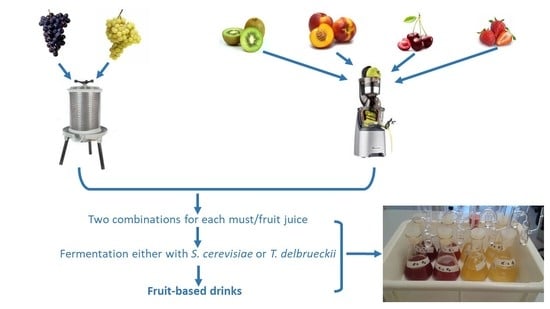
Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice
Abstract
1. Introduction
2. Results and Discussion
2.1. Flask Trials
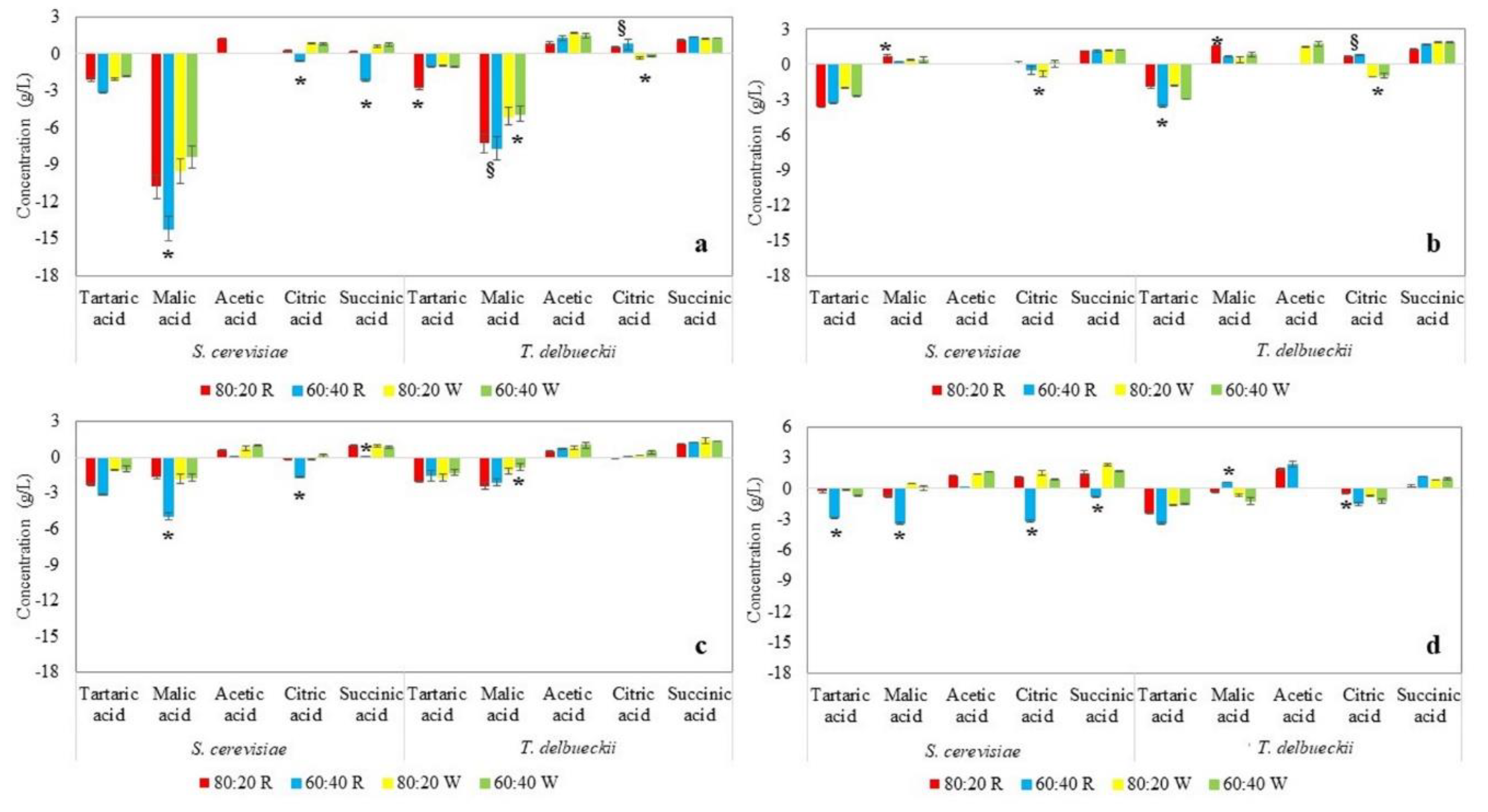
2.1.1. Fermentation Trends and Chemical Composition
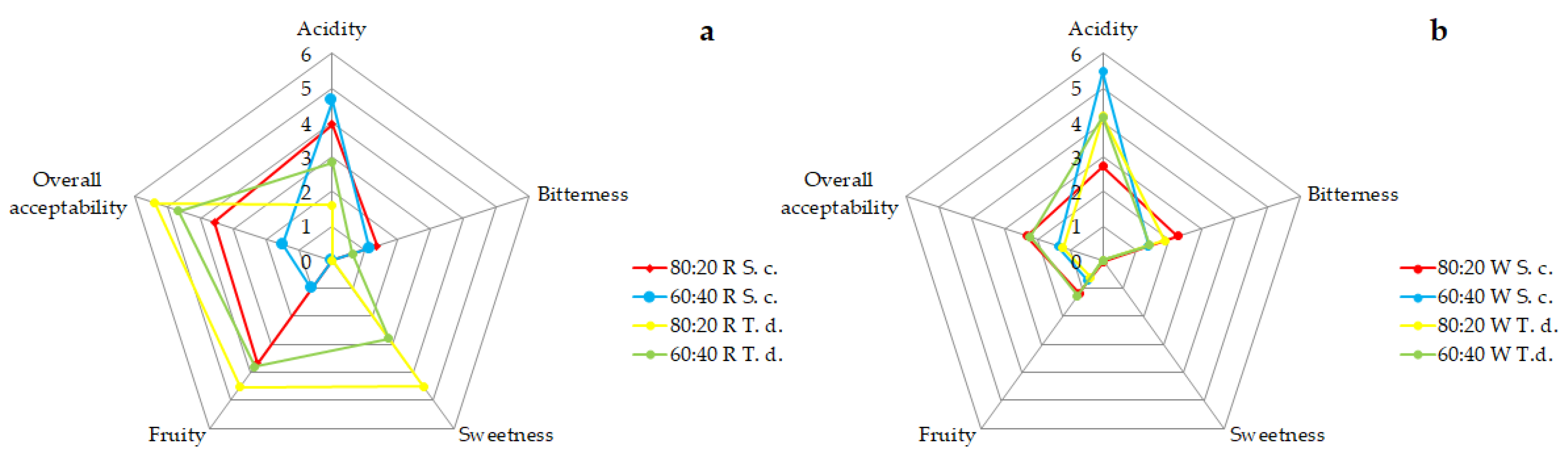
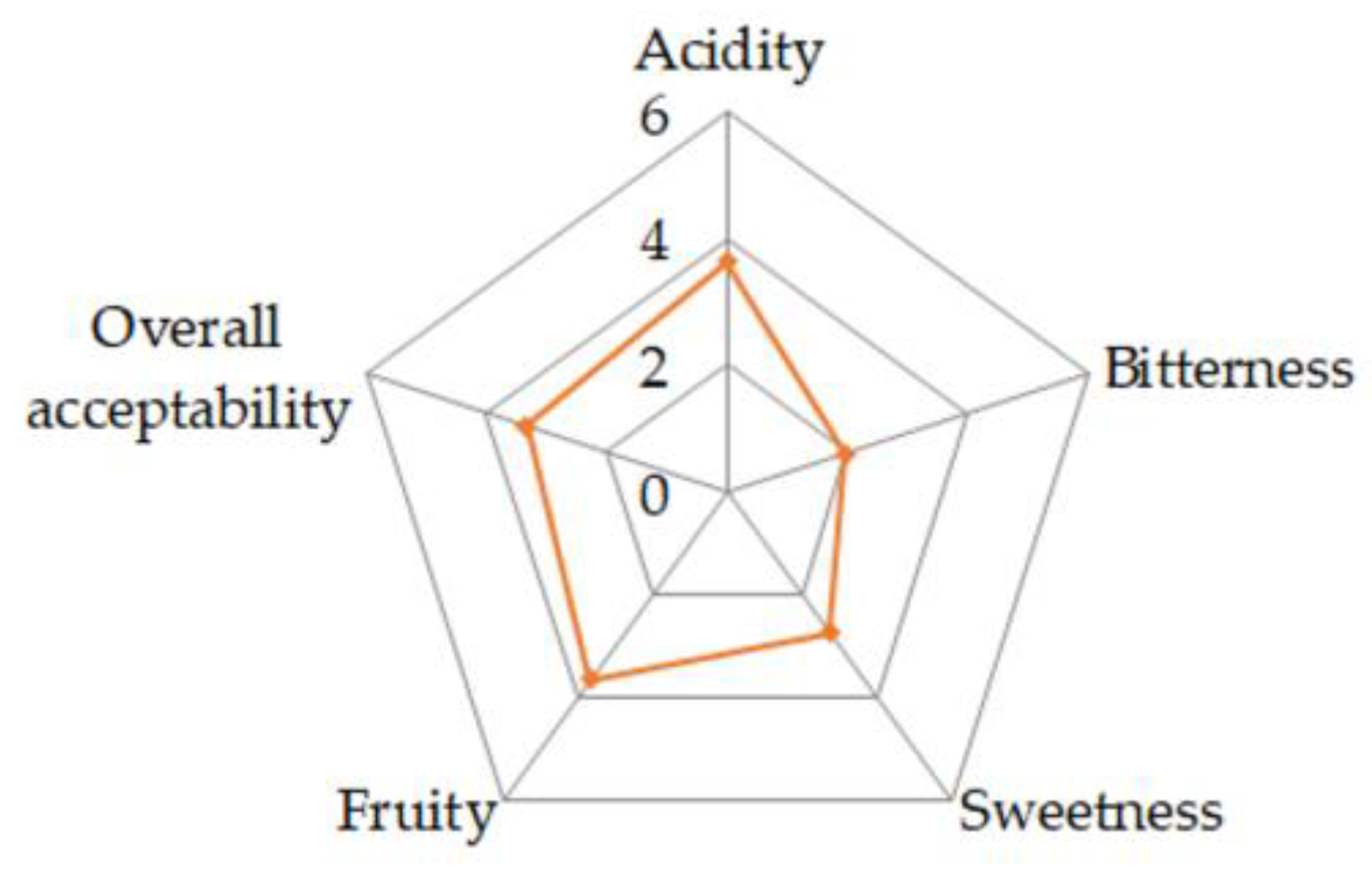
2.1.2. Sensory Analysis
2.2. Batch Experiment
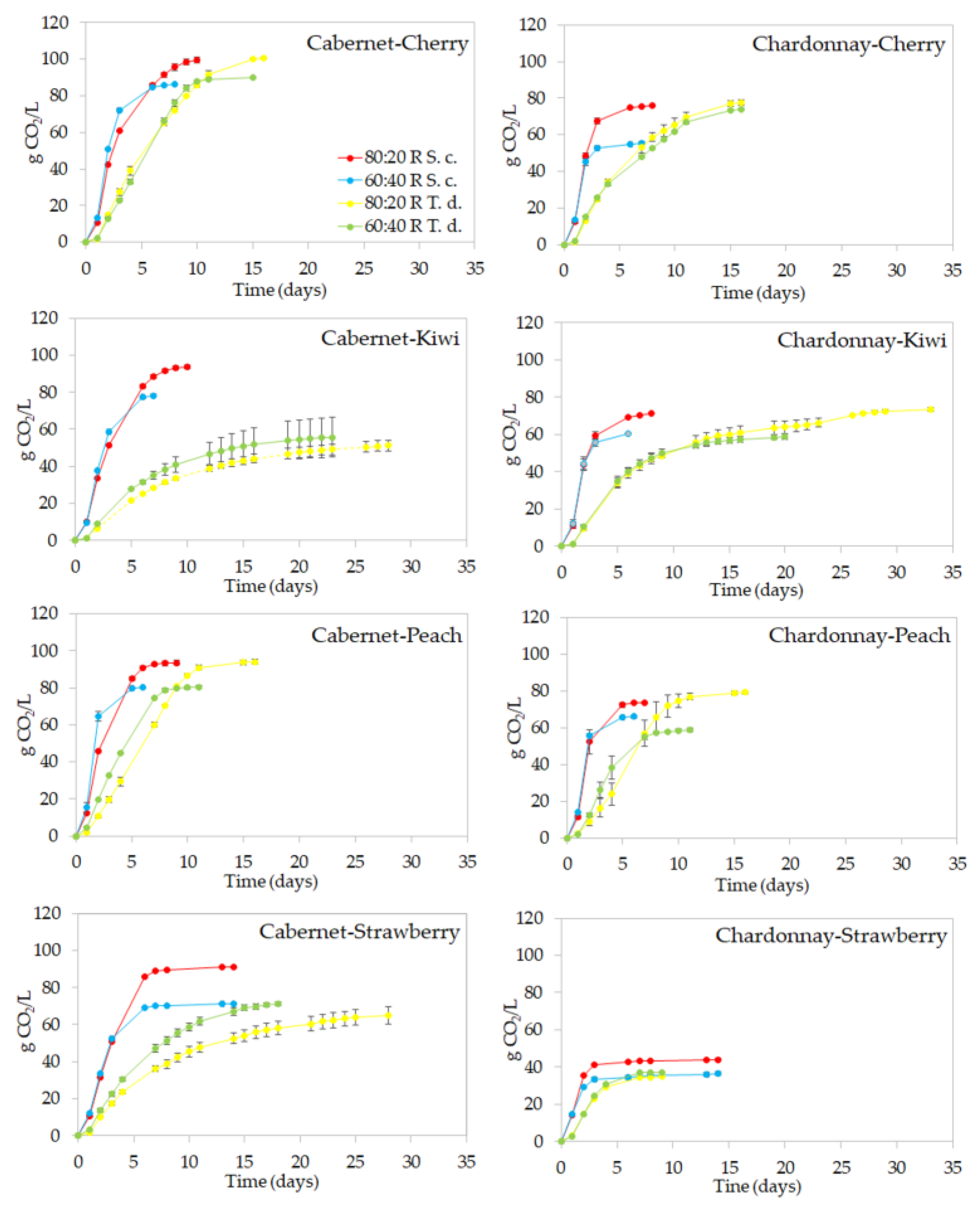
2.2.1. Alcoholic Fermentation Trend
2.2.2. Aroma Profile
2.3. Microvinification
2.3.1. Fermentation Trend and Chemical Composition
2.3.2. Chemical Composition of Kiwi-Based Drink
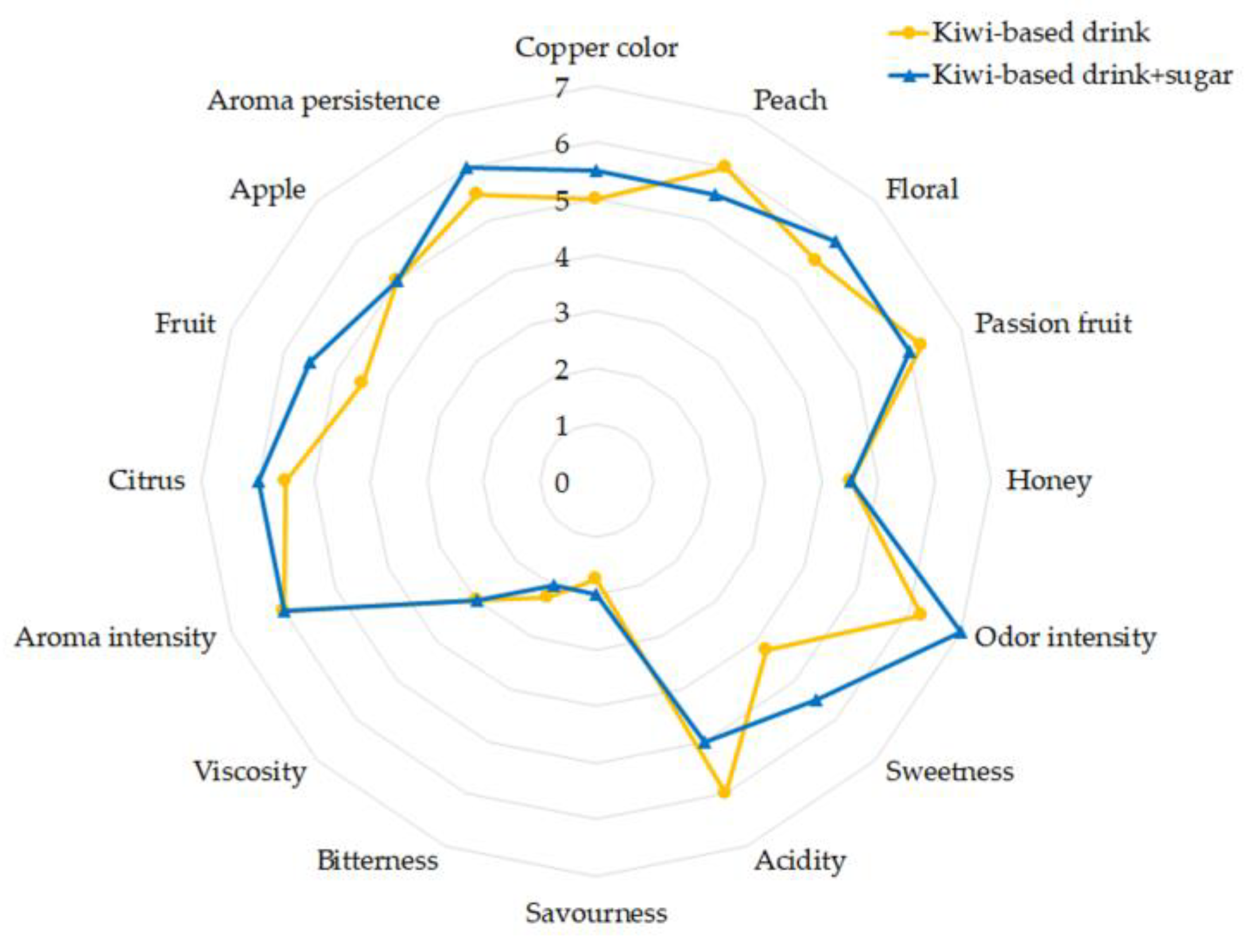
2.3.3. Sensory Analysis
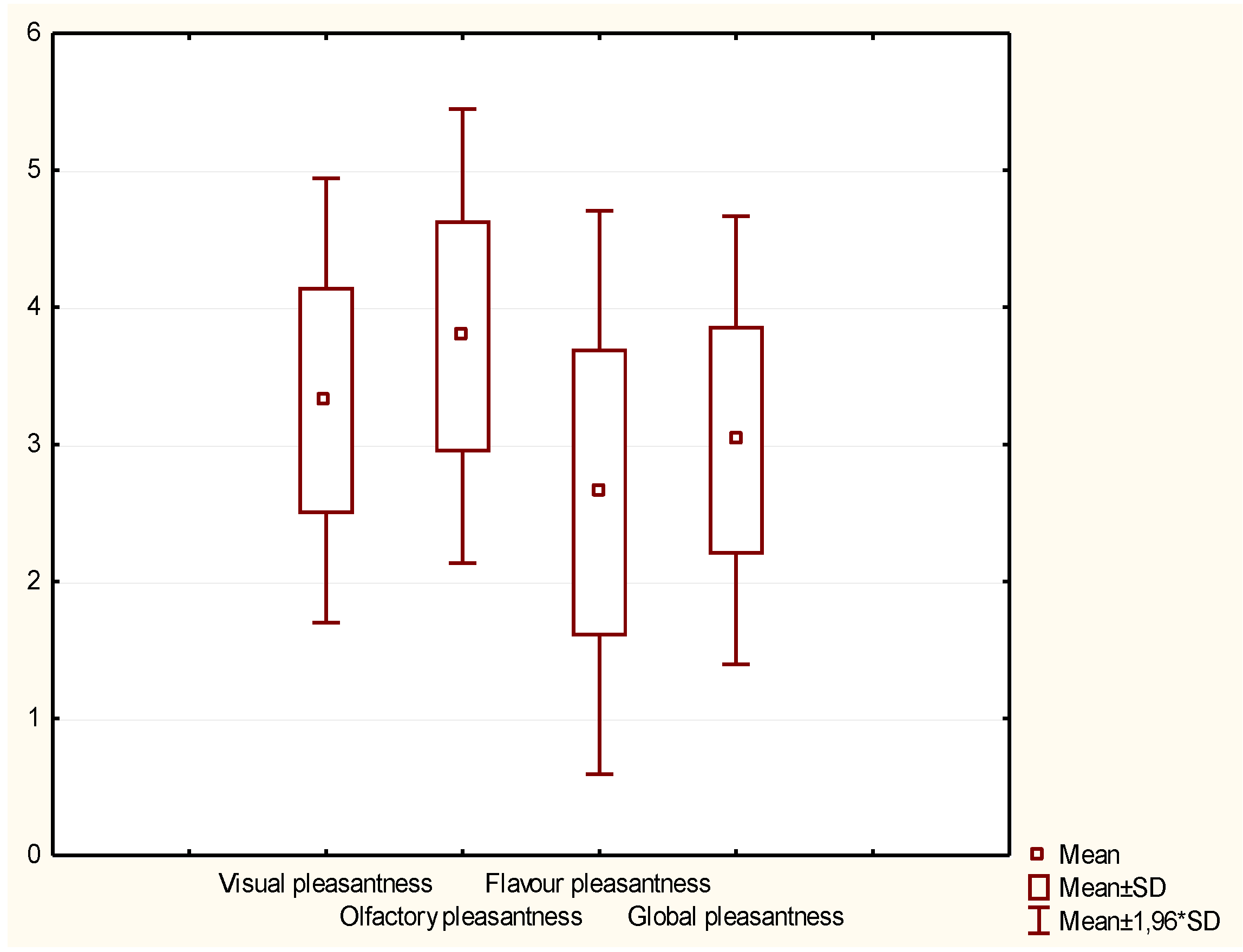
2.3.4. Consumer Acceptability
3. Materials and Methods
3.1. Preparation of Fruit Juices and Musts
3.2. Yeast Strains
3.3. Fermentation Trials
3.4. Microbial and Chemical Analysis
3.5. Sensory Analysis and Acceptability of Grape/Fruit-Based Drinks
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Fruit-Based Drink | Chemical Characteristic of the Final Product |
|---|---|
