Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire
Abstract
1. Introduction
2. Results
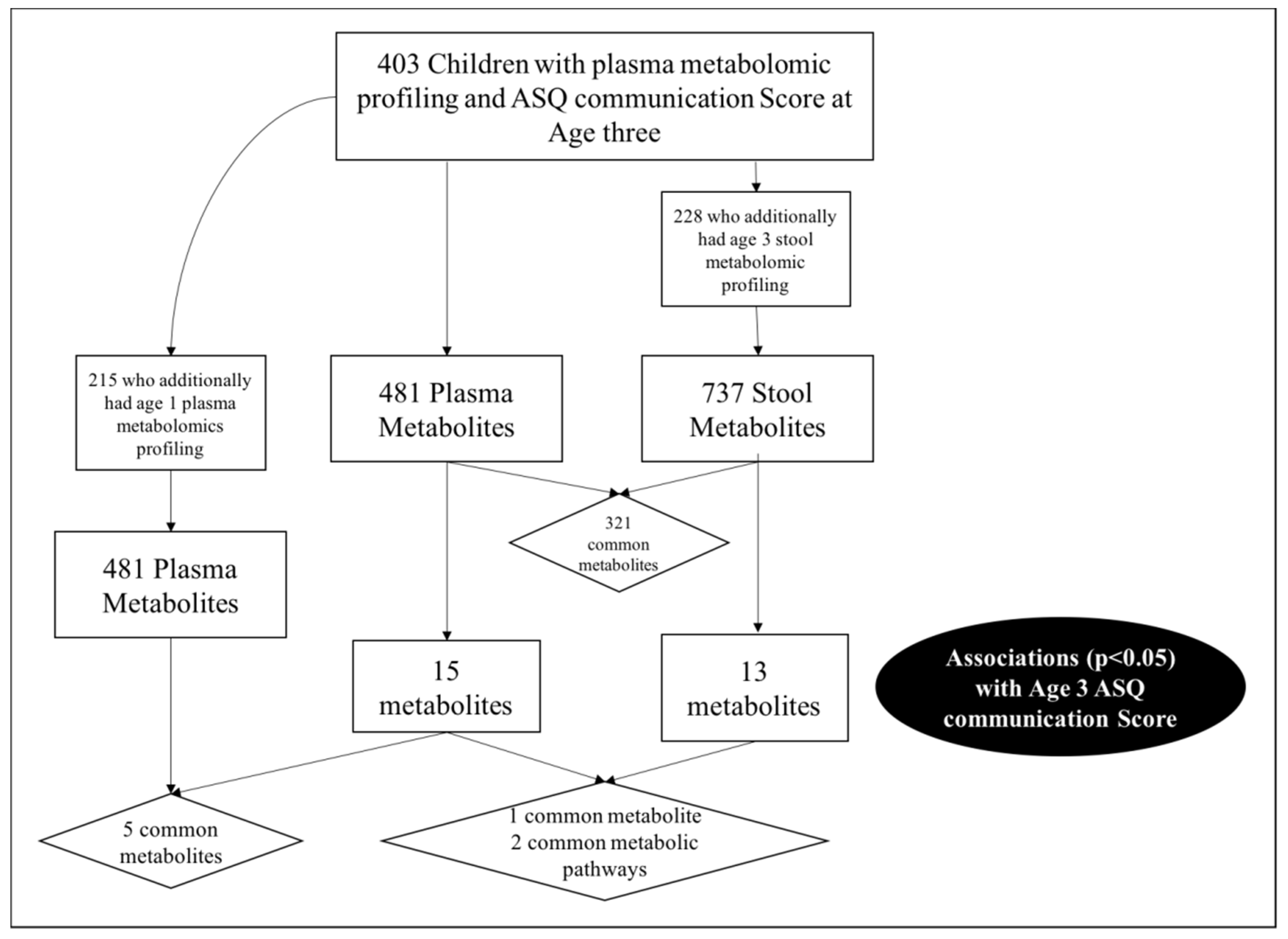
2.1. Study Population
2.2. Plasma Metabolome
2.3. Stool Metabolome
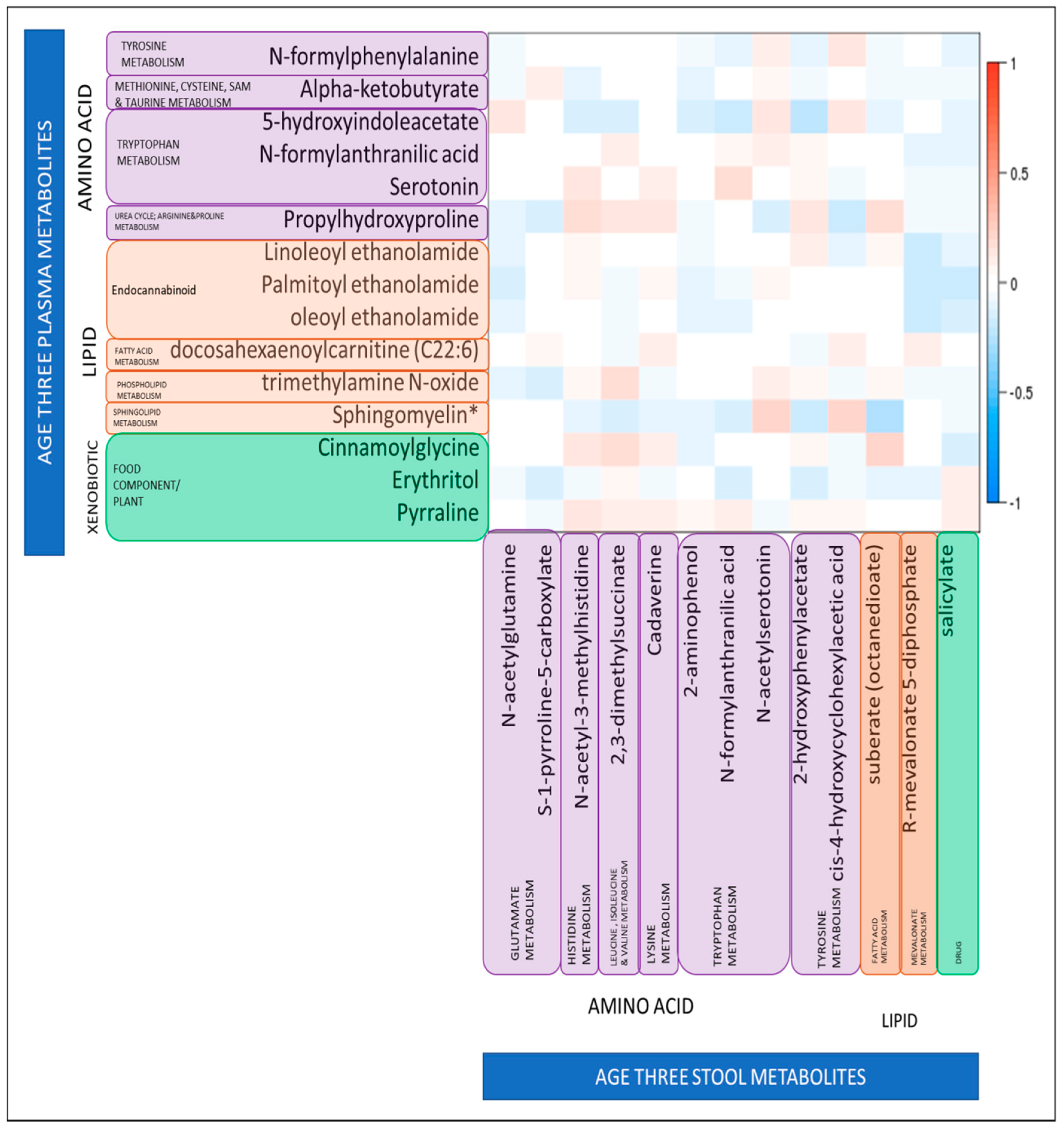
2.4. Correlation between the Plasma and Stool Metabolomic Results
2.5. Autism
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Metabolomic Profiling
4.3. Ages and Stages Questionnaire (ASQ) Assessment
4.4. Statistical Analysis
4.4.1. Plasma and Stool Metabolome
4.4.2. Correction for Multiple Testing
4.4.3. Diagnosing and Predicting Autism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar]
- Developmental Disabilities Monitoring Network Surveillance Year Principal, I.; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2014, 63, 1–21. [Google Scholar]
- Gray, K.M.; Tonge, B.J.; Brereton, A.V. Screening for Autism in Infants, Children, and Adolescents. In International Review of Research in Mental Retardation; Academic Press: Cambridge, MA, USA, 2006; Volume 32, pp. 197–227. [Google Scholar]
- Myers, S.M.; Johnson, C.P. Management of Children With Autism Spectrum Disorders. Pediatrics 2007, 120, 1162. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.; Twombly, E.; Bricker, D.; Potter, L. Ages & Stages Questionnaires, 3rd ed.; Paul H. Brookes: Baltimore, MD, USA, 2009. [Google Scholar]
- Hardy, S.; Haisley, L.; Manning, C.; Fein, D. Can Screening with the Ages and Stages Questionnaire Detect Autism? J. Dev. Behav. Pediatr. 2015, 36, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Smith, K.S.; Rosen, N.; Anderson, M.C.; Grether, J.K.; Coolman, R.B.; Harris, S. Autism and developmental screening in a public, primary care setting primarily serving Hispanics: Challenges and results. J. Autism Dev. Disord. 2014, 44, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.; Sarkans, U.; Schumann, G.; Persico, A.M. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- West, P.R.; Amaral, D.G.; Bais, P.; Smith, A.M.; Egnash, L.A.; Ross, M.E.; Palmer, J.A.; Fontaine, B.R.; Conard, K.R.; Corbett, B.A.; et al. Metabolomics as a Tool for Discovery of Biomarkers of Autism Spectrum Disorder in the Blood Plasma of Children. PLoS ONE 2014, 9, e112445. [Google Scholar] [CrossRef] [PubMed]
