2-aminohexanoic acid | 31.8 ± 1.7 a | 31.8 ± 1.7 a | 11.5 ± 0.3 b | 8.3 ± 1.6 c | 5.6 ± 0.4 d | 9.6 ± 1.2 c |
| 3,5-Dimethoxymandelic acid | 16.9 ± 0.8 a | 16.9 ± 0.8 a | 4.3 ± 0.1 b | 5.7 ± 1.5 b | 4.2 ± 0.5 b | 3.6 ± 0.7 b |
| Ethanedioic acid | 1101.4 ± 260.7 a | 1101.4 ± 260.7 a | 641.6 ± 13.7 c | 625.5 ± 57.5 c | 503.0 ± 19.4 d | 740.9 ± 20.0 b |
| Lipids | ||||||
| Hexadecanoic acid | 98.4 ± 3.3 a | 98.4 ± 3.3 a | 41.6 ± 6.6 b | 41.8 ± 11.4 bc | 57.8 ± 4.0 c | 29.2 ± 3.8 d |
| Octadecanoic acid | 60.8 ± 18.9 a | 60.8 ± 18.9 a | 19.7 ± 3.6 b | 22.2 ± 6.8 bc | 30.7 ± 3.0 c | 14.0 ± 1.2 d |
| Alcohols | ||||||
| 2,5-Monoformal-l-rhamnitol | 3.2 ± 0.7 d | 3.2 ± 0.7 d | 5.3 ± 0.09 b | 4.6 ± 0.4 c | 6.3 ± 0.4 a | 5.5 ± 0.2 b |
| 3,5-Dimethoxymandelic acid | 16.9 ± 0.9 d | 16.9 ± 0.9 d | 4.3 ± 0.1 b | 5.7 ± 1.5 c | 4.4 ± 0.5 a | 3.6 ± 0.7 c |
| Others | ||||||
| Acetamide | 48.1 ± 2.4 a | 48.1 ± 2.4 a | 29.4 ± 0.5 c | 30.8 ± 1.2 c | 43.0 ± 3.2 b | 30.9 ± 0.4 c |
| Monoethanolamine | 10.0 ± 1.6 a | 10.0 ± 1.6 a | 5.0 ± 0.05 d | 6.5 ± 0.4 b | 6.9 ± 0.5 b | 5.4 ± 0.3 c |
| Tetradecane | 0.9 ± 0.2 d | 0.9 ± 0.2 d | 5.3 ± 0.05 b | 6.7 ± 1.5 a | 7.0 ± 1.4 a | 5.1 ± 0.2 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, F.; Liang, L.; Jiang, Y.; Chen, J. Fibroin Delays Chilling Injury of Postharvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage. Metabolites 2019, 9, 152. https://doi.org/10.3390/metabo9070152
Liu J, Li F, Liang L, Jiang Y, Chen J. Fibroin Delays Chilling Injury of Postharvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage. Metabolites. 2019; 9(7):152. https://doi.org/10.3390/metabo9070152
Chicago/Turabian StyleLiu, Juan, Fengjun Li, Lei Liang, Yueming Jiang, and Junjia Chen. 2019. "Fibroin Delays Chilling Injury of Postharvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage" Metabolites 9, no. 7: 152. https://doi.org/10.3390/metabo9070152
APA StyleLiu, J., Li, F., Liang, L., Jiang, Y., & Chen, J. (2019). Fibroin Delays Chilling Injury of Postharvest Banana Fruit via Enhanced Antioxidant Capability during Cold Storage. Metabolites, 9(7), 152. https://doi.org/10.3390/metabo9070152