| Cherry-based drinks (Table S2) | Residual sugars: <1.0 ± 0.0 g/L in all the combinations. Ethanol: It was the highest compared to the others investigated combinations, reaching the maximum in combination with Cabernet Sauvignon 80:20 (12.7 ± 0.1% (v/v) for S. cerevisiae and 12.3 ± 0.7% (v/v) for T. delbrueckii). pH: 3.27 ± 0.02 to 3.54 ± 0.00. Total acidity: Higher in beverages produced with T. delbrueckii. Organic acids: Malic acid decreased during the alcoholic fermentation, although it was the major organic acid in the drinks produced. Acetic acid was found only in the drinks fermented with T. delbrueckii (maximum 1.8 ± 0.0 g/L in the 80:20 mix with Cabernet Sauvignon). |
| Kiwi-based drinks (Table 1) | Residual sugars: Higher in the mixes Cabernet Sauvignon fermented by T. delbrueckii (71 ± 2 g/L and 52 ± 17 g/L for 80:20 and 60:40 combinations, respectively). Ethanol: 6.5 ± 0.3% (v/v) and 7.0 ± 1.3% (v/v) for mixes 80:20 and 60:40, respectively, fermented by T. delbrueckii in comparison to the respective fermented drinks with S. cerevisiae (10.8 ± 0.5% (v/v) for 80:20 and 9.6 ± 0.3% (v/v) for 60:40. pH: Slightly higher in drinks fermented with T. delbrueckii (3.13 ± 0.08 to 3.50 ± 0.01) in the mix 60:40 with