- Schain, R.J.; Freedman, D.X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961, 58, 315–320. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Lange, N.E.; Carey, V.J.; Brown, S.; Laranjo, N.; Harshfield, B.J.; O’Connor, G.T.; Sandel, M.; Strunk, R.C.; Bacharier, L.B.; et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp. Clin. Trials 2014, 38, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Yeung, J.M.; Cherny, S.S.; Sham, P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 2004, 74, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Autism Spectrum Disorder (ASD)—Data and Statistics; Centers for Disease Control and Prevention: Antlanta, GA, USA, 2017. [Google Scholar]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Chen, C.-F.; Pittman, A.R.; Skinner, C.D.; McCartney, H.J.; Jones, K.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol. Autism 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. A Perspective on the Safety of Supplemental Tryptophan Based on Its Metabolic Fates. J. Nutr. 2016, 146, 2601s–2608s. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F. The neuropathology of autism. Brain Pathol. 2007, 17, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009, 19, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; de Bildt, A.; van Lang, N.D.; den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, Z.; Li, G.; Piechowicz, M.; Anderson, M.; Uddin, Y.; Wu, J.; Qiu, S. The autism-associated MET receptor tyrosine kinase engages early neuronal growth mechanism and controls glutamatergic circuits development in the forebrain. Mol. Psychiatry 2016, 21, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Parolaro, D. The Endocannabinoid System and Autism Spectrum Disorders: Insights from Animal Models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Spector, T.D.; Menni, C. Blood, urine and faecal metabolite profiles in the study of adult renal disease. Arch. Biochem. Biophys. 2016, 589, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.; Moody, D.B.; Mola, A.R.; Cheng, T.-Y.; Comstock, L.E.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; et al. Intestinal microbial-derived sphingolipids are inversely associated with childhood food allergy. J. Allergy Clin. Immunol. 2018, 142, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | Age 3ASQ Communication Score | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| On Schedule (n = 365) | Needs Monitoring (n = 23) | Requires Further Evaluation (n = 15) | ||||||