Chardonnay must. Total acidity: 11.5 ± 0.7 to 16.3 ± 0.3 g tartaric acid/L in the tests with T. delbrueckii. Organic acids: The most abundant was citric acid, deriving from kiwi juice. Succinic acid was detected at the end of fermentation, while acetic acid was found only in drinks based on Chardonnay must and fermented by T. delbrueckii. |
| Peach-based drinks (Table S3) | Residual sugars: <1.0 ± 0.0 g/L in all the combinations. Ethanol: Higher in the 80:20 combinations for both musts and yeasts used. pH: Slightly higher in drinks fermented with T. delbrueckii (3.30 ± 0.01 to 3.72 ± 0.01) in the mix 60:40 with Chardonnay must. Total acidity: Slight differences between drinks fermented with S. cerevisiae and T. delbrueckii. Organic acids: Both tartaric and malic acids decreased during the fermentation. The drinks contained succinic acid (highest amount 1.40 ± 0.23 g/L), as well as acetic acid (highest amount 0.98 ± 0.03 g/L). |
| Strawberry-based drinks (Table S4) | Residual sugars: <2.5 ± 1.5 g/L, except for the trial with Cabernet Sauvignon must at the proportion 80:20 fermented by T. delbrueckii in which the residual sugar was 44 ± 5 g/L. Ethanol: From 4.4 ± 1.0% (v/v) in the trial with Chardonnay must at the proportion 60:40 fermented by T. delbrueckii to 9.9 ± 0.9% (v/v) in the test with Cabernet Sauvignon must at the proportion 80:20 fermented by S. cerevisiae. The higher concentrations were detected in drinks obtained from mixes with Cabernet Sauvignon, attributable to the higher sugar content. pH: No significant differences among the different combinations must/strawberry juice (range 2.97 ± 0.00 to 3.22 ± 0.02). Total acidity: Higher in the fermented mixes with T. delbrueckii. Organic acids: A significant decrease in tartaric acid occurred. Acetic acid was in the range 1.3 ± 0.0 g/L to 2.4 ± 0.3 g/L, except for the drinks based on Chardonnay must and fermented with T. delbrueckii. |
References
- McGovern, P.E.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Wines from fruits other than grapes: Current status and future prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- State of the Viticulture World Market. April 2018. Available online: http://www.oiv.int/public/medias/5958/oiv-state-of-the-vitiviniculture-world-market-april-2018.pdf (accessed on 19 March 2019).
- Swami, S.B.; Thakor, N.J.; Divate, A.D. Fruit Wine Production: A Review. J. Food Res. Technol. 2014, 2, 93–100. [Google Scholar]
- Kosseva, M.R.; Joshi, V.K.; Panesar, P.S. Science and Technology of Fruit Wine Production, 2nd ed.; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Elsevier: London, UK, 2016. [Google Scholar]
- Duarte, F.W.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Coelho, E.; Vilanova, M.; Genisheva, A.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT Food Sci. Technol. 2015, 62, 1043–1052. [Google Scholar] [CrossRef]
- Lipinski, B.; Hanson, C.; Lomax, J. Reducing food loss and waste. In Installment of “Creating a Sustainable Food Future”; Working Paper; World Resources Institute: Washington, DC, USA, 2013; Available online: http://www.worldresourcesreport.org (accessed on 20 March 2019).
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity: A review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- McMahon, K.M.; Diako, C.; Aplin, J.; Mattinson, D.S.; Culver, C.; Ross, C.F. Trained and consumer panel evaluation of sparkling wines sweetened to brut or demi sec residual sugar levels with three different sugars. Food Res. Int. 2017, 99, 173–185. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, J.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. The Microbiology of Wine and Vinifications, Volume 2, 3rd ed.; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Vigentini, I.; Maghradze, D.; Petrozziello, M.; Bonello, F.; Mezzapelle, V.; Valdetara, F.; Failla, O.; Foschino, R. Indigenous Georgian wine-associated yeasts and grape cultivars to edit the wine quality in a precision oenology perspective. Front. Microbiol. 2016, 7, 352. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Ferreira, V.; Hernández, P.; Cacho, J.F. Identification of impact odorants of young red wines made with Merlot, Cabernet Sauvignon and Grenache grape varieties: A comparative study. J. Sci. Food Agric. 1999, 79, 1461–1467. [Google Scholar] [CrossRef]
- Jordán, M.J.; Margaría, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Farina, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Gamero, A.; Ferreira, V.; Pretorius, I.S.; Querol, A. Wine, beer and cider: Unrevelling the aroma profile. In Molecular Mechanisms in Yeast Carbon Metabolism; Piskur, J., Compagno, C., Eds.; Springer: Berlin/Hidelberg, Germany, 2014; pp. 261–297. [Google Scholar]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cersosimo, M.; Loscos, N.; Cacho, J.; Garcia-Moruno, E.; Ferreira, V. The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem. 2008, 107, 1064–1077. [Google Scholar] [CrossRef]
- European Community. Regulation (EC) No 110/2008 of the European Parliament and of the council on the definition, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No. 1576/89. Off. J. Eur. Union 2008, L 39/16, 16–54. [Google Scholar]
- Resolution Oeno 551-2015. Compendium of international methods of analysis—OIV. Method OIV-MA-AS313-01 for the determination of total acidity. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 29 April 2019).
- Cavaglioni, A.; Ferrari, S. Confronto tra alcuni metodi per la determinazione dell’azoto prontamente assimilabile. Vignevini 2002, 11, 119–123. [Google Scholar]
- Falqué López, E.; Fernández Gómez, E. Simultaneous determination of the major organic acids, sugars, glycerol, and ethanol by HPLC in grape musts and white wines. J. Chromatogr. Sci. 1996, 34, 254–257. [Google Scholar] [CrossRef]
- Fracassetti, D.; Gabrielli, M.; Corona, O.; Tirelli, A. Characterisation of Vernaccia Nera (Vitis vinifera L.) grapes and wine. S. Afr. J. Enol. Vitic. 2017, 38, 72–81. [Google Scholar]
- ISO 11035:1994. Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach; ISO: Geneva, Switzerland, 1994. [Google Scholar]