| n | % | n | % | n | % | |||
| Sex | Female | 176 | 48.2% | 9 | 39.1% | 3 | 20.0% | 0.076 |
| Males | 189 | 51.8% | 14 | 60.9% | 12 | 80.0% | ||
| Site | San Diego | 131 | 35.9% | 6 | 26.1% | 0 | 0.0% | 0.002 |
| Boston | 72 | 19.7% | 5 | 21.7% | 9 | 60.0% | ||
| St Louis | 162 | 44.4% | 12 | 52.2% | 6 | 40.0% | ||
| Race | Black | 173 | 47.4% | 13 | 56.5% | 9 | 60.0% | 0.725 |
| White | 122 | 33.4% | 6 | 26.1% | 5 | 33.3% | ||
| Other | 70 | 19.2% | 4 | 17.4% | 1 | 6.7% | ||
| Treatment | Vitamin D | 186 | 51.0% | 12 | 52.2% | 7 | 46.7% | 0.940 |
| Placebo | 179 | 49.0% | 11 | 47.8% | 8 | 53.3% | ||
| Asthma/Wheeze | Yes | 93 | 25.5% | 5 | 21.7% | 6 | 40.0% | 0.414 |
| No | 272 | 74.5% | 18 | 78.3% | 9 | 60.0% | ||
| Maternal Marital Status | Married | 179 | 49.0% | 9 | 39.1% | 4 | 26.7% | 0.449 |
| Not married/not living together | 88 | 24.1% | 8 | 34.8% | 6 | 40.0% | ||
| Not married - living together | 86 | 23.6% | 6 | 26.1% | 5 | 33.3% | ||
| Separated/Divorced | 12 | 3.3% | 0 | 0.0% | 0 | 0.0% | ||
| Maternal Educational Level | Less than high school | 45 | 12.3% | 3 | 13.0% | 3 | 20.0% | 0.057 |
| High school, Technical school | 96 | 26.3% | 10 | 43.5% | 5 | 33.3% | ||
| Some college | 84 | 23.0% | 5 | 21.7% | 6 | 40.0% | ||
| College graduate/Graduate school | 140 | 38.4% | 5 | 21.7% | 1 | 6.7% | ||
| Body Mass Index (BMI) at age 3 | Mean (standard deviation, SD) | 16.7 (1.9) | 16.4 (1.3) | 17.2 (2.3) | 0.528 | |||
| Age 1 Communication Score | On Schedule | 308 | 84.4% | 18 | 78.3% | 14 | 93.3% | 0.200 |
| Needs monitoring | 5 | 1.4% | 1 | 4.3% | 1 | 6.7% | ||
| Requires further evaluation | 2 | 0.5% | 0 | 0.0% | 0 | 0.0% | ||
| Missing | 50 | 13.7% | 4 | 174% | 0 | 0.0% | ||
| Age 2 Communication Score | On Schedule | 315 | 86.3% | 11 | 47.8% | 5 | 33.3% | 8.0 × 10−9 |
| Needs monitoring | 24 | 6.6% | 5 | 21.7% | 5 | 33.3% | ||
| Requires further evaluation | 7 | 1.9% | 3 | 13.0% | 4 | 26.7% | ||
| Missing | 19 | 5.2% | 4 | 17.4% | 1 | 6.7% | ||
| Stool samples available | Yes | 204 | 55.9% | 14 | 60.9% | 10 | 66.7% | - |
| Metabolite | Super Pathway | Sub Pathway | HMDB ID a | OR (95% Confidence Interval, CI) | p-Value |