| Must | Proportion * | Sugar (g/L) | Ethanol (v/v) | Total Acidity (g Tartaric Acid/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Fermenting Yeast | S. c. | T. d. | S. c. | T. d. | S. c. | T. d. | ||
| Cabernet Sauvignon | 80:20 | T0 | 180 ± 17 | 196 ± 18 | - | - | 7.8 ± 0.3 | 7.5 ± 0.3 |
| EF | 0.80 ± 0.0 | 71 ± 2 | 10.8 ± 0.5 | 6.5 ± 0.3 | 8.6 ± 0.7 | 11.5 ± 0.7 | ||
| 60:40 | T0 | 153 ± 14 | 169 ± 16 | - | - | 9.5 ± 0.4 | 10.1 ± 0.4 | |
| EF | 0.10 ± 0.0 | 52 ± 17 | 9.6 ± 0.3 | 7.0 ± 1.3 | 11.0 ± 0.2 | 15.0 ± 0.5 | ||
| Chardonnay | 80:20 | T0 | 147 ± 14 | 160 ± 15 | - | - | 9.3 ± 0.4 | 9.2 ± 0.4 |
| EF | 0.15 ±0.07 | 0.33 ± 0.00 | 8.7 ± 0.2 | 9.1 ± 0.1 | 10.2 ± 0.4 | 13.9 ± 0.5 | ||
| 60:40 | T0 | 127 ± 12 | 135 ± 13 | - | - | 11.1 ± 0.4 | 11.4 ± 0.5 | |
| EF | 0.05 ± 0.02 | 3.2 ± 2.6 | 7.6 ± 0.1 | 7.4 ± 0.3 | 12.2 ± 0.2 | 16.3 ± 0.3 | ||
| Chemical Parameter | Must/Kiwi Juice | Kiwi-Based Drink |
|---|---|---|
| Sugar (g/L) | 190.1 ± 8.0 | 31.1 ± 8.8 |
| Ethanol (v/v) | - | 7.6 ± 0.4 |
| pH | 3.2 ± 0.0 | 3.2 ± 0.1 |
| Total acidity (g tartaric acid/L) | 10.2 ± 0.3 | 14.9 ± 1.4 |
| Tartaric acid (g/L) | 1.70 ± 0.06 | 1.66 ± 0.05 |
| Malic acid (g/L) | 2.58 ± 0.39 | 3.00 ± 0.22 |
| Lactic acid (g/L) | n.d. | n.d. |
| Acetic acid (g/L) | n.d. | 0.21 ± 0.06 |
| Citric acid (g/L) | 6.03 ± 0.22 | 5.86 ± 0.14 |
| Succinic acid (g/L) | n.d. | 1.83 ± 0.11 |
| Compound | Perception Threshold (μg/L) | Descriptor | Cabernet Sauvignon Must/Kiwi Juice | Days of Fermentation | ||
|---|---|---|---|---|---|---|
| 3 | 11 | 17 * | ||||
| Acids | ||||||
| Isobutyric acid | 2300 c | Rancid, butter, cheese | 4.12 ± 1.04 | 89.77 ± 78.06 | n.d. | n.d. |
| Isopentanoic acid | 33 e | Sweat, rancid | n.d. | 100.72 ± 95.63 | 35.92 ± 2.95 | 36.41 ± 6.74 |
| Pentanoic acid | 17 d | Sweat | n.d. | 3.84 ± 0.94 | 3.00 ± 1.25 | 2.00 ± 0.59 |
| Hexanoic acid | 420 c | Sweat | 32.02 ± 11.50 | 603.73 ± 57.96 | 465.00 ± 51.79 | 388.51 ± 33.64 |
| trans-2-Hexenoic acid | - | Must, fat | 25.31 ± 8.52 | 42.69 ± 10.35 | 35.55 ± 5.43 | 27.84 ± 2.18 |
| Octanoic acid | 500 c | Cheese, sweat | 13.13 ± 0.87 | 563.38 ± 15.85 | 479.39 ± 43.96 | 455.36 ± 29.24 |
| Decanoic acid | 1000 d | Rancid, fat | n.d. | 319.58 ± 32.81 | 155.78 ± 41.90 | 112.86 ± 54.34 |
| 9-Decenoic acid | 2 d | Fat | n.d. | 112.98 ± 64.23 | 79.20 ± 14.41 | 71.54 ± 33.18 |
| 2-Methylbutanoic acid | 33 d | Cheese, sweat | n.d. | 130.16 ± 21.04 | 125.10 ± 16.46 | 89.07 ± 21.52 |
| 2-Butenoic acid | - | Milky | 52.51 ± 9.48 | 72.45 ± 8.05 | 76.33 ± 22.06 | 60.12 ± 8.34 |
| Total | 127.09 ± 31.41 | 2039.31 ± 384.93 | 1455.26 ± 200.20 | 1243.71 ± 189.78 | ||
| Alcohols | ||||||
| Isobutanol | 40,000 d | Wine, solvent, bitter | 60.27 ± 9.53 | 138.66 ± 17.84 | 94.70 ± 18.77 | 76.05 ± 7.76 |
| 3-Penten-2-ol | - | Green, vinyl | 7.70 ± 1.42 | 32.98 ± 15.11 | 32.91 ± 7.54 | 27.04 ± 6.73 |
| 1-Pentanol | - | Balsamic | n.d. | 10.62 ± 1.70 | 6.72 ± 1.80 | 5.83 ± 0.96 |
| 1-Hexanol | 1110 g | Resin, flower, green | 48.55 ± 15.96 | 396.08 ± 70.77 | 328.74 ± 23.61 | 351.68 ± 56.33 |
| 2-Hexanol | - | Resin, flower, green | 20.77 ± 5.06 | 29.54 ± 3.40 | 26.19 ± 4.83 | 24.54 ± 0.64 |
| 3-Ethoxy-1-propanol | - | Fruit | n.d. | 184.45 ± 28.60 | 169.08 ± 4.77 | 152.96 ± 19.25 |
| cis-3-Hexen-1-ol | 400 e | Grass | 29.70 ± 10.07 | 35.87 ± 2.94 | 25.83 ± 0.50 | 20.34 ± 2.47 |
| 2-Ethyl-1-decanol | - | Fat | 25.65 ± 4.03 | 20.32 ± 14.26 | 2.21 ± 0.48 | 2.29 ± 0.21 |
| 4-Hepten-1-ol | - | Green, grassy odor | n.d. | 4.66 ± 4.47 | 4.54 ± 2.23 | 5.25 ± 0.92 |
| Isoamyl alcohol | 30,000 d | Spirit, alcoholic | 52.30 ± 6.27 | 16569 ± 1348 | 12909 ± 860 | 11224 ± 953 |
| 2-Methyl-4-octanol | - | Cucumber | n.d. | 4.85 ± 1.19 | 2.72 ± 0.89 | 4.28 ± 0.44 |
| 2,3-Butanediol | - | Fruit, onion | n.d. | 2.49 ± 1.62 | 11.07 ± 2.17 | 10.71 ± 0.78 |
| Linalool | 15 d | Flower, lavender | n.d. | 8.39 ± 4.80 | 2.59 ± 0.55 | 2.78 ± 0.40 |
| 3,4-Dimethyl pentanol | - | - | 2.95 ± 1.24 | 2.23 ± 0.25 | 107.98 ± 11.40 | 92.08 ± 15.65 |
| α-Terpineol | 250 d | Oil, anise, mint | 8.08 ± 1.04 | 10.54 ± 2.10 | 9.31 ± 2.06 | 9.93 ± 1.50 |
| 2-Phenyl-2-hexanol | - | - | n.d. | 6.33 ± 3.12 | 3.78 ± 0.83 | 4.65 ± 0.82 |
| Citronellol | 100 d | Rose | n.d. | 3.26 ± 0.46 | 3.37 ± 0.84 | 3.72 ± 1.37 |
| Geraniol | 30 d | Rose, geranium | n.d. | 9.32 ± 2.71 | 8.61 ± 0.97 | 8.54 ± 1.08 |
| 2-Phenylethanol | 10,000 d | Honey, spice, rose, lilac | n.d. | 15978 ± 504 | 16286 ± 1805 | 14660 ± 690 |
| p-Tyrosol | - | - | n.d. | 258.13 ± 59.94 | 271.51 ± 20.35 | 223.25 ± 13.96 |
| Total | 255.97 ± 54.62 | 33704 ± 2089 | 30307 ± 2770 | 26256 ± 2174 | ||
| Aldehydes | ||||||
| Nonanal | 8 d | Fat, citrus, green | 66.71 ± 22.51 | 12.21 ± 2.44 | 10.99 ± 1.30 | 12.45 ± 3.18 |
| Benzaldehyde | 5 f | Almond, sugar | 16.27 ± 3.80 | 7.78 ± 3.27 | 7.10 ± 1.14 | 9.26 ± 4.13 |
| Phenylethanal | 1 e | Honey, sweet, hawthorn | n.d. | 5.73 ± 2.88 | 4.27 ± 0.40 | 3.25 ± 1.24 |
| 2,4-Dimethyl benzaldehyde | - | Sweet | 4.37 ± 1.11 | 4.25 ± 0.53 | 6.41 ± 2.61 | 7.23 ± 2.46 |
| Total | 87.35 ± 27.42 | 29.97 ± 9.12 | 28.77 ± 5.45 | 32.19 ± 11.00 | ||
| Benzenoids | ||||||
| 4-Vinyl guaiacol | 40 d | Clove, curry | 8.84 ± 1.91 | 100.32 ± 3.32 | 108.49 ± 12.39 | 83.19 ± 8.35 |
| Guaiacol | 9.5 e | Smoke, sweet, medicine | 4.20 ± 2.21 | 10.45 ± 2.97 | 9.22 ± 1.42 | 6.10 ± 3.94 |
| Syringol | - | Medicine, phenol, smoke | 88.84 ± 24.29 | 65.25 ± 2.79 | 61.30 ± 12.28 | 51.05 ± 7.30 |
| Total | 101.88 ± 28.41 | 176.01 ± 9.08 | 179.01 ± 26.09 | 146.28 ± 19.59 | ||
| Esters | ||||||
| Isoamyl acetate | 12,270 d | Banana | n.d. | 19.52 ± 2.66 | 17.18 ± 1.29 | 21.81 ± 3.48 |
| Ethyl hexanoate | 14 a | Apple, peach | n.d. | 59.77 ± 3.26 | 37.93 ± 6.20 | 35.84 ± 3.75 |
| Ethyl octanoate | 2 f | Fruit, fat | n.d. | 47.79 ± 7.05 | 44.75 ± 1.55 | 42.12 ± 2.66 |
| Ethyl decanoate | 200 d | Grape | 5.15 ± 1.04 | 105.10 ± 57.31 | 36.54 ± 10.77 | 29.11 ± 16.62 |
| Diethyl succinate | 200,000 e | Wine, fruit | n.d. | 6.21 ± 2.14 | 18.72 ± 2.34 | 26.21 ± 2.96 |
| Ethyl-9-decenoate | - | Fruity | n.d. | 45.76 ± 27.65 | 26.45 ± 14.37 | 29.94 ± 12.11 |
| Ethyl acetate | 7500 d | Pineapple | n.d. | 28.19 ± 11.67 | 15.78 ± 4.43 | 14.21 ± 2.03 |
| α-Isoamyl-γ-butyrolactone | - | Coumarin, sweet | n.d. | 33.19 ± 6.15 | 35.56 ± 6.72 | 30.75 ± 3.19 |
| Phenylethyl acetate | 250 d | Rose, honey, tobacco | n.d. | 77.32 ± 10.94 | 109.03 ± 18.45 | 118.19 ± 18.44 |
| Butyl isobutyrate | Fruity, green, apple, banana | n.d. | 112.44 ± 13.31 | 100.93 ± 15.08 | 82.38 ± 9.51 | |
| γ-Nonalactone | 25 d | Coconut, peach | 4.71 ± 2.87 | 5.29 ± 0.87 | 6.24 ± 0.99 | 6.92 ± 0.26 |
| Diethyl malate | - | Brown sugar, sweet | n.d. | 7.05 ± 2.25 | 7.47 ± 0.63 | 8.57 ± 1.56 |
| Methyl hexadecanoate | - | Fat, wax | 44.59 ± 6.38 | 40.93 ± 2.45 | 37.31 ± 4.58 | 33.74 ± 5.44 |
| Ethyl hexadecanoate | - | Wax | n.d. | 24.95 ± 10.29 | 70.72 ± 16.86 | 53.35 ± 19.71 |
| Ethyl hydrogen succinate | - | Wine, fruit | n.d. | 43.36 ± 29.15 | 99.77 ± 15.86 | 87.70 ± 19.43 |
| Phenethyl propionate | - | Fruit | n.d. | 18.16 ± 2.35 | 15.54 ± 2.32 | 14.09 ± 1.21 |
| Total | 54.46 ± 10.28 | 675.03 ± 189.51 | 679.92 ± 122.45 | 634.95 ± 122.37 | ||
| Furanoids | ||||||
| cis-Linalool oxide | - | Flower | n.d. | 1.96 ± 0.50 | 197.44 ± 27.48 | 175.09 ± 4.73 |
| Ketones | ||||||
| 6-Methyl-2-heptanone | - | Soap | n.d. | 8.16 ± 4.18 | 6.73 ± 4.97 | 6.01 ± 1.16 |
| Norisoprenoids | ||||||
| 3-Hydroxy-β-damascone | - | Apple, tea, tobacco | 11.31 ± 6.25 | 146.28 ± 106.57 | 65.23 ± 30.63 | 87.69 ± 8.09 |
| Thiols | ||||||
| 3-(Methylthio)-propanol | 1000 b | Sweet, potato | n.d. | 51.62 ± 8.23 | 63.92 ± 6.73 | 53.89 ± 6.25 |
| Compound | Perception Threshold (μg/L) | Descriptor | Cabernet Sauvignon Must/Kiwi Juice | Days of Fermentation | ||
|---|---|---|---|---|---|---|
| 3 | 11 | 17 * | ||||