|---|---|---|---|---|---|
| N-formylphenylalanine * | Amino Acid | Tyrosine Metabolism | - | 4.1 × 10−3 (4.1 × 10−2,0.11) | 0.002 |
| trimethylamine N-oxide * | Lipid | Phospholipid Metabolism | HMDB00925 | 30.13 (2.74,337.23) | 0.005 |
| cinnamoylglycine * | Xenobiotics | Food Component/Plant | HMDB11621 | 4.41 (1.52,12.80) | 0.006 |
| linoleoyl ethanolamide * | Lipid | Endocannabinoid | HMDB12252 | 12.38 (1.67,92.73) | 0.013 |
| palmitoyl ethanolamide | Lipid | Endocannabinoid | HMDB02100 | 141.67 (2.25,9324.32) | 0.019 |
| 5-hydroxyindoleacetate * | Amino Acid | Tryptophan Metabolism | HMDB00763 | 0.08 (0.01,0.64) | 0.023 |
| erythritol | Xenobiotics | Food Component/Plant | HMDB02994 | 21.39 (1.31,300.33) | 0.024 |
| pyrraline * | Xenobiotics | Food Component/Plant | HMDB33143 | 0.11 (0.01,0.68) | 0.024 |
| sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0) * | Lipid | Sphingolipid Metabolism | - | 0.02 (3.3 × 10−4,0.62) | 0.034 |
| docosahexaenoylcarnitine (C22:6) | Lipid | Fatty Acid Metabolism (Acyl Carnitine) | - | 6.28 (1.07,34.75) | 0.037 |
| prolylhydroxyproline | Amino Acid | Urea cycle; Arginine and Proline Metabolism | HMDB06695 | 95.30 (1.29,7378.98) | 0.038 |
| alpha-ketobutyrate | Amino Acid | Methionine, Cysteine, SAM and Taurine Metabolism | HMDB00005 | 3.98 (1.04,14.72) | 0.040 |
| N-formylanthranilic acid * | Amino Acid | Tryptophan Metabolism | HMDB04089 | 0.05 (3.3 × 10−4,0.75) | 0.043 |
| Serotonin * | Amino Acid | Tryptophan Metabolism | HMDB00259 | 5.75 (1.03,32.63) | 0.046 |
| oleoyl ethanolamide | Lipid | Endocannabinoid | HMDB02088 | 12.75 (0.99,161.42) | 0.048 |
| Metabolite | Super Pathway | Sub Pathway | HMDB ID | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| salicylate * | Xenobiotics | Drug | HMDB01895 | 0.3 (0.13,0.64) | 0.003 |
| R-mevalonate 5-diphosphate * | Lipid | Mevalonate Metabolism | HMDB01981 | 0.38 (0.18,0.69) | 0.004 |
| N-acetylglutamine * | Amino Acid | Glutamate Metabolism | HMDB06029 | 0.25 (0.09,0.65) | 0.005 |
| 2-hydroxyphenylacetate | Amino Acid | Tyrosine Metabolism | HMDB00669 | 0.44 (0.22,0.83) | 0.014 |
| suberate (octanedioate) * | Lipid | Fatty Acid, Dicarboxylate | HMDB00893 | 0.31 (0.12,0.78) | 0.015 |
| 2-aminophenol | Amino Acid | Tryptophan Metabolism | - | 0.43 (0.21,0.85) | 0.016 |