| Acids | ||||||
| Nonanoic acid | - | Green, fat | 20.20 ± 1.22 | 11.13 ± 1.23 | 17.10 ± 2.61 | 17.89 ± 3.22 |
| Geranic acid | - | Green, floral | 28.77 ± 9.87 | 26.22 ± 10.76 | 21.65 ± 10.08 | 20.65 ± 2.81 |
| Total | 48.97 ± 11.09 | 37.25 ± 11.99 | 38.75 ± 12.69 | 38.54 ± 6.03 | ||
| Alcohols | ||||||
| 3-Penten-2-ol | - | Green, vinyl | 41.32 ± 2.79 | 41.41 ± 4.60 | 30.93 ± 9.42 | 38.67 ± 6.20 |
| 1-Hexanol | 1110c | Resin, flower, green | 34.95 ± 4.95 | 33.81 ± 1.92 | 32.93 ± 5.49 | 28.73 ± 1.36 |
| 2-Hexanol | - | Resin, flower, green | 27.31 ± 2.96 | 24.95 ± 0.83 | 25.61 ± 5.46 | 26.56 ± 3.20 |
| 3-Octanol | - | Moss, nut, mushroom | 79.09 ± 4.60 | 98.55 ± 4.14 | 99.64 ± 2.55 | 106.25 ± 2.74 |
| Linalool | 15a | Flower, lavender | 12.10 ± 2.69 | 11.09 ± 1.49 | 10.19 ± 0.78 | 7.87 ± 3.44 |
| α-Terpineol | 250a | Oil, anise, mint | 9.34 ± 1.07 | 7.99 ± 0.71 | 9.21 ± 0.40 | 10.16 ± 1.85 |
| Nerol | - | Sweet | 112.53 ± 5.18 | 125.19 ± 8.13 | 130.25 ± 4.49 | 131.72 ± 13.58 |
| Benzyl alcohol | - | Sweet, flower | 334.71 ± 13.38 | 113.71 ± 6.47 | 110.66 ± 8.20 | 78.00 ± 9.63 |
| 8-Hydroxygeraniol | - | - | 8.34 ± 1.82 | 8.38 ± 1.22 | 8.55 ± 0.63 | 16.24 ± 3.92 |
| Total | 659.69 ± 39.46 | 465.08 ± 29.51 | 457.97 ± 37.41 | 444.20 ± 45.92 | ||
| Aldehydes | ||||||
| 2-Hexanal | - | Grass, tallow, fat | 164.99 ± 19.71 | 42.11 ± 7.92 | 34.88 ± 8.33 | 33.52 ± 5.48 |
| Nonanal | 8a | Fat, citrus, green | 4.31 ± 1.16 | 3.08 ± 0.19 | 2.45 ± 0.57 | 12.78 ± 2.33 |
| Benzaldehyde | 5b | Almond, sugar | 9.22 ± 2.66 | 4.09 ± 2.22 | 2.63 ± 0.61 | 2.52 ± 0.91 |
| Total | 178.51 ± 23.54 | 49.28 ± 10.34 | 39.96 ± 9.52 | 48.82 ± 8.72 | ||
| Benzenoids | ||||||
| 4-Vinyl guaiacol | 40a | Clove, curry | 154.10 ± 36.96 | 84.51 ± 13.28 | 100.87 ± 10.01 | 95.61 ± 5.82 |
| Eugenol | - | Clove, honey | 14.20 ± 2.32 | 17.10 ± 2.98 | 16.96 ± 1.85 | 17.52 ± 0.27 |
| Syringol | - | Medicine, phenol, smoke | 49.85 ± 19.09 | 31.19 ± 8.11 | 58.55 ± 22.44 | 44.61 ± 9.42 |
| Total | 218.16 ± 58.37 | 132.80 ± 24.36 | 176.38 ± 34.29 | 157.73 ± 15.52 | ||
| Norisoprenoids | ||||||
| 3-oxo-α-damascone | - | Apple | 35.24 ± 11.16 | 39.86 ± 1.83 | 38.49 ± 3.05 | 37.47 ± 3.72 |
| 3-Oxo-α-ionol | - | Spice, tea, tobacco | 118.34 ± 32.57 | 83.13 ± 27.57 | 81.43 ± 2.23 | 70.78 ± 8.83 |
| Total | 153.58 ± 58 | 123.00 ± 29.39 | 119.92 ± 5.28 | 108.25 ± 12.55 | ||
| Furanoids | ||||||
| cis-Linalool oxide | - | Flower | 11.18 ± 1.72 | 11.34 ± 1.09 | 12.41 ± 0.24 | 12.26 ± 0.82 |
| Chemical Parameter | Must/Kiwi Juice | Kiwi-Based Drink |
|---|---|---|
| Sugar (g/L) | 180 ± 17 | 8.6 ± 0.7 |
| Ethanol (v/v) | - | 9.5 ± 0.2 |
| Methanol (mg/L) | - | 75.0 |
| pH | 3.3 ± 0.01 | 3.5 ± 0.01 |
| Total acidity (g tartaric acid/L) | 9.2 ± 0.4 | 9.4 ± 0.6 |
| Tartaric acid (g/L) | 0.79 ± 0.03 | 0.51 ± 0.10 |
| Malic acid (g/L) | 4.09 ± 0.29 | 2.63 ± 0.54 |
| Lactic acid (g/L) | n.d. | n.d. |
| Acetic acid (g/L) | n.d. | 0.11 ± 0.07 |
| Citric acid (g/L) | 5.37 ± 0.28 | 6.05 ± 0.81 |
| Succinic acid (g/L) | n.d. | n.d. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fracassetti, D.; Bottelli, P.; Corona, O.; Foschino, R.; Vigentini, I. Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites 2019, 9, 86. https://doi.org/10.3390/metabo9050086
Fracassetti D, Bottelli P, Corona O, Foschino R, Vigentini I. Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites. 2019; 9(5):86. https://doi.org/10.3390/metabo9050086
Chicago/Turabian StyleFracassetti, Daniela, Paolo Bottelli, Onofrio Corona, Roberto Foschino, and Ileana Vigentini. 2019. "Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice" Metabolites 9, no. 5: 86. https://doi.org/10.3390/metabo9050086
APA StyleFracassetti, D., Bottelli, P., Corona, O., Foschino, R., & Vigentini, I. (2019). Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites, 9(5), 86. https://doi.org/10.3390/metabo9050086