| cadaverine * | Amino Acid | Lysine Metabolism | HMDB02322 | 0.55 (0.33,0.89) | 0.017 |
| N-acetyl-3-methylhistidine | Amino Acid | Histidine Metabolism | - | 0.57 (0.34,0.96) | 0.034 |
| N-formylanthranilic acid | Amino Acid | Tryptophan Metabolism | HMDB04089 | 0.38 (0.15,0.93) | 0.039 |
| cis-4-hydroxycyclohexylacetic acid | Amino Acid | Tyrosine Metabolism | HMDB00451 | 0.54 (0.29,1.00) | 0.043 |
| 2,3-dimethylsuccinate | Amino Acid | Leucine, Isoleucine and Valine Metabolism | - | 0.55 (0.31,1.00) | 0.044 |
| S-1-pyrroline-5-carboxylate | Amino Acid | Glutamate Metabolism | HMDB01301 | 0.55 (0.30,1.00) | 0.048 |
| N-acetylserotonin | Amino Acid | Tryptophan Metabolism | HMDB01238 | 0.50 (0.24,0.97) | 0.050 |
| Classifier. | Area under the Curve (AUC) (95% CI) | Performance Compared to Model 1 | Sensitivity | Specificity |
|---|---|---|---|---|
| Model 1: Binary ASQ Communication Score | 0.736 (0.563, 0.909) | - | 55.6% | 91.6% |
| Model 2: Metabolite Summary Score | 0.759 (0.603, 0.915) | p = 0.635 | 66.7% | 78.7% |
| Model 3: Metabolite Levels | 0.924 (0.867, 0.980) | p = 0.034 | 88.9% | 84.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, R.S.; Boulin, A.; Laranjo, N.; Lee-Sarwar, K.; Chu, S.H.; Yadama, A.P.; Carey, V.; Litonjua, A.A.; Lasky-Su, J.; Weiss, S.T. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites 2019, 9, 42. https://doi.org/10.3390/metabo9030042
Kelly RS, Boulin A, Laranjo N, Lee-Sarwar K, Chu SH, Yadama AP, Carey V, Litonjua AA, Lasky-Su J, Weiss ST. Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites. 2019; 9(3):42. https://doi.org/10.3390/metabo9030042
Chicago/Turabian StyleKelly, Rachel S., Adrianna Boulin, Nancy Laranjo, Kathleen Lee-Sarwar, Su H. Chu, Aishwarya P. Yadama, Vincent Carey, Augusto A. Litonjua, Jessica Lasky-Su, and Scott T. Weiss. 2019. "Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire" Metabolites 9, no. 3: 42. https://doi.org/10.3390/metabo9030042
APA StyleKelly, R. S., Boulin, A., Laranjo, N., Lee-Sarwar, K., Chu, S. H., Yadama, A. P., Carey, V., Litonjua, A. A., Lasky-Su, J., & Weiss, S. T. (2019). Metabolomics and Communication Skills Development in Children; Evidence from the Ages and Stages Questionnaire. Metabolites, 9(3), 42. https://doi.org/10.3390/metabo9030